Abstract
The activation of nuclear factor κB (NF-κB) contributes to muscle degeneration that results from dystrophin deficiency in human Duchenne muscular dystrophy (DMD) and in the mdx mouse. In dystrophic muscle, NF-κB participates in inflammation and failure of muscle regeneration. Peptides containing the NF-κB Essential Modulator (NEMO) binding domain (NBD) disrupt the IκB kinase complex, thus blocking NF-κB activation. The NBD peptide, which is linked to a protein transduction domain to achieve in vivo peptide delivery to muscle tissue, was systemically delivered to mdx mice for 4 or 7 weeks to study NF-κB activation, histological changes in hind limb and diaphragm muscle and ex vivo function of diaphragm muscle. Decreased NF-κB activation, decreased necrosis and increased regeneration were observed in hind limb and diaphragm muscle in mdx mice treated systemically with NBD peptide, as compared to control mdx mice. NBD peptide treatment resulted in improved generation of specific force and greater resistance to lengthening activations in diaphragm muscle ex vivo. Together these data support the potential of NBD peptides for the treatment of DMD by modulating dystrophic pathways in muscle that are downstream of dystrophin deficiency.
Keywords: Duchenne Muscular Dystrophy, mdx mouse, histopathology, muscle necrosis, muscle regeneration, specific force, lengthening activation, protein transduction domain, NEMO binding domain peptide, NF-κB
Introduction
Dystrophin deficiency causes muscle degeneration in Duchenne muscular dystrophy (DMD) patients and in the mdx mouse, a murine model for DMD (Bulfield et al., 1984). Dystrophin, a 427kDa cytoskeletal protein expressed from the X-linked dystrophin gene, provides structural stability and functional signaling from the internal cytoskeleton to the extracellular matrix through the dystrophin-glycoprotein complex, (DGC) (Ervasti et al., 1990; Ervasti and Campbell, 1991; Zubrzycka-Gaarn et al., 1988). Disruption of the DGC, due to absence or truncation of dystrophin protein, causes the dystrophic phenotype of progressive muscle necrosis, inflammation, and fibrosis. Critical intracellular pathways that mediate the inflammatory responses to muscle sarcolemmal damage and attempted regeneration are central to the pathogenesis of dystrophic changes in muscle. By these mechanisms, the detrimental structural defects of dystrophin deficiency are compounded (Guttridge, 2004). The only treatments currently available for DMD can, at best, delay progression of the disease.
Nuclear factor κB (NF-κB) is a transcription factor that is crucial for development, cell survival and innate immunity, and can regulate genes encoding a wide range of targets from growth factors to cytokines (Verma, 2004). The 5 family members of the NF-κB family are p50, p52, RelA/p65, c-Rel and RelB, and they exist as homo- or hetero-dimers. When bound to the inhibitor protein IκB in the cytoplasm of mammalian cells, NF-κB remains in an inactive state. However, upon induction by specific cell stimuli, such as TNF-α or IL-1β, the IκB kinase (IKK) complex phosphorylates the IκB inhibitory protein (IκB). Once phosphorylated, IκB is targeted for ubiquitin-mediated proteosomal degradation, exposing the NF-κB subunits' nuclear localization signal, leading to nuclear translocation whereby the NF-κB dimers regulate transcription (Häcker H and Karin M, 2006).
Increased levels of NF-κB activation are observed in muscle of DMD patients and the mdx mouse. A heterozygous deletion of the p65 subunit of NF-κB on the mdx genetic background (mdx;p65+/−) resulted in improved muscle histopathology characterized by decreased necrosis and increased regeneration, supporting the crucial role that the NF-κB p65 subunit plays in muscle health and points to a potential target for DMD therapy (Acharyya et al., 2007). Treatment of mdx mice in vivo with therapies targeted to interrupt NF-κB activation have been previously reported to improve the dystrophic phenotype, including infliximab (Grounds and Torrisi, 2004), N-acetylcysteine (NAC) (Whitehead et al., 2008), and pyrrolidine dithiocarbamate (PDTC) (Messina et al., 2004).
Peptide-based approaches to interrupt NF-κB activation have been developed to test for therapeutic efficacy in disease models. The NF-κB Essential Modulator (NEMO) binding domain (NBD) peptide utilized in this study shares sequence homology with the IKKβ subunit of the IKK complex. NBD peptide prevents formation of the IKK complex and thereby decreases the activation of NF-κB (May et al., 2000). The NBD peptide is synthesized as a fusion peptide with a protein transduction domain (PTD) to facilitate intra-cellular delivery (Strickland and Ghosh, 2006).
PTDs are small, naturally occurring or synthetic peptides with the robust ability to cross cell membranes and transport larger molecules into cells. PTDs can be either cationic or hydrophobic and may be tissue-specific (Tilstra et al., 2007). Cationic PTDs, such as the HIV-transactivator of transcription (TAT) or 8 lysines (8K), bind heparin on the cell surface (Hakansson et al., 2001; Rusnati et al., 1997). Another naturally occurring cationic PTD, Drosophila Antennapedia (Antp), also binds to cell surface glycosaminoglycans (Tilstra et al., 2007). After cell surface binding, the PTD and fused cargo are internalized by macropinocytosis and released into the cytoplasm (Noguchi and Matsumoto, 2006).
Prior biodistribution studies have been performed with PTD-delivered peptides. The uptake of streptavidin-Cy3-linked PTD into cells has been shown previously in cultured cells (Dave et al., 2007; Mi et al., 2000). One in vivo study showed uptake of streptavidin-Cy3-linked PTD into spleen and mesenteric lymph nodes within 30 minutes of intraperitoneal administration (Dave et al., 2007). To complement PTD-peptide uptake studies with functional uptake studies, Khaya and Robbins (Khaja and Robbins, 2010) linked different PTDs to the NEMO binding domain peptide (NBD) and performed studies of PTD delivery of the functional NBD cargo. Schwarze et al. showed uptake of a TAT-FITC peptide in skeletal muscle after intraperitoneal administration to mice (Schwarze et al., 1999).
In this study, we explore the ability of NBD, fused to different cationic PTDs, to inhibit activation of NF-κB in muscle of the mdx murine model for DMD, to ameliorate the pathology of skeletal muscle in both hind limb and diaphragm in treated mdx mice and to improve physiological functioning of diaphragm muscle, tested by analysis of ex vivo force production and resistance to lengthening activations.
Materials and Methods
Synthesis of fusion peptides
Fusion peptides of wild-type or mutant NBD sequence with PTD sequence (different PTD sequences are described below) were synthesized at the Peptide Synthesis Facility (University of Pittsburgh, Pittsburgh, PA). The PTDs utilized in this study are the naturally occurring TAT-NBD (amino acid sequence: GYGRKKRRQRR), the Antp-NBD (amino acid sequence: RQIKIWFQNRRMKWKK) and 8K-NBD (amino acid sequence: KKKKKKKK). PTDs were linked to either an 11-amino acid wild-type NBD peptide, representing the binding site of IKKβ subunit, (amino acid sequence: TALDWSWLQTE) or mutant NBD peptide in which the tryptophan residues were replaced with alanine residues (amino acid sequence: TALDASALQTE). Fusion peptides contained an N-terminal PTD linked to a C-terminal NBD wild-type or mutant peptide by a 2 glycine spacer (GG).
Mice and administration of fusion peptides
C57BL/10/J and C57BL/10ScSn-Dmdmdx/J (mdx) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were housed at the University of Pittsburgh, Biomedical Science Tower South Animal Housing Facility in specific pathogen-free, controlled light and humidity environmental conditions and received unrestricted access to food and water. Mice were sacrificed by carbon dioxide inhalation, followed by cervical dislocation to ensure death, prior to muscle harvesting. Studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Control EMSA analysis was performed on untreated C57BL/10J and mdx mice at 4.5, 9, and 12 weeks of age. For EMSA studies on muscle from PTD-NBD peptide treated mdx mice, muscle was collected from 8K- and TAT-NBD and saline treated 9 week old mice after a single intraperitoneal injection of 10mg/kg peptide or saline. For EMSA studies on muscle from Antp-NBD treated mdx mice, muscle was collected after treatment with 2µg peptide, 3 times per week for 4 weeks (n=3 per group).
In the 4 week experimental peptide study, sex-matched groups of 4.5 week old mdx mice were each administered intraperitoneal injections of PTD-NBD peptide dosed at 10mg/kg 3 times per week (every other day) for 4 weeks (n=8). For the 7 week experimental peptide study, sex-matched, 4 week old mdx mice were each administered intraperitoneal injections of PTD-NBD peptide dosed at 10mg/kg or saline diluent alone 3 times per week for 7 weeks (n=8). At the time of sacrifice, diaphragm muscle from experimental and control animals in the 7 week study was analyzed ex vivo for force production and resistance to lengthening activations as described below.
Ex vivo force analysis of costal diaphragm
To perform the ex vivo functional analysis, experimental and control mice were anesthetized with 50mg/kg pentobarbital followed by costal diaphragm excision with the rib origin and central tendon insertion intact. Diaphragm specific force (peak isometric tetanic force normalized for muscle cross-sectional area) and isometric force generation during repetitive isovelocity lengthening activations were performed as previously described (Watchko et al., 2002; Watchko et al., 1994).
Immunohistochemical analysis of treated and control mice
For 4 week peptide treatment studies, tibialis anterior and whole diaphragm muscles were harvested and snap frozen in dry ice-cooled isopentane from mice sacrificed 24 hours after final fusion peptide injection (treated) or from age-matched mdx and C57BL/10 untreated control animals. Tissues were sectioned at 10µm and analyzed for necrosis by labeling with a fluorescein-labeled horse anti-mouse (H+L) IgG antibody (1:200) (Vector Laboratories, Burlingame, CA). Tissue sections were analyzed for regeneration by immunohistological detection of embryonic myosin heavy chain (eMyHC) expression. For immunohistology detecting eMyHC, endogenous biotin was blocked with the Biotin-Avidin Blocking Kit and mouse IgG was blocked using the Vector M.O.M. Kit (Vector Laboratories, Burlingame, CA) followed by incubation with eMyHC supernatant antibody (1:10), biotinylated goat anti-mouse IgG secondary and streptavidin alexa 488 (1:1500) (Invitrogen, Carlsbad, CA) tertiary antibodies. The eMyHC monoclonal antibody, F1.652, developed by Helen Blau, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. All sections were co-immunostained with rabbit anti-collagen IV (Millipore Corporation, Billerica, MA) primary and fluorescein-goat anti-rabbit (Sigma-Aldrich, St. Louis, MO) secondary antibodies to visualize muscle fiber membranes to facilitate total fiber counts. For quantitative analysis, 2 independent sections from separate regions of the mid-section of the muscle were counted to determine the percentage of necrotic and regenerating fibers per cross section.
For analysis of 7 week PTD-NBD peptide-treated and control, sham-treated mice, tibialis anterior muscles were harvested and snap frozen as described above. Two 10µm sections of tibialis anterior were collected from the midsection of the muscle ~500µm apart with approximately 1000 fibers per section for quantitative analysis. After harvesting costal diaphragm, the muscle was divided into 1cm lengths that were plunged into dry ice-cooled isopentane, as described above. Frozen diaphragm segments were sectioned to yield tissue cross-sections with approximately 1000 fibers per section. Two sections from each diaphragm muscle were analyzed for IgG labeling and eMyHC expression, performed as described above for 4 week peptide treatment studies.
Sections of tibialis anterior and diaphragm from mice in all treatment and control groups at both time points were also stained with hematoxylin and eosin.
Electrophoretic mobility shift assay (EMSA)
Cytoplasmic and nuclear cellular fractions were extracted from tissue samples from collected tibialis anterior and diaphragm muscles using the NE-PER Cytoplasmic and Nuclear Extraction Reagent Kit (ThermoFisher Scientific, Rockford, IL). NF-κB activity was measured by pre-incubating 5µL of muscle nuclear extract (30µg) with 5X Gel Shift Binding Buffer (Promega) and nuclease-free distilled water in a 9µL final volume, followed by incubation with an α–32P-deoxycytodine triphosphate labeled double-stranded DNA probe containing the NF-κB binding domain to detect NF-κB activity (Perkin Elmer, Waltham, MA). Probes were added at a count per minute (cpm) of ~100,000 in 1µL, followed by separation on a 6% non-denaturing polyacrylamide gel. The NF-κB probe was designed previously (Guttridge et al., 1999). Briefly, 15bp annealing oligonucleotides were annealed to 31bp template oligonucleotides at the 3' end of the template strand and the overhang was filled in with dNTPs in conjunction with 32P dCTP using DNA Polymerase I, Large (Klenow) Fragment (Invitrogen, Carlsbad, CA) with incorporation of ~7 radioactive dCTPs per DNA molecule. Labeling reactions were purified using illustra MicroSpin G50 columns (GE Healthcare, Piscataway, NJ). Oligonucleotide sequences are as follows, with the DNA binding sequence underlined: NF-κB template: 5'-cagggctggggattccccatctccacagtttcacttc-3'; NF-κB annealing: 5'-gaagtgaaactgtgg-3' (Integrated DNA Technologies, Inc, Coralville, IA).
Statistics
For the 4 week peptide treatment and control data, Dunnett's test was used for pair wise comparisons with the untreated, age-matched mdx group as the control. In the event that normal assumptions were violated, the Wilcoxon Rank Sum test was used for pair wise comparisons. For the 7 week peptide treatment, control and densitometry data, a log10 transformation was applied prior to statistical analysis. Unless otherwise stated, Scheffe’s test was used for pair-wise comparisons. In the event that the normality and homogeneity of variance assumptions were violated, and transformation did not correct the assumption violations, the Wilcoxon Rank Sum test was used for pair-wise comparisons. The MIXED procedure in SAS with repeated measures was used to model % Baseline (response) for lengthening activations. A contrast statement in the MIXED procedure was utilized to make regression inference (differences in slopes) with respect to treatment groups. SAS version 9.1 was used for the statistical analyses of the data.
Results
NF-κB activity in mouse muscle nuclear extracts
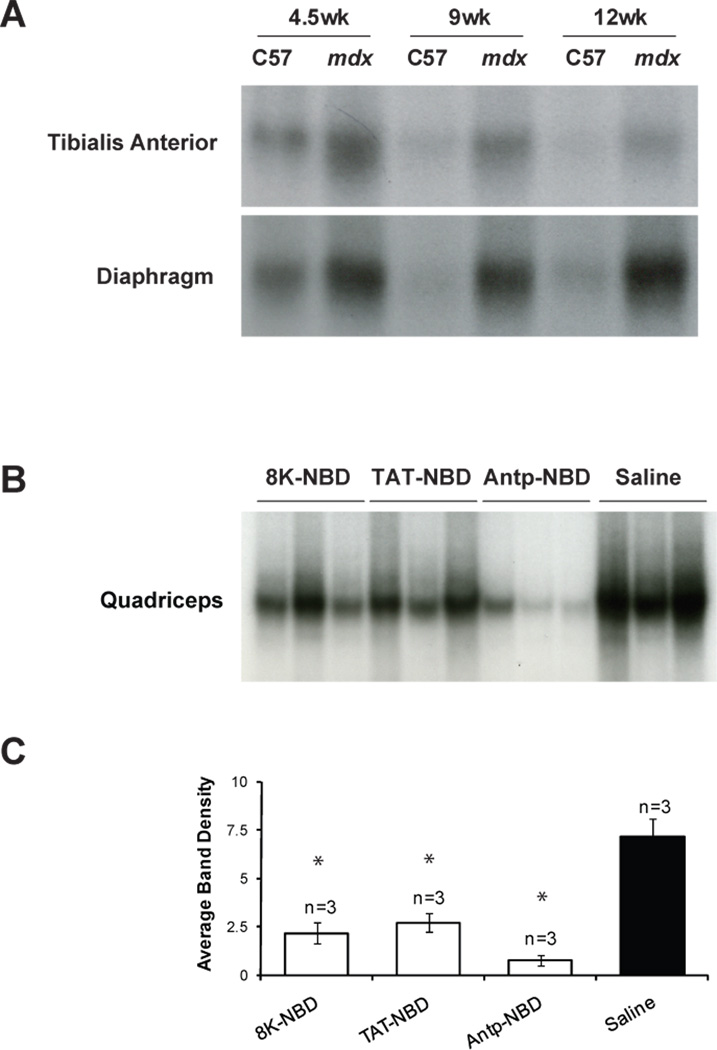
EMSA analysis of NF-κB nuclear translocation in C57BL/10 and mdx mice at 4.5, 9, and 12 weeks of age was performed to determine levels of activity at different time points relevant to this study. As shown in Figure 1A, C57BL/10 mice exhibit a basal level of NF-κB activation as part of development, but over time, there is a decrease in NF-κB activation in both hind limb skeletal muscle and diaphragm. In mdx mice, we observed high levels of NF-κB activation throughout the time frame studied in both hind limb skeletal muscle and diaphragm.
Figure 1. EMSA analysis of NF-κB activation of control and PTD-NBD peptide treated mdx mice.
(A) Nuclear extracts from tibialis anterior and diaphragm muscle tissue were assessed for NF-κB activation by electrophoretic mobility shift assay (EMSA) in untreated C57BL/10 and mdx mice at 4.5, 9, and 12 weeks of age. (B) Nuclear extracts from quadriceps muscles of 8–9-week-old 8K-, TAT- and Antp-NBD peptide treated mdx mice (n=3) and saline treated mdx mice (n=3) were assessed for NF-κB activation by EMSA. (C) Densitometric analysis of the blots shown in B represented as mean ± standard error. 8K- and TAT-NBD peptide treated mdx mice received a single injection of 10mg/kg peptide. Antp-NBD peptide treated mdx mice received intraperitoneal injections of 200µg peptide 3 times per week for 4 weeks, beginning at 4–5 weeks of age. * indicates that PTD-NBD peptide treatment group is significantly different from saline mdx control animals (p<0.05). n=number of animals analyzed.
To demonstrate that the PTD-NBD peptides suppress activation of NF-κB in muscle in vivo, muscle tissue was collected from 9 week old mdx mice treated with a single 10mg/kg intraperitoneal injection of either 8K- or TAT-NBD peptide. We also collected tissue from Antp-NBD peptide treated mdx mice that had received 200µg peptide by intraperitoneal injection 3 times per week for 4 weeks, beginning at 4–5 weeks of age. Administration of each of the PTD-NBD peptides to mdx mice resulted in a reduction in NF-κB activation compared to saline mdx controls (Figure 1 B,C). The larger reduction in NF-κB activation observed in muscle from Antp-NBD peptide treated mdx mice may reflect the sustained administration over 4 weeks as compared to a single administration with either 8K- or TAT-NBD peptide.
Analysis of tibialis anterior muscle after 4 weeks of PTD-NBD treatment
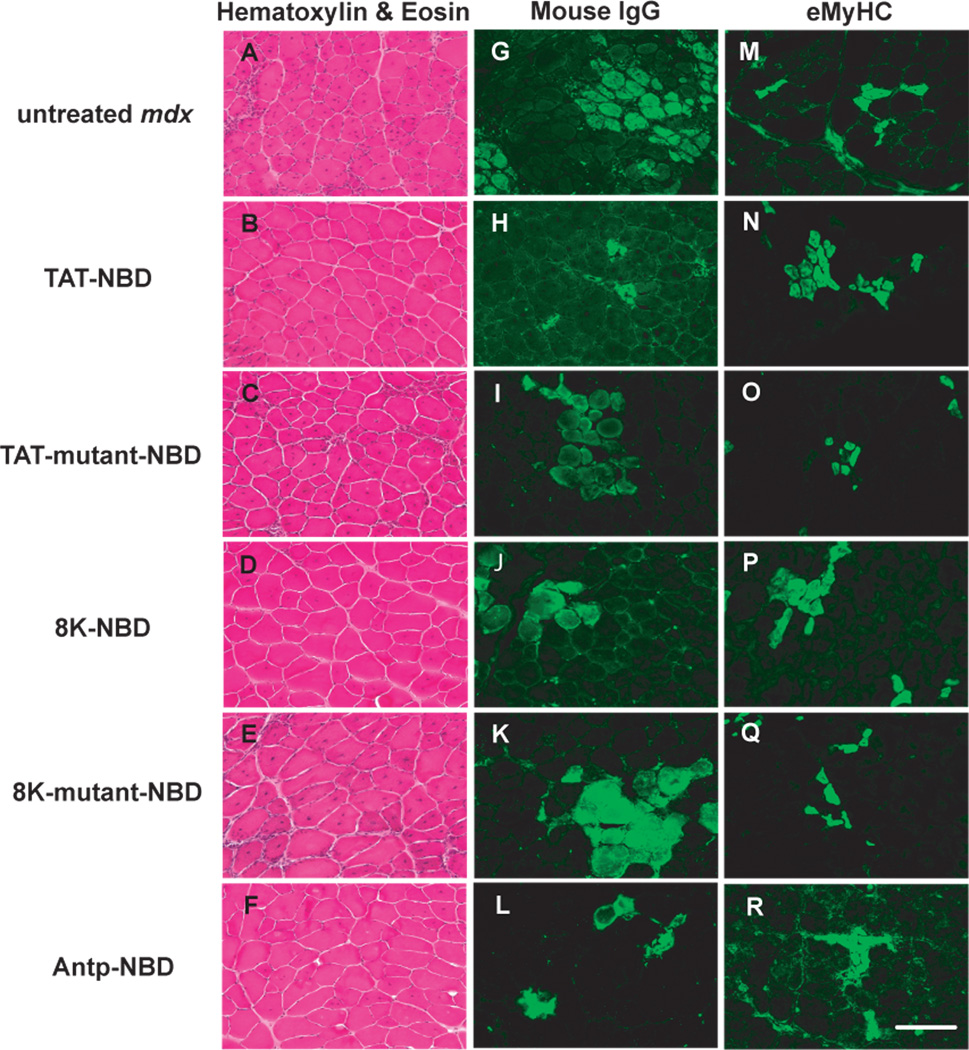
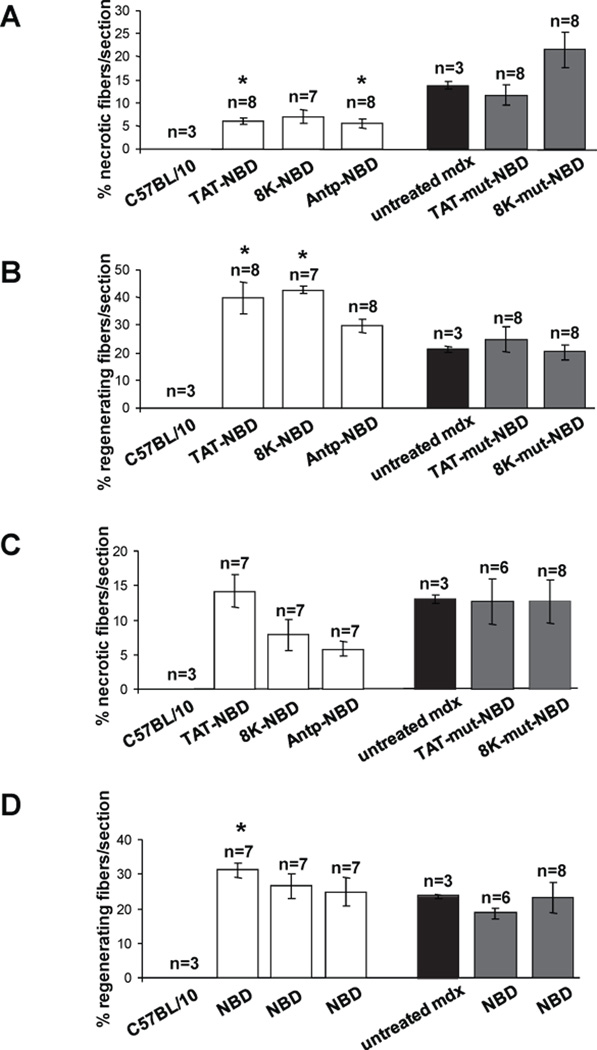
Systemic treatment of 4.5-week-old mdx mice with the 3 different wild-type PTD-NBD fusion peptides for a period of 4 weeks demonstrated improvement in dystrophic muscle histopathology when compared to age-matched mdx controls and mutant PTD-NBD peptide treated animals. Wild-type PTD-NBD treated mdx mice showed decreased necrosis in the hind limb tibialis anterior muscle after examination of hematoxylin and eosin stained and mouse IgG labeled sections (Figure 2). Untreated age-matched mdx mice had an average of 13.6% ± 0.9% necrotic fibers per section (Figure 3A). Treatment of mdx mice with TAT-NBD, 8K-NBD, and Antp-NBD fusion peptides led to decreases of 56.1%, 49.1% and 60.0%, respectively, compared to age-matched mdx control animals. Statistical significance was reached in the TAT-NBD and Antp-NBD treated groups (p=0.0091 and p=0.0121, respectively). Mutant peptide treatment did not result in significant changes in the levels of necrosis.
Figure 2. Histological analysis of mdx tibialis anterior muscle histopathology after 4 weeks of PTD-NBD peptide-mediated therapy.
10µm tibialis anterior muscle sections from age-matched control mdx mice, PTD-NBD wild type peptide-treated mdx mice, and PTD-NBD mutant peptide treated mdx mice were stained with hematoxylin and eosin for gross morphology (A–F), labeled with mouse IgG for detection of necrotic fibers (G–L), and immunostained for embryonic myosin heavy chain for detection of regenerating fibers (M–R). Representative images for each treatment group are shown. Bar represents 100µm.
Figure 3. Quantification of mdx tibialis anterior and diaphragm histopathology after 4 weeks of PTD-NBD peptide-mediated therapy.
10µm muscle sections from age-matched control C57BL/10 and mdx mice, PTD-NBD wild type peptide-treated mdx and PTD-NBD mutant peptide-treated mdx mice were quantified to assess levels of necrosis (A) and regeneration (B) in tibialis anterior muscle and necrosis (C) and regeneration (D) in diaphragm muscle. Data is represented as mean % necrotic or regenerating fibers ± standard error quantified from 2 representative sections per muscle. * indicates that PTD-NBD peptide treatment group is significantly different from untreated mdx control animals (p<0.05). n=number of animals analyzed.
Furthermore, wild-type PTD-NBD peptide treated mdx mice displayed increased numbers of regenerating fibers in hind limb skeletal muscles in response to the different PTD-NBD fusion peptides (Figure 2 and 3B). Immunostaining for eMyHC to identify regenerating muscle fibers in untreated age-matched mdx mice demonstrated an average of 21.3% ± 1.1% regenerating fibers per section. Wild-type PTD-NBD treated groups showed increased numbers of eMyHC-positive fibers compared to mdx control animals in the TAT-NBD, 8K-NBD and Antp-NBD fusion peptide treated groups, with increases of 85.6%, 100.3%, and 38.7%, respectively. TAT-NBD and 8K-NBD fusion peptide treated groups showed statistically significant (p=0.0494 and p=0.0232, respectively) increases in the number of regenerating muscle fibers compared to untreated age-matched mdx control mice. Similar to the assay of necrosis, mutant peptide treatment did not result in significant changes in the levels of regenerating muscle fibers.
Analysis of diaphragm following 4 weeks of PTD-NBD treatment
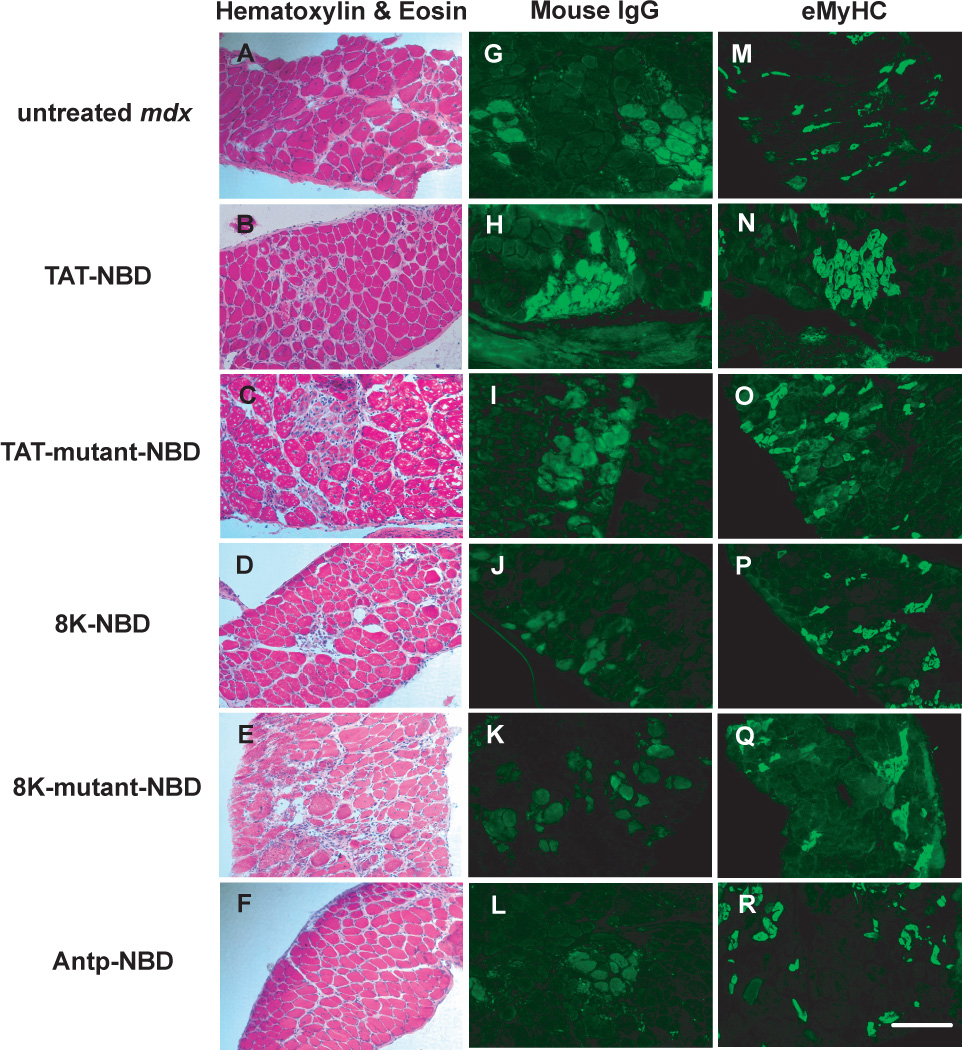
Diaphragm tissue from untreated age-matched, wild-type and mutant PTD-NBD peptide treated mdx mice were analyzed for levels of necrosis and regeneration after 4 weeks of treatment. Histological analysis with hematoxylin and eosin staining and IgG labeling of necrotic fibers are shown in Figure 4. Untreated, age-matched control mdx mice had an average of 13.0% ± 0.66% necrotic fibers per section (Figure 3C). In the 4 week wild-type PTD-NBD peptide groups, the number of necrotic fibers in the mdx diaphragm decreased by 39.8% and 55.4% in response to the 8K-NBD and Antp-NBD peptide, respectively. TAT-NBD peptide and mutant PTD-NBD peptide treatment did not alter the levels of necrosis in the diaphragm of treated mdx mice.
Figure 4. Histological analysis of mdx diaphragm muscle histopathology after 4 weeks of PTD-NBD peptide-mediated therapy.
10µm sections of diaphragm muscle from age-matched control mdx mice, 8K-, TAT-, and Antp-NBD wild type peptide-treated mdx mice, and 8K- and TAT-NBD mutant peptide-treated mdx mice were stained with hematoxylin and eosin for gross morphology (A–F), labeled with mouse IgG for detection of necrotic fibers (G–L), and immunostained for embryonic myosin heavy chain for detection of regenerating fibers (M–R). Representative images for each treatment group are shown. Bar represents 100µm.
Analysis of eMyHC immunostaining revealed that untreated age-matched mdx mice had 23.6% ± 0.52% regenerating fibers per section in the diaphragm (Figure 3D and 4). Only the wild-type TAT-NBD peptide demonstrated an effect on regeneration, showing a 32.4% increase compared to mdx untreated control animals. Mutant PTD-NBD peptide treatment had no significant effect on levels of regeneration in the diaphragm compared to age-matched control mdx mice.
Analysis of tibialis anterior muscle after 7 weeks of PTD-NBD treatment
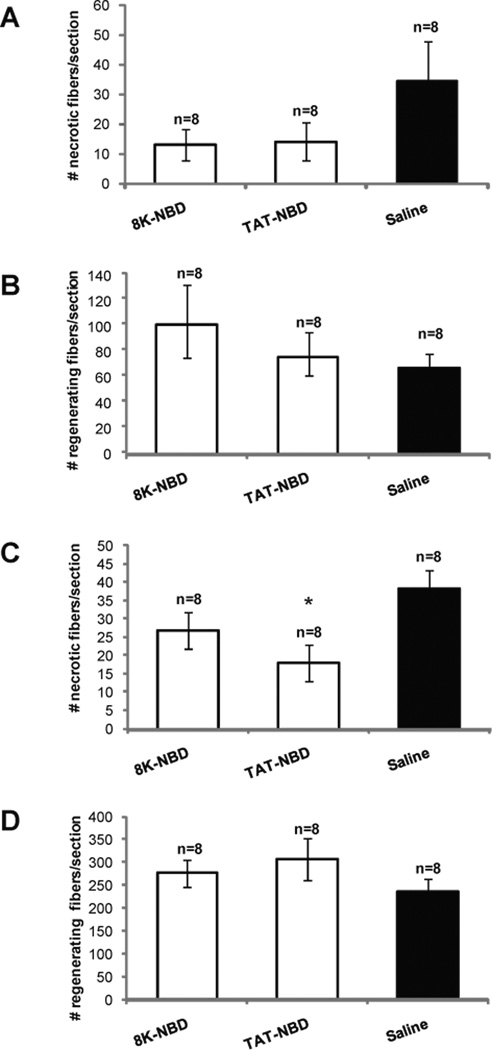
Following 7 weeks of PTD-NBD peptide treatment, we observed improved histopathology in the tibialis anterior muscle of dystrophic mdx mice treated with 8K- and TAT-NBD peptides, as compared to saline treated mdx controls, which correlated with decreased NF-κB activation in muscle tissue (Figure 5 A,C). Hematoxylin and eosin stained muscle sections revealed less cellular infiltrate and necrosis in PTD-NBD peptide treated mdx mice (Figure 6 A–C). Quantitative analysis of saline treated mdx control tibialis anterior sections averaged 34±14 necrotic fibers/section, while tissue from 8K- and TAT-NBD peptide treated mdx mice average 13±5 and 14±6 necrotic fibers/section, corresponding to decreases in necrotic fibers by 61.8% and 58.8%, respectively (Figure 7A). 8K- and TAT-NBD peptide treated mdx mice demonstrated a 51.2% and 13.5% increase in eMyHC positive, regenerating fibers in the tibialis anterior muscle when compared to saline treated mdx controls (Figure 7B).
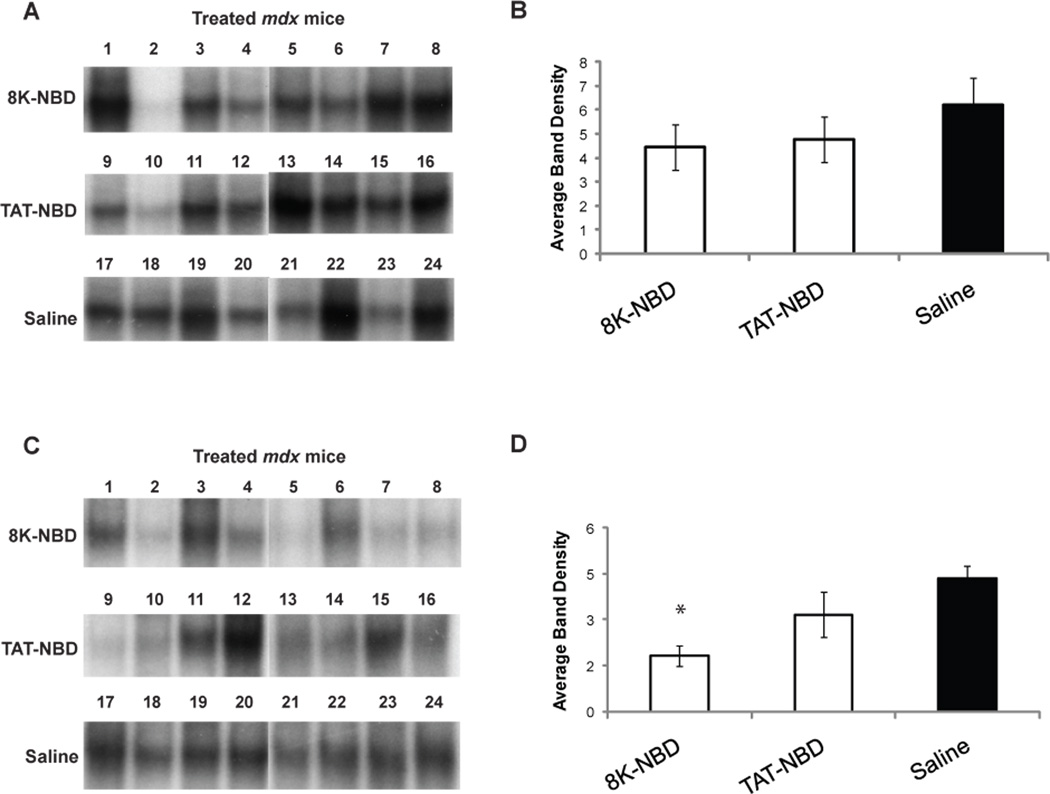
Figure 5. EMSA analysis of NF-κB activation after 7 weeks of PTD-NBD peptide or saline treatment of mdx mice.
Nuclear extracts of muscle tissue collected from mdx mice after treatment for 7 weeks with 8K- or TAT-NBD peptide, or saline were analyzed for NF-κB activation in the tibialis anterior (A,C) and costal diaphragm (B,D) muscles by electrophoretic mobility shift assay (EMSA). Individual lanes in A and B each show the level of NF-κB in nuclear extract from one animal in the study (n=8 mice/group). Mouse numbers indicate corresponding tibialis anterior and diaphragm samples taken from the same mouse. Panels C and D show densitometric analysis of blots shown in A and B, respectively, represented as mean ± standard error. * indicates that PTD-NBD peptide treatment group is significantly different from saline mdx control animals (p<0.05). n=number of animals analyzed.
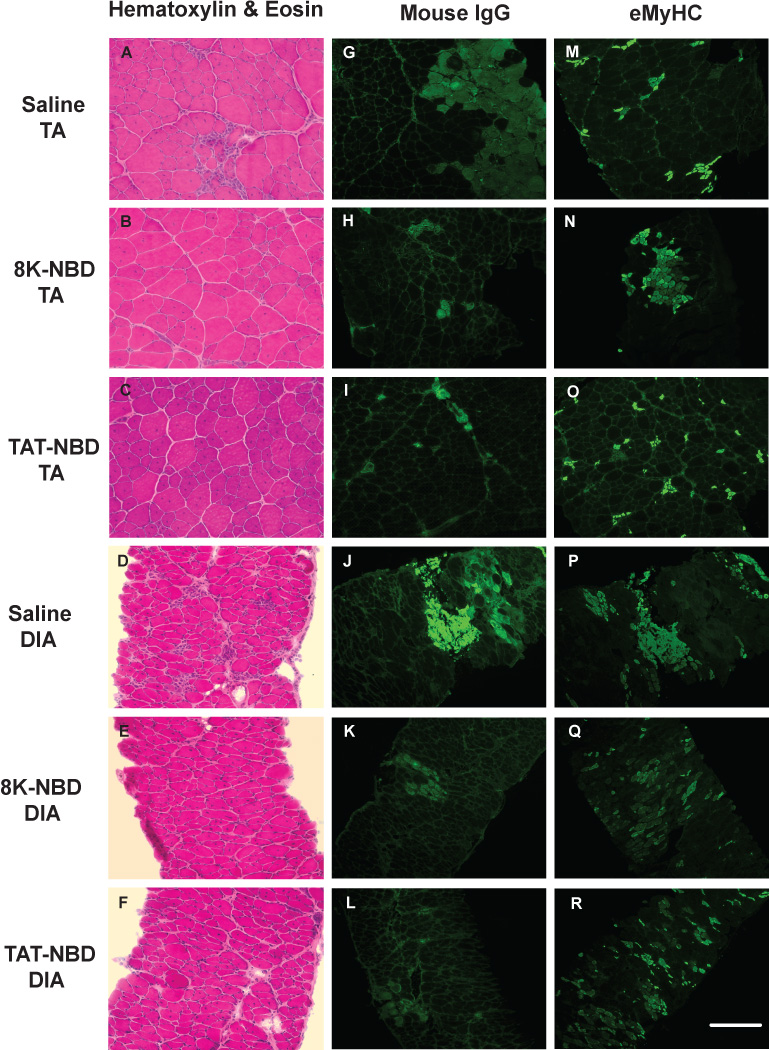
Figure 6. Histological analysis of mdx tibialis anterior and costal diaphragm muscle histopathology after 7 weeks of PTD-NBD peptide-mediated therapy.
10µm sections of tibialis anterior and costal diaphragm muscle from age-matched saline treated mdx mice and 8K- and TAT-NBD peptide-treated mdx mice were stained with hematoxylin and eosin for gross morphology (A–F), labeled with mouse IgG for detection of necrotic fibers (G–L), and immunostained for embryonic myosin heavy chain for detection of regenerating fibers (M–R). Representative images for each treatment group are shown. Bar represents 100µm. TA=tibialis anterior. DIA=diaphragm.
Figure 7. Quantification of mdx tibialis anterior and costal diaphragm muscle histopathology after 7 weeks of PTD-NBD peptide-mediated therapy.
10µm muscle sections from age-matched, saline treated mdx mice, and 8K- and TAT-NBD wild type peptide-treated mdx mice were immunostained and quantified to assess levels of necrosis (A) and regeneration (B) in tibialis anterior muscle and necrosis (C) and regeneration (D) in diaphragm muscle. Data is represented as mean total number of necrotic or regenerating fibers ± standard error quantified from 2 representative sections per muscle. * indicates that PTD-NBD peptide treatment group is significantly different from saline treated mdx control animals (p<0.05). n=number of animals analyzed.
Analysis of costal diaphragm after 7 weeks of PTD-NBD treatment
In the costal diaphragm of 7 week PTD-NBD peptide treated mdx mice, we observed improved dystrophic histopathology characterized by decreased cellular infiltrate, smaller regions of necrosis, and less fiber size variability, as compared to saline treated mdx mice (Figure 6 D–F). The observed improvements in muscle histology in PTD-NBD peptide treated mdx mice correlated with decreased levels of NF-κB activation in diaphragm tissue (Figure 5 B,D). Quantitative assessments of necrotic fibers in saline treated mdx mice revealed 38±10 necrotic fibers per section. 8K- and TAT-NBD peptide treated mdx mice had 27±6 and 18±5 necrotic fibers per section, a decrease of 30.2% and 52.5%, respectively, compared to saline treated mdx mice (Figure 7C). Muscle regeneration observed in the costal diaphragms after 7 weeks of 8K- and TAT-NBD peptide treated mdx mice revealed increases of 17.5% and 30.1%, respectively, compared to saline treated mdx mice (Figure 7D). TAT-NBD fusion peptide treated mdx mice showed statistically significant (p=0.0009) decreases in the number of necrotic muscle fibers compared to saline treated mdx control mice. Otherwise, an overall trend toward decreased number of necrotic fibers and increased number of regenerating fibers in the diaphragm with 8K- and TAT-NBD fusion peptide treatment compared to saline treated mdx control animals was observed. However, these treatment effects were not statistically significant.
Ex vivo costal diaphragm functional assay
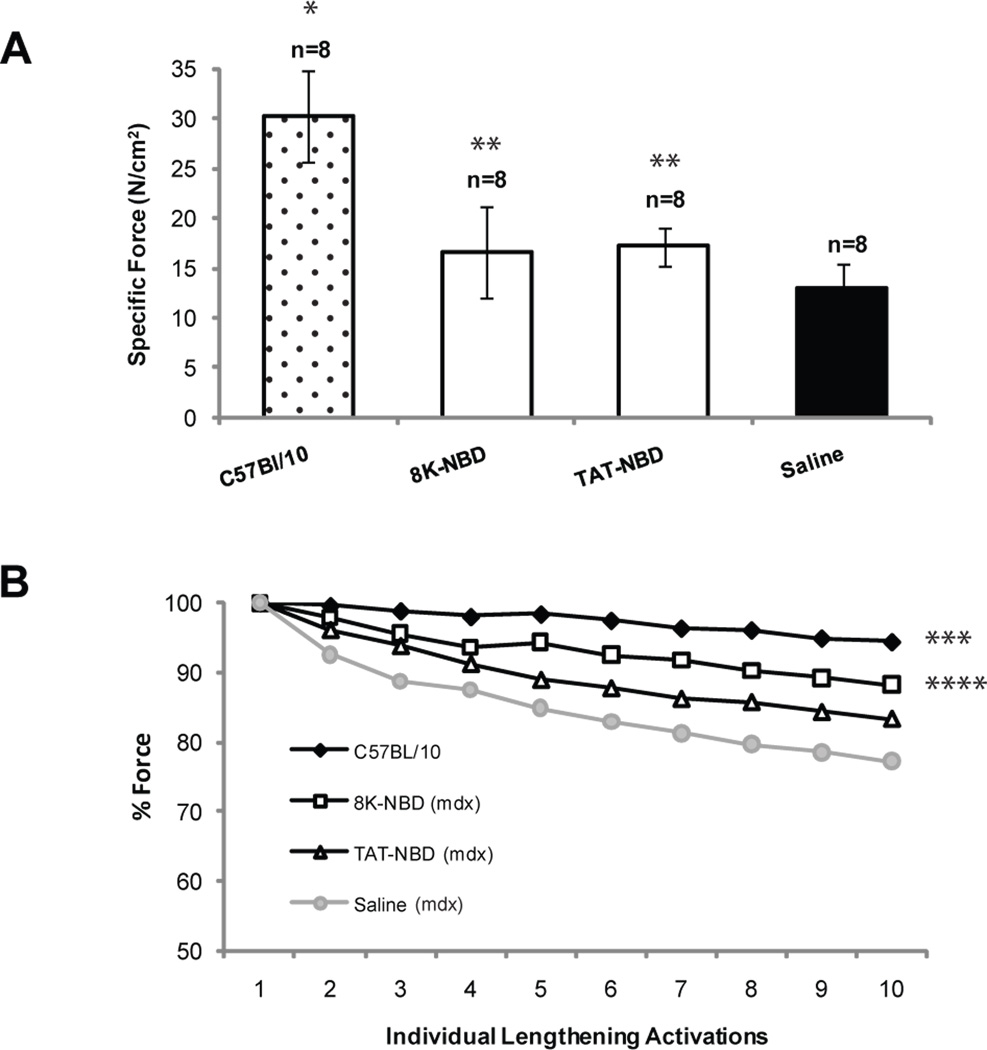
The observed improvements in muscle histopathology with PTD-NBD peptide treatment prompted us to explore the effect of PTD-NBD peptide treatment on physiological function of diaphragm compared to age-matched, saline treated mdx and C57BL/10 controls. Diaphragm from age-matched C57BL/10 normal mice demonstrated the highest average specific force of 30.2±5N/cm2, which was significantly higher than all other groups (p=<0.0001) (Figure 8A). Diaphragm from saline treated mdx mice showed an average specific force of 13±2N/cm2, representing a 57.1% decrease from C57BL/10 mice. Diaphragm from mdx mice treated with 7 weeks of 8K-NBD or TAT-NBD peptide treated mdx mice had an average specific force of 16.6±5N/cm2 and 17.1±2N/cm2, respectively, representing a 28.2% and 32.1% increase, respectively, over saline treated mdx controls. The specific force of the costal diaphragm of 8K- and TAT-NBD peptide treated mdx mice were both significantly higher than saline mdx controls (p=0.0086 and p=0.0234, respectively).
Figure 8. Force generation properties in mdx costal diaphragm after 7 weeks of PTD-NBD peptide therapy.
Costal diaphragm muscles were collected after 7 weeks of treatment with 8K- or TAT-NBD peptide or saline. Diaphragms from age-matched control C57BL/10 mice, age-matched saline treated mdx mice, and 8K- and TAT-NBD peptide-treated mdx mice were analyzed for (A) specific force (N/cm2) and (B) residual force following 10 repetitive lengthening activations. Lengthening activation data is expressed as a percentage of the force generated in response to the first lengthening activation. The data is shown as mean ± standard error. * indicates that C57BL/10 group is significantly different from 8K-NBD, TAT-NBD and saline treated mdx mice (p<0.05). ** indicates that PTD-NBD peptide treated groups are significantly different from saline treated mdx mice (p<0.05). ***indicates that C57BL/10 group is significantly different from TAT-NBD and saline treated mdx mice (p<0.05). **** indicates that 8K-NBD peptide group is significantly different from saline treated mdx mice (p<0.05), but not significantly different from C57BL/10 mice. n=number of animals analyzed.
The response of muscle to repetitive lengthening activations reflects the muscle’s ability to sustain force in a paradigm of physiological stress. Relative changes (% baseline) in force during the isometric phase of ten repetitive isovelocity lengthening activations in which the diaphragm was stretched to 110% of resting length were determined (Watchko et al., 2002; Watchko et al., 1994). The lowest decrement of force with repetitive lengthening activations was observed in normal C57BL/10 mouse diaphragm (Figure 8B). The average force generated by the final activation was 94.5% of the initial activation, significantly different from TAT-NBD peptide treated mdx mice (p=0.0261) and saline treated mdx controls (p=0.0002). Saline treated mdx mice exhibited the greatest decrement of force, with the average force generated by the final activation at only 77.2% of the initial activation. The average force generated by the final activation of diaphragm from mdx mice after 7 weeks of treatment with 8K- and TAT-NBD peptide was 88.3% and 83.4% of the initial activation, respectively, demonstrating an increased protection from contraction-induced injury as compared to saline treated mdx mice. 8K-NBD peptide treated mdx mice demonstrated significantly greater force compared with saline treated mdx mice (p=0.0086) and was not significantly different from C57BL/10 mice (p=0.2212).
Discussion and Conclusions
The decline in function of dystrophin-deficient skeletal muscle is due to progressive muscle necrosis and the ultimate failure of muscle regeneration. Prior studies demonstrated that NF-κB signaling is central to the processes of inflammation, necrosis and regeneration in dystrophin-deficient muscle (Acharyya et al., 2007). Therefore, we expanded on a prior study demonstrating the potential of the Antp-NBD peptide to ameliorate the pathology of dystrophin-deficient limb muscle (Acharyya et al., 2007). In this study we demonstrated that systemic delivery of the NBD peptide fused to multiple different PTDs was effective in decreasing necrosis and increasing regeneration of dystrophin-deficient muscle. The therapeutic effect was observed in diaphragm muscle and was confirmed in limb muscle of the mdx mouse model of DMD. The observed effect on diaphragm muscle histology and our demonstration of improved ex vivo physiological function, as measured by specific force and response to repetitive lengthening activations, is significant because of the more severe histological phenotype in the mdx mouse diaphragm, which provides the best histological model of the changes observed in human DMD muscle (Stedman et al., 1991).
The rationale for delivering PTD-NBD peptides for the treatment of DMD is based on increased levels of activated NF-κB and cytokine production in dystrophic muscle (Acharyya et al., 2007; Dogra et al., 2008; Hnia et al., 2007; Kumar and Boriek, 2003; Messina et al., 2004; Messina et al., 2006; Whitehead et al., 2008). Furthermore, heterozygous deletion of the NF-κB p65 subunit on the mdx genetic background demonstrated improved muscle pathology compared to age matched mdx mice (Acharyya et al., 2007). In this study, we experimentally demonstrated that multiple PTD-NBD peptides interrupt NF-κB activation, presumably by the disruption of the IKK complex. This disruption is predicted to modulate downstream events in the NF-κB signaling pathway, leading to decreased expression of NF-κB dependent pro-inflammatory genes and decreasing NF-κB-mediated repression of genes whose expression is required for muscle regeneration.
Analysis of NF-κB activation by EMSA in normal C57BL/10 and mdx mouse muscle confirmed previous studies (Acharyya et al., 2007; Kumar and Boriek, 2003; Messina et al., 2004; Messina et al., 2006). Interestingly, both the diaphragm and hind limb skeletal muscle of 4-week-old C57BL/10 mice revealed increased levels of activated NF-κB, presumably due to the role of NF-κB in key developmental cellular processes. However, by 9 and 12 weeks of age very little NF-κB activation was detected in normal mouse muscle, while the levels of NF-κB activation increased in mdx muscle.
Treatment of mdx mice with PTD-NBD peptides, either with a single injection or over a period of 4 or 7 weeks, effectively decreased NF-κB activation in hind limb skeletal muscle and diaphragm, as detected by EMSA, highlighting a critical role that NF-κB plays in dystrophic muscle. From previous studies in other disease models, including neonatal hypoxia-ischemia (Nijboer et al., 2008), collagen induced arthritis (Jimi et al., 2004), and carrageenan-induced paw edema (Di et al., 2005), treatment with PTD-NBD peptide therapy was shown to decrease NF-κB activation, as determined by EMSA, in tissue extracts from brain, ankle joints, and mouse paws, respectively.
In the prior study that demonstrated use of a single PTD-NBD peptide and its effect on hind limb skeletal muscle, 200µg of Antp-NBD peptide was administered by intraperitoneal injection to 23 day old mdx mice every other day for 27 days. Improved muscle histopathology and increased force output was observed in the wild type Antp-NBD peptide treated mdx mice compared to those receiving mutant peptide (Acharyya et al., 2007). The present study builds on this prior data to demonstrate that 8K and TAT are also effective PTDs for the systemic delivery of NBD peptide to skeletal muscle. All 3 PTDs were effective and no single PTD stood out as consistently superior to any other when viewed over 2 muscle types (limb and diaphragm) and 2 lengths of treatment (4 and 7 weeks).
Some observed differences across the experiments reported here provide further insights into the treatment of dystrophic muscle with NBD peptide-mediated inhibition of NF-κB activation. In the tibialis anterior muscle of mice treated for 4 weeks, there was improvement in dystrophic histopathology, decreased necrotic fibers and increased levels of muscle fiber regeneration, compared to age-matched untreated mdx mice. Following 7 weeks of treatment, we observed continued protection of the tibialis anterior muscle from necrosis with each of the PTD-NBD peptides, but there was a lower effect at this time point. The temporal pattern of degeneration and regeneration in mdx skeletal muscle is characterized by an increase in the degree of pathological changes of limb muscle degeneration and regeneration between 4 and 10 weeks of age in mdx mice (Grounds et al., 2008). By 12 weeks of age, progressive degeneration and regeneration in mdx limb muscle continues at a lower level. Consistent with this, we did not observe an increase in activated NF-κB levels by EMSA in hind limb muscle between 9 and 12 weeks of age.
In contrast in diaphragm muscle, we demonstrated that NF-κB activation levels increase between 9 and 12 weeks of age. In diaphragm, we observed a trend towards improved histopathology after 4 weeks of PTD-NBD peptide delivery and this trend continued following 7 weeks of PTD-NBD peptide treatment. Diaphragm pathology in mdx mice more closely models the dystrophic pathology of human DMD muscle with progressive degeneration, attempted regeneration that ultimately fails and replacement with connective tissue (Lynch et al., 1997; Niebroj-Dobosz et al., 1997; Stedman et al., 1991). Since we observed increasing levels of NF-κB activation in diaphragm muscle tissue between 9 and 12 weeks of age, the ongoing benefit seen over time in mdx diaphragm with continued treatment by PTD-NBD peptide in our study is encouraging for its ultimate therapeutic utility.
The improvements observed in the functional properties of the costal diaphragm reflected in specific force production and the response to repetitive lengthening activations demonstrates a functional correlate to the improvements observed in histopathology. We demonstrate that mice treated with either the 8K- or TAT-NBD peptide show significantly higher specific force production compared to saline treated mdx mice. Additionally, both of the peptide treated mdx mice groups had greater sustained force production following repetitive lengthening activations compared to saline treated mdx mice after each individual lengthening activation. However, mdx mice treated with 8K-NBD peptide achieved the greatest functional correction, as measured by repetitive lengthening activations; 8K-NBD peptide treated mdx mice were significantly different from saline treated mdx mice, and not significantly different from the C57BL/10 control mice group. Provision of a functional benefit to respiration, which is a vital physiological process, by systemic administration of PTD-NBD peptide to a murine model of dystrophin deficiency suggests a clinically significant treatment effect in this preclinical model of DMD.
Our findings strongly support an in vivo mechanism of NBD peptide therapy in muscle. The beneficial effect on necrosis and regeneration in dystrophic muscle reflects the effect of decreased NF-κB activation on downstream targets of the NF-κB pathway. We hypothesize that a lower level of NF-κB activation decreases the inflammatory microenvironment in dystrophic skeletal muscle and this in turn plays a role in the decreased necrosis and increased regeneration observed in PTD-NBD peptide treated mdx mouse muscle. Reactive oxygen species (ROS) are increased in pre-necrotic dystrophic muscles (Disatnik et al., 1998), and have been shown to activate the NF-κB pathway (Kumar and Boriek, 2003; Whitehead et al., 2006). A previous study utilizing the anti-oxidant N-acetylcysteine in mdx mice resulted in significant reduction in ROS in muscle cells in conjunction with increased force output following stretch-induced injury and decreased centralized nuclei in hind limb muscle (Whitehead et al., 2008). The increased levels of ROS in dystrophic muscle coupled with the ability of ROS to activate NF-κB, in addition to knowledge that NF-κB regulates pro-inflammatory cytokines, such as TNF-α and IL-1β, which are also elevated in dystrophic tissue, presents a complex picture of activation and feedback loops that contribute to the inflammatory response in muscular dystrophy (Kumar and Boriek, 2003). Future studies will need to comprehensively examine these relationships to determine how PTD-NBD fusion peptide treatment affects this balance.
Administration of PTD-NBD peptides by intraperitoneal injection is a relatively non-invasive means of therapy and offers the advantage of treating a disease of widespread muscle tissue by systemic delivery. However, it is difficult to determine the biodistribution of PTD-NBD peptides in treated mice and whether there are effects on other non-target organs. Evaluation of serum creatine kinase levels from mdx mice treated with PTD-NBD peptide for 4 weeks did not show different results from untreated mdx controls (data not shown). The demonstration that NF-κB activation is decreased in muscle of treated mice indicates that the NBD peptide is active in muscle tissue following intraperitoneal delivery. Dosage studies will be required to determine the optimal treatment effect and the potential effects on non-target tissues.
In this study, the use of PTD-NBD peptide therapy for treatment of dystrophic muscle of mdx mice supports continued research toward its use for DMD patients. While the primary cause of DMD is the lack of a functional dystrophin protein to maintain structural and signaling links from the internal cytoskeleton to the extracellular matrix of muscle cells, therapies that limit inflammation associated with dystrophy and promote muscle regeneration could serve a critical therapeutic purpose. Highly effective treatment of DMD has been elusive to date due to the extent of affected muscle tissue, eventual exhaustion of muscle satellite cells and persistence of inflammation. PTD-NBD peptide therapy has potential both as a primary treatment for DMD and as an adjunct to dystrophin gene transfer.
Research Highlights.
Systemic PTD-NBD therapy of mdx mice improves diaphragmatic muscle function.
NBD peptide effectively blocks NF-κB activation in dystrophic mdx mice in vivo.
NBD peptide treatment improves histopathology in dystrophic mdx mice.
Both hindlimb and diaphragm muscles show improvement with NBD peptide therapy.
Inhibition of NF-κB signaling pathway has therapeutic potential for dystrophic muscle.
Acknowledgements
This work was supported by a Department of Defense grant (PRC), a VA Merit Review grant, a Muscular Dystrophy Association grant (PDR), U54 AR50733 (PDR) and U01 NS069562 (DCG, PDR and PRC). The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PML, Carathers M, et al. Interplay of IKK/NF-kappa B signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulfield, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dave SH, Tilstra JS, Matsuoka K, Li F, Karrasch T, Uno JK, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–7859. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di MP, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-kappaB activation. Arthritis Rheum. 2005;52:951–958. doi: 10.1002/art.20960. [DOI] [PubMed] [Google Scholar]
- 5.Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, et al. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. doi: 10.1016/s0022-510x(98)00258-5. [DOI] [PubMed] [Google Scholar]
- 6.Dogra C, Srivastava DS, Kumar A. Protein-DNA array-based identification of transcription factor activities differentially regulated in skeletal muscle of normal and dystrophin-deficient mdx mice. Mol Cell Biochem. 2008;312:17–24. doi: 10.1007/s11010-008-9716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ervasti J, Campbell K. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 8.Ervasti J, Ohlendieck K, Kahl S, Gaver M, Campbell K. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 9.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De LA. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grounds MD, Torrisi J. Anti-TNF alpha (Remicade (R)) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- 11.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. NF-kappa B controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttridge D. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:443–450. doi: 10.1097/01.mco.0000134364.61406.26. [DOI] [PubMed] [Google Scholar]
- 13.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci Signal. 2006;357:1–19. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 14.Hakansson S, Jacobs A, Caffrey M. Heparin binding by the HIV-1 tat protein transduction domain. Protein Sci. 2001;10:2138–2139. doi: 10.1110/ps.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient mdx mice. Am J Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 17.Khaja K, Robbins P. Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide. Pharmaceuticals. 2010;3:110–124. doi: 10.3390/ph3010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 19.Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol. 1997;272:C2063–C2068. doi: 10.1152/ajpcell.1997.272.6.C2063. [DOI] [PubMed] [Google Scholar]
- 20.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 21.Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, et al. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messina S, Seminara P, Aguennouz M, Monici MC, Marini H, Squadrito F, et al. NF-kB blockade reduces skeletal muscle degeneration and enhances muscle function in mdx mice. Neuromuscul Disord. 2004;14:624–624. [Google Scholar]
- 23.Mi Z, Mai J, Lu X, Robbins PD. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol Ther. 2000;2:339–347. doi: 10.1006/mthe.2000.0137. [DOI] [PubMed] [Google Scholar]
- 24.Niebroj-Dobosz I, Fidzianska A, Glinka Z. Comparative studies of hind limb and diaphragm muscles of mdx mice. Basic Appl Myol. 1997;7:381–386. [Google Scholar]
- 25.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van BF, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi H, Matsumoto S. Protein transduction technology: A novel therapeutic perspective. Acta Med Okayama. 2006;60:1–11. doi: 10.18926/AMO/30757. [DOI] [PubMed] [Google Scholar]
- 27.Rusnati M, Coltrini D, Oreste P, Zoppetti G, Albini A, Noonan D, et al. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J Biol Chem. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 28.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 29.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 30.Strickland I, Ghosh S. Use of cell permeable NBD peptides for suppression of inflammation. Ann Rheum Dis. 2006;65:75–82. doi: 10.1136/ard.2006.058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilstra J, Rehman KK, Hennon T, Plevy SE, Clemens P, Robbins PD. Protein transduction: identification, characterization and optimization. Biochem Soc Trans. 2007;35:811–815. doi: 10.1042/BST0350811. [DOI] [PubMed] [Google Scholar]
- 32.Verma I. Nuclear factor (NF)-kappa B proteins: therapeutic targets. Ann Rheum Dis. 2004;63:57–61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watchko J, O'Day T, Wang B, Zhou L, Tang Y, Li J, et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- 34.Watchko JF, Johnson BD, Gosselin LE, Prakash YS, Sieck GC. Age-related differences in diaphragm muscle injury after lengthening activations. J Appl Physiol. 1994;77:2125–2133. doi: 10.1152/jappl.1994.77.5.2125. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 37.Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988;333:466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]