Abstract
Following a stroke, reduced level of physical activity and functional use of the paretic leg may lead to bone loss and muscle atrophy. These factors and the high incidence of falls may contribute to hip fractures in the stroke population. This study was the first to examine total proximal femur bone mineral content (BMC) and bone mineral density (BMD) and their relationship to stroke-specific impairments in ambulatory individuals with chronic stroke (onset >1 year). We utilized dual-energy X-ray absorptiometry (DXA) to acquire proximal femur and total body scans on 58 (23 women) community-dwelling individuals with chronic stroke. We report total proximal femur BMC (g) and BMD (g/cm2) derived from the proximal femur scans, and lean mass (g) and fat mass (g) for each leg derived from the total body scans. Each subject was evaluated for ambulatory capacity (Six Minute Walk Test), knee extension strength (hand-held dynamometry), physical fitness [Maximal oxygen uptake (VO2max)], and spasticity (Modified Ashworth Scale). Results showed that the paretic leg had significantly lower proximal femur BMD, lean mass, and percent lean mass but higher fat mass than the non-paretic leg for both men and women. Proximal femur BMD of the paretic leg was significantly related to ambulatory capacity (r=0.33, p=0.011), muscle strength (r=0.39, p=0.002), physical fitness (r=0.57, p<0.001) but not related to spasticity (r=−0.23, p=0.080). Multiple regression analysis showed that lean mass in the paretic leg was a major predictor (R2=0.371, p<0.001) of the paretic proximal femur BMD. VO2max was a significant predictor of both paretic proximal femur BMD (R2=0.325, p<0.001) and lean mass in the paretic leg (R2=0.700, p<0.001). Further study is required to determine whether increasing physical fitness and lean mass is important to improve hip bone health in chronic stroke.
Keywords: Aerobic capacity, cerebrovascular accident, osteoporosis, physical activity, rehabilitation
Introduction
Following a stroke, individuals sustain a higher incidence of falls than the reference population [1,2]. Balance deficits, muscle weakness, poor motor recovery and reduced functional mobility are contributing factors [3–5]. Following a stroke, the reduction of weight-bearing physical activity and functional use of the paretic lower extremity may lead to other devastating complications, such as osteoporosis and muscle atrophy [6–9]. Reduction in bone mineral levels and muscle atrophy, coupled with a high incidence of falls, may contribute to the 2 to 4 times higher risk of hip fractures in individuals with stroke when compared with the reference population [8,10,11].
Stroke was first used as a model of disuse osteoporosis by Prince et al. [12] and Iversen et al. [13]. It is well known that removal of the mechanical stimuli from muscle forces and weight-bearing activity leads to bone loss [13,14]. Individuals with stroke typically suffer varying extent of motor paralysis on one side of the body (hemiparesis). By comparing the affected and the non-affected limbs, one can study the effects of disuse on bone demineralization while providing appropriate control for the different cofactors (i.e. environmental, genetic) which may affect bone metabolism in different subjects [15].
Ramnemark et al. [16] have previously reported a 12.2% decrease in proximal femur bone mineral density (BMD) on the paretic side within the first year following a stroke. A significant 7% decrease in total leg bone mineral content (BMC) and 3% decrease in lean mass of the paretic leg have also been reported at 1 year post-stroke [7]. However, very few studies have examined changes in BMC, BMD and soft tissue composition in the legs beyond 1 year following stroke.
There are several important reasons to investigate bone health and soft tissue composition changes in community dwelling, ambulatory chronic stroke survivors (onset >1 year). First, most fractures following stroke occur in the chronic stage (median time for first fracture: 2.4 years) [10]. Second, independent ambulators make up a large proportion (80%) of chronic stroke survivors [17]. These individuals may have a higher risk of falls because the majority of falls occur during walking [1]. Third, the percentage of fallers is higher among individuals with stroke who live in the community [2,18] when compared with both those who live in stroke rehabilitation settings [5,19] and the older adult reference population [2]. Finally, BMD at various sites at the hip continued to decrease on the paretic side during the second half of the first year following stroke despite recovering walking function [7,16,20]. It is very possible that bone health may continue to decline beyond the first year post-stroke and thus further increase the risk of hip fractures.
Most studies in chronic stroke included relatively small samples of subjects and consisted of individuals with a wide range of capabilities [21–23]. No study has examined both proximal femur bone mineral and soft tissue composition in the lower extremities of community-dwelling, ambulatory individuals with chronic stroke. Further, it is unknown how these parameters relate to various stroke-specific impairments. As bone formation is stimulated by muscle forces [24], lean mass may potentially be an important predictor of proximal femur BMD. In addition, stroke-specific impairments such as reduced ambulatory capacity, leg muscle weakness, physical inactivity and spasticity may also be important predictors. Identification of the stroke-related factors that influence proximal femur BMD and soft tissue composition of the paretic leg may help clinicians devise appropriate treatment to enhance bone health in this group. The purposes of this study were to: (1) compare the total proximal femur BMC and BMD, lean mass and fat mass between the paretic and non-paretic leg in ambulatory individuals with chronic stroke and to healthy reference values, and (2) determine the relationship of these parameters to common stroke-specific impairments such as reduced ambulatory capacity, muscle weakness, decreased physical fitness and spasticity. We studied the total proximal femur BMD as it is the best predictor for hip fractures [25,26].
Materials and Methods
Sample size calculation
The computer program G Power was used to calculate the sample size required for multiple regression analyses [27]. If up to 6 variables were modeled at an effect size=0.35 (large) at an alpha level of 0.05 and power of 0.90, a minimum of 57 subjects are required.
Subjects
Community-dwelling individuals with stroke were recruited on a volunteer basis. All subjects had to fulfill the following inclusion criteria: (1) had one single stroke only, (2) had a post-stroke interval of one year or more, (3) were independent in ambulation with or without a walking aid (i.e. no supervision or physical assistance from a person), (4) were 50 years of age or older, and (5) were living at home (not institutionalized). Potential subjects were excluded if they (1) had other neurological conditions in addition to stroke, (2) had significant musculoskeletal conditions (i.e. amputations, total knee or hip replacements), (3) had unstable cardiovascular disease, (4) had a Folstein Mini Mental Status Examination (MMSE) score <22 [28], (5) had any metal implants in the lower extremities or (6) were taking prescribed medications that affect bone metabolism. Eligible subjects gave informed, written consent to participate in the study. Written permission was also obtained from the primary care physician before an individual was accepted into the study. The study was approved by the local hospital and university research ethics committees. The study was conducted according to the Helsinki Declaration for human experiments.
Dual-energy X-ray Absorptiometry
All subjects underwent 3 separate scans on the same day – total body and bilateral proximal femurs using dual-energy X-ray absorptiometry (DXA; Hologic QDR 4500, Hologic Inc., Waltham, MA, USA). All scans were performed by the same technician using standard procedures (Hologic Users Manual). In order to maintain the standard position of the legs throughout the scanning procedures, a Velcro strap was placed around the ankles. We reported total proximal femur BMC (g) and BMD (g/cm2) as the primary outcomes. Lean mass (g) and fat mass (g) of the paretic and non-paretic leg were derived from the total body scan. Percent lean mass of each leg was then calculated [lean mass × 100%/(lean mass + fat mass)]. In terms of precision of our DXA scanner, the coefficients of variation (CV) for proximal femur BMC and BMD were 0.90% and 0.44%, respectively. The CV for left leg and right leg lean mass, left and right leg fat mass were 1.0%, 0.7%, 3.1% and 2.9%, respectively.
Six Minute Walk Test
Although the majority of stroke survivors regain the ability to ambulate independently [17], ambulatory capacity is compromised to varying extents in these individuals. Eng et al. [29] has previously reported that functional walk distances in older individuals with chronic stroke were less than 50% of those for the reference population. The reduced ambulatory capacity in these individuals may significantly impact on bone health. Six Minute Walk Test (6MWT) was used to assess ambulatory capacity [30]. The total distance walked (meters) was recorded.
Leg Muscle Strength
Another factor that may influence bone health in stroke is reduced muscle strength. For example, in individuals with chronic stroke, the torque generated by the knee extensors of the paretic side has been reported to be only half of that generated by the non-paretic side [31]. We used hand-held dynamometry (Nicholas MMT, Lafayette Instruments, Lafayette, IN, USA) to evaluate isometric knee extension strength. Subjects were instructed to sit upright in a chair with back support. The knee was placed in 90° flexion and subjects were asked to perform a maximal isometric contraction of knee extension. Three trials were performed on each side. The force values obtained (N) in each leg were averaged. Hand-held dynamometry has been shown to be a reliable method to measure leg muscle strength in stroke [32].
Physical fitness
Different subjective or self-report measures have been used to estimate the level of physical activity, which has been related to bone health [33,34]. In this study, we used a standardized and objective measure, namely maximal oxygen uptake (VO2max), as an indictor of physical work capacity. VO2max is accepted as the standard measure of cardiorespiratory fitness (ACSM, 2000) and is associated with level of habitual physical activity [35,36]. As low VO2 max is commonly observed in people with stroke, it may be an important factor in bone health in this group [37].
To measure VO2max, each subject underwent a maximal cycle ergometer test on the Excalibur cycle ergometer (Lode B.V. Medical Technology, Groningen, Netherlands). Subjects wore a face mask and oxygen consumption (VO2) was continuously measured using a portable metabolic unit (Cosmed K4 b2 system, COSMED Srl, Rome, Italy). A 12-lead electrocardiography system was used to monitor the cardiac activity by a physician (Quark C12, COSMED Srl, Rome, Italy). The testing protocol was dependent upon the capability of the individual [38]. The workload started at 20W with increments of 20W/min for 27 subjects who were mildly impaired whereas the workload started at 10W with increments of 10W/min for the other 31 subjects who were more severely impaired [35,38]. Subjects were instructed to pedal at approximately 60 revolutions per minute (rpm). The test was terminated when maximal effort was attained, which was indicated by a respiratory exchange ratio of >1.0 or a plateau in VO2 (<150 ml/min) and volitional fatigue (decline in cycling rate <30 rpm) [35]. Since the metabolic unit performed breath by breath gas analysis, the VO2 data were averaged at a rate of every 15s to obtain a more accurate measure of the VO2max. The maximal value (ml/min) obtained was considered to be the VO2max.
Spasticity
Increased spasticity is related to a higher degree of asymmetry in walking, characterized by decreased single limb support time of the paretic leg [39] and may thus potentially lead to decreased mechanical loading of the paretic leg. Resistance to passive movements in the paretic foot was evaluated by Modified Ashworth Scale of Spasticity (0: no increase in muscle tone, 4: affected part rigid in flexion and extension). The Modified Ashworth Scale of Spasticity is a reliable tool to assess muscle tone in individuals with stroke [40].
Statistical Analysis
The data from the third National Health and Nutrition Examination Survey (NHANES III) were used as the reference sample to calculate the T-score [41], as recommended by Kanis and Gluer [42]. According to the criterion set by the World Health Organization (WHO), a subject was classified as having osteopenia or osteoporosis if proximal femur BMD was below 1.0 (T-score <−1.0) or 2.5 standard deviations (T-score <−2.5) below the young reference population, respectively [42]. Gender-specific reference data were used to calculate the T-scores, in accordance with the official positions adopted by the International Society for Clinical Densitometry [43]. Data from the NHANES III study were also used to calculate total proximal femur Z-scores, to compare our subjects with the age and gender-matched population [44]. For example, a Z-score of −1 indicates a proximal femur BMD value of 1.0 standard deviation below the age and gender-matched population.
Paired t-tests were used to examine whether there were side-to-side differences in proximal femur BMC, BMD, lean mass and fat mass. Normal distribution of different variables was checked by descriptive statistics (e.g. skewness, kurtosis) and visual inspection of histograms. Pearson’s moment correlations were used to determine the relationship among the variables that were normally distributed: (1) paretic proximal femur BMC and (2) BMD, (3) lean mass and (4) fat mass in the paretic leg, (5) 6MWT distance, (6) paretic knee extension strength, (7) VO2max, (8) age, and (9) height. Spearman’s rho correlations were used for (1) spasticity, and (2) post-stroke duration (not normally distributed). A point-biserial correlation coefficient was used to quantify the relationship between gender (a dichotomous variable) (male=0, female=1) and other variables.
Stepwise multiple regression analyses were performed to identify significant predictors of proximal femur BMD and lean mass on the paretic side. To further examine the relative contribution of lean mass and fat mass to the difference in proximal femur BMD between the two sides, an additional stepwise multiple regression analysis was performed to predict the side-to-side difference in proximal femur BMD, using the side-to-side difference in lean mass and fat mass as predictors. A predictor was entered into the model at p≤0.05 and was removed at p>0.1. All statistical analyses were performed using SPSS11.5 software (SPSS Inc.) using a significance level of 0.05 (2-tailed).
Results
Subject characteristics
Sixty three subjects (36 men, 27 women) volunteered to participate in the study. Of these, five (1 men, 4 women) were taking bone resorption inhibitors and were excluded from the study. As a result, data from 58 community-dwelling individuals with chronic stroke were included. Subject characteristics are listed in Table 1. Seventeen subjects used a walking aid (wheeled walker, n= 5; crutch, n=1; quad cane, n=3; cane, n=8) and 9 subjects used an ankle foot orthosis for ambulation. On average, the knee extension strength of the paretic leg was 72.9% of the non-paretic leg.
Table 1.
Subject Characteristics
| Variable | Mean±SD |
|---|---|
| Subject demographics | |
| Gender (male/female) | 35/23 |
| Paretic side (left/right) | 38/20 |
| Race (White/Asian/Black) | 37/20/1 |
| Post-stroke duration (years) | 5.6±5.1 |
| Age (years) | 65.5±8.8 |
| Mass (kg) | 77.7±15.8 |
| Height (cm) | 169.3±10.7 |
| Secondary outcome measures | |
| 6MWT distance (m) | 312.2±131.5 |
| Knee extension strength (N) | |
| Paretic leg | 188.7±71.3 |
| Non-paretic leg | 256.9±86.4 |
| VO2max (ml/min) | 1372.7±515.9 |
| VO2max (ml/kg/min) | 22.3±4.7 |
| Spasticity (median) | 1 |
Bone mineral levels and soft tissue composition
Proximal femur BMD on the paretic side were significantly lower than on the non-paretic side for both male and female subjects (Table 2). A similar trend exists for proximal femur BMC but the difference was not statistically significant. The distribution of proximal femur BMD T-scores for both legs is illustrated in Figure 1. In the paretic proximal femur, 52% and 17% of female subjects met the criteria for osteopenia and osteoporosis, respectively. The corresponding values for the non-paretic side were 65% and 4 %. The proportion of male subjects with osteoporosis was lower. Only 2 men (6%) had osteoporosis on the paretic side compared to none on the non-paretic side. A large percentage of male subjects had osteopenia, however. Osteopenia was present in 43% and 40% of male subjects in the paretic and non-paretic proximal femur, respectively.
Table 2.
Comparison between paretic leg and non-paretic leg
| Variable | Paretic leg | Non-paretic leg | Δ between legs (95%CI) | p | Percent Δ between legs |
|---|---|---|---|---|---|
| Proximal femur BMC, g | |||||
| Male | 40.805±9.005 | 41.619±7.952 | −0.882 (−2.08,0.323) | 0.146 | −2.3 |
| Female | 24.271±6.117 | 25.095±5.578 | −0.824 (−1.716, 0.068) | 0.069 | −3.6 |
| Proximal femur BMD, g/cm2 | |||||
| Male | 0.91±0.14 | 0.95±0.11 | −0.033 (−0.051, −0.014) | 0.001 | −3.7 |
| Female | 0.76±0.16 | 0.80±0.15 | −0.037 (−0.062, −0.012) | 0.005 | −4.8 |
| Lean mass, g | |||||
| Male | 9076.63±1552.03 | 9550.73±1307.98 | −474.10 (−696.89, −251.30) | <0.001 | −5.2 |
| Female | 6080.19±1155.48 | 6354.75±1251.30 | −274.55 (−469.69, −79.42) | 0.008 | −4.0 |
| Fat mass, g | |||||
| Male | 3038.75±1385.38 | 2959.30±1411.94 | 79.44 (4.66, 154.22) | 0.038 | 3.6 |
| Female | 4349.81±1384.83 | 4229.17±1430.18 | 120.64 (31.80, 209.49) | 0.010 | 3.8 |
| Percent lean mass, % | |||||
| Male | 75.53±5.96 | 77.08±5.77 | −1.55 (−2.30, −0.796) | <0.001 | −2.0 |
| Female | 59.15±6.73 | 60.98±6.84 | −1.82 (−2.84, −0.80) | 0.001 | −2.9 |
difference
CI: confidence interval
Figure 1. Distribution of proximal femur T-scores.
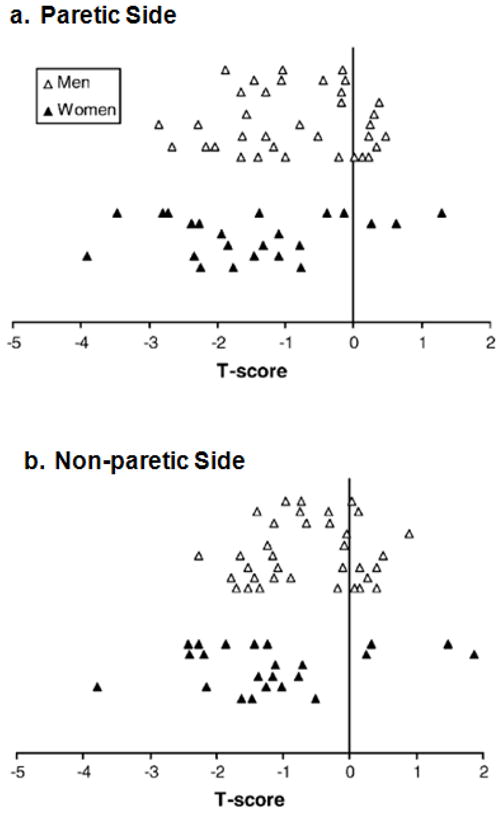
Proximal femur T-scores for the (a) Paretic Side and (b) the Non-paretic Side were indicated by open triangles (men), and solid triangles (women). Each triangle represents a single subject.
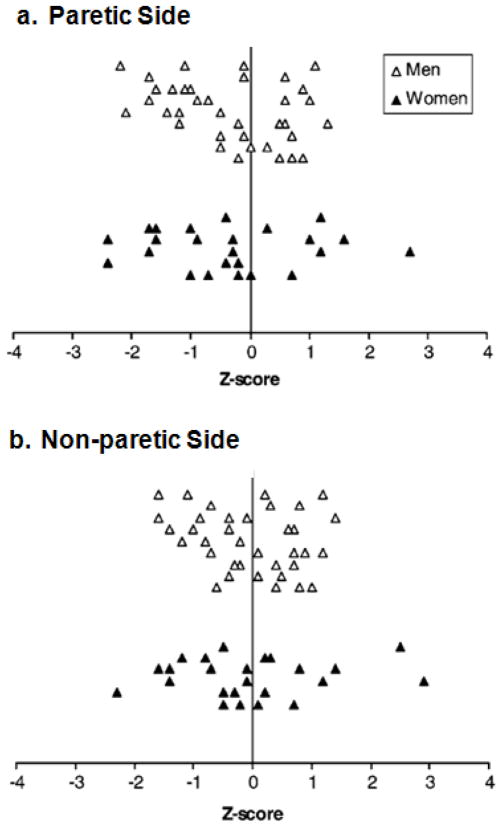
The Z-score distribution is shown in Figure 2. Approximately 26% of women and 26% of men had a Z-score < −1 in the paretic proximal femur (Figure 2a). A lower percentage of women (13%) and men (9%) had a Z-score < −1 in the non-paretic proximal femur (Figure 2b).
Figure 2. Distribution of proximal femur Z-scores.
Proximal femur Z-scores for the (a) Paretic Side and (b) the Non-paretic Side were indicated by open triangles (men), and solid triangles (women). Each triangle represents a single subject.
Soft tissue composition was also significantly different between the two legs. Both lean mass and percent lean mass were significantly lower in the paretic leg than in the non-paretic leg for both male and female subjects. In contrast, fat mass was significantly higher in the paretic leg than in the non-paretic leg (Table 2).
In general, the side-to-side differences in bone mineral level and soft tissue composition were consistent across ethnicities. In both Caucasian (n=37) and Asian subjects (n=20), significantly lower proximal femur BMD (Caucasian: p=0.014; Asian: p<0.001), lean mass (Caucasian: p=0.007, Asian: p<0.001), and percent lean mass (Caucasian: p=0.002; Asian: p<0.001) but higher fat mass (Caucasian: p=0.007; Asian: p=0.010) were obtained in the paretic leg when compared to the non-paretic leg. The side-to-side difference in proximal femur BMC was significantly different in Asian subjects (p=0.004), but not in Caucasian subjects (p=0.597).
Influence of stroke-specific impairments on bone mineral levels and soft tissue composition
Proximal femur BMD on the paretic side was significantly correlated with height, gender, paretic leg lean mass, knee extension strength, 6MWT distance, and VO2max (Table 3) and these factors were used as the independent variables in the first stepwise multiple regression model. Although age is related to hip BMD in healthy older adults [41,44,45], it was not included in the model due to a non-significant pair-wise correlation. Results showed that paretic leg lean mass was the only significant predictor of proximal femur BMD on the paretic side, accounting for 37.1% of its variance [F(1,56)=32.991, p<0.001] (Table 4, model 1). Height, gender, 6MWT distance, knee extension strength, and VO2max were excluded from the stepwise regression model (p>0.1).
Table 3.
Correlations with proximal femur BMC, BMD, lean mass and fat mass of the paretic leg
| Variables | Proximal Femur BMC | Proximal Femur BMD | Lean mass | Fat mass |
|---|---|---|---|---|
| Demographics/Soft tissue composition | ||||
| Height | 0.77** | 0.52** | 0.82** | −0.22 |
| Post-stroke duration | 0.01 | 0.04 | −0.02 | 0.17 |
| Age | −0.01 | −0.11 | −0.14 | −0.17 |
| Gender | −0.72** | −0.47** | −0.73** | 0.43** |
| Lean mass | 0.78** | 0.61** | — | 0.01 |
| Fat mass | −0.03 | 0.17 | 0.01 | — |
| Stroke-related impairments | ||||
| Ambulatory Capacity (Six Minute Walk Test) | 0.25 | 0.33* | 0.22 | −0.04 |
| Muscle strength (Paretic Knee extension) | 0.41** | 0.39** | 0.50** | −0.05 |
| Physical activity (VO2max) | 0.69** | 0.57** | 0.84** | −0.05 |
| Spasticity (Modified Ashworth Scale) | −0.21 | −0.23 | −0.21 | 0.05 |
p<0.05,
p<0.005
Table 4.
Multiple regression analyses for predicting proximal femur BMD and lean mass of the paretic leg
| Dependent variable | Predictors | R2 | R2 Change | β | p for each predictor |
|---|---|---|---|---|---|
| 1. Proximal femur BMDa | Paretic leg lean mass | 0.371 | 0.371 | 0.609 | <0.001 |
| 2. Proximal femur BMDb | VO2max | 0.325 | 0.325 | 0.570 | <0.001 |
| 3. Paretic leg lean massc | VO2max | 0.700 | 0.700 | 0.507 | <0.001 |
| Height | 0.794 | 0.094 | 0.450 | <0.001 | |
| 4. Side to side difference in proximal femur BMDd | Side-to-side difference in lean mass | 0.099 | 0.099 | 0.315 | 0.016 |
Excluded variables: height, gender, 6MWT distance, paretic knee extension strength, VO2max
Excluded variables: height, gender, 6MWT distance, paretic knee extension strength
Excluded variables: gender, paretic knee extension strength
Excluded variable: side-to-side difference in fat mass
Because of the significant relationships of lean mass to knee extension strength (r=0.495, p<0.001) and VO2max (r=0.837, p<0.001), there is the potential that the regression procedure may have removed these factors from the model due to their close relationship to the best predictor (lean mass). Thus, in the second stepwise regression model, the same variables in model 1 were entered except paretic leg lean mass (Table 4, model 2). In this model, VO2max was the only significant predictor of paretic proximal femur BMD, accounting for 32.5% of the variance [F(1,56)=26.926, p<0.001]. Height, gender, 6MWT distance, and knee extension strength were removed from this model (p>0.1).
The third regression model was used to predict lean mass based on its significant correlations with height, gender, knee extension strength, and VO2max (Table 3). VO2max and height were significant predictors of lean mass in the paretic leg, with VO2max accounting for 70.0% of its variance (Table 4, model 3). Adding height to the model accounted for an additional 9.4% of the variance in paretic leg lean mass [F(2,55)=105.828, p<0.001]. Gender and knee extension strength were removed from the regression model (p>0.1). Regarding fat mass in the paretic leg, it was significantly correlated with gender only (Table 3) and hence no multiple regression analysis was performed.
In the last regression model, we used the side-to-side difference in lean mass and fat mass to predict the side-to-side difference in paretic proximal femur BMD (Table 3, model 4). Side-to-side difference in lean mass, but not side-to-side difference in fat mass, was a significant predictor of side-to-side difference in paretic proximal femur BMD, accounting for 9.9% of its variance [F(1,56)=6.161, p=0.016]
Discussion
Comparison with the reference population
Our data showed that many individuals with chronic stroke had osteopenia or osteoporosis in proximal femur, with a higher degree of bone loss in the paretic leg (Figure 1). It may be related to the preferential use of the non-paretic leg during functional activities. For example, it is common that more weight is borne by the non-paretic leg during standing or walking activities in individuals with stroke [46,47]. A substantial proportion of our subjects also had low proximal femur BMD values when compared to the age and gender-matched population (Figure 2). The results indicate that stroke has major impact on bone health.
Lower hip BMD is associated with a higher risk of hip fracture. An individual with a T-score of −1 standard deviation at the hip would have a 2.6-fold higher relative risk of hip fracture [42,48]. Based on the prevalence of osteopenia and osteoporosis in the proximal femur on the paretic side (Figure 1), 43% of men and 52% of women would have at least a 2.6-fold increase in hip fracture risk (osteopenia; T-score between −1.0 and −2.5). An additional 6% of men and 17% of women would have greater than 10-fold (2.62.5) increase in hip fracture risk (osteoporosis; T-score ≤ 2.5) [42]. However, the actual risk of hip fractures would presumably be much higher due to the presence of additional risk factors such as high incidence of falls [19] and physical impairments (i.e. poor balance, reduced motor control) [3–5]. This is of particular concern on the paretic side, because proximal femur BMD is lower in the paretic leg (Table 2) and the majority of fractures occur on the paretic side [11,49].
Side-to-side differences in bone mineral levels and soft tissue composition
Previous studies in stroke patients have reported 1.3–8.8% side-to-side difference in BMD at various sites in the legs [15,16,20,22,23,50–52]. However, comparison with other studies is extremely difficult for several reasons. First, these studies reported data from patients in different stages of stroke recovery such as acute [22], subacute [15,23,50,51], and chronic [15,50]. Second, many studies included subjects who were non-ambulatory [16,20,22,51,52]. Third, BMD was measured at different sites in the lower extremity in different studies such as femoral neck [15,20,51,52], trochanter [20], proximal femur [16], total femur [16], first metatarsus [21] and whole leg [6,50]. This is the first study to examine total proximal femur BMC and BMD in community-dwelling, independent ambulators with chronic stroke.
Overall, we found a 4.1% side-to-side difference in proximal femur BMD, respectively (Table 2). This is in contrast to previous studies in healthy subjects, which found no significant (0.1–1.5%) side-to-side difference in proximal femur BMD [53,54]. Stroke-related impairments thus have considerable impact on bone health in the paretic leg.
Our results showed a significant 4.7% side-to-side difference in lean mass, indicating muscle atrophy in the paretic leg. This finding is comparable to those found by Ryan et al. [55] (3.5%; post-stroke duration=3 years) and Iversen et al. [6] (5.4%; post-stroke duration = 23–38 weeks). We also found that the paretic leg had a significant 3.7% greater fat mass when compared with the non-paretic leg. In contrast, no significant change in fat mass at 1 year post-stroke was reported in a previous study [7]. Nevertheless, our results lend support to the findings by Ryan et al. [55], who used computed tomography to demonstrate that in individuals with chronic stroke, there was increased fat content in and around the muscle fibers in the paretic leg. The observed soft tissue composition changes in the paretic leg (i.e. muscle atrophy, increased fat content) may be a function of the reduced fitness level secondary to a sedentary lifestyle following stroke [55] and reduced weightbearing during daily functional activities [46,47].
Influence of stroke-specific impairments
Paretic leg lean mass was the most significant predictor of total proximal femur BMD on the paretic side. Moreover, side-to-side difference in paretic leg lean mass was also the best predictor for side-to-side difference in proximal femur BMD. This muscle-bone relationship has been found in other populations, such as post-menopausal women [56], middle-aged [57] and older adults [58,59]. Our results thus suggested that increasing or maintaining muscle mass in the paretic leg may have a protective effect on proximal femur bone health in individuals with chronic stroke.
It has been previously reported that VO2max is a major predictor of proximal femur BMD in elderly women [60]. We identified VO2max as a strong determinant of both the paretic proximal femur BMD and paretic leg lean mass in older individuals with chronic stroke. Reduction in VO2max is related to decreased habitual physical activity in healthy populations [36]. Our finding thus highlights the importance of physical activity and fitness on muscle and bone health in individuals with stroke. Low VO2max is common following stroke [37] and the mean VO2max value obtained in this study was only at the 10th percentile of the age-matched healthy population for both men and women [35]. The observed low fitness level reflects a sedentary lifestyle or decreased physical activity level, despite that all subjects are independent in ambulation. Reduced physical activity, when coupled with the preferential use of the non-paretic side for weightbearing [46,47], may explain the more compromised bone and muscle health status in the paretic leg when compared to the non-paretic leg.
Ambulation has been identified as a significant determinant of hip BMD in older adults [59]. The importance of ambulatory ability on bone and muscle health in stroke was shown by Jorgensen et al. [20], who reported that those who stayed wheelchair bound at 1 year post-stroke had 13% reduction in femoral neck BMD on the paretic side whereas those who relearned to walk at 2 months only had a 8% decrease of the same. Another study in stroke showed that those who relearned to walk at 2 months post-stroke had no significant reduction of lean mass on the paretic side at 1 year post-stroke [7]. In contrast, those who remained non-ambulatory at 2 months lost 5–6% of lean mass on the paretic side at 1 year [7].
Our results, however, showed that ambulatory capacity (6MWT distance) only had a low correlation with proximal femur BMD on the paretic side (r=0.33) and had no significant correlation with paretic leg lean mass (r=0.22). The difference in results may be due to several reasons. First, all of our subjects were independent ambulators whereas the subjects in their study included those who were non-ambulatory or who required physical assistance by another person. Second, different outcome measures were used to indicate ambulatory capacity. Functional Ambulation Category (FAC) was used in their study to classify the level of ambulatory independence based on the amount of human assistance needed (1=wheelchair bound; 6=independent on both level and uneven surfaces) whereas we used the 6MWT to measure walking distance. Lastly, the stronger determinant of VO2max may have reduced the effect of the 6MWT distance in the model. Improvement in VO2max is related to the interactions among factors such as frequency, duration and intensity of physical activities [35]. A longer distance walked during the 6MWT does not necessarily mean that an individual regularly participates in activities at a level sufficient to induce an increase in VO2max.
Previous studies in older adults have found a positive correlation between leg muscle strength and hip BMD [58, 61]. Our results has demonstrated a similar relationship between muscle strength and hip BMD in stroke (r=0.39). This is in agreement with a previous study in patients with subacute stroke (mean onset=63 days), which showed a significant relationship between femoral neck BMD loss and degree of motor paralysis in the leg (r=−0.41) [52]. Overall, the results again point to the importance of muscle health on hip BMD.
Our results only showed a trend for a negative relationship between spasticity and paretic proximal femur BMD but it did not reach statistical significance (r=−0.23, p=0.080). A simple explanation is that our study may be insufficiently powered to detect a significant relationship. However, the effects of spasticity on hip BMD may be quite complex. Spasticity is related to reduced walking velocity [29] in individuals with stroke, which would in turn cause a reduction in the peak ground reaction force [62]. Moreover, those with more severe spasticity tend to walk with more asymmetry, resulting in a reduced single limb support time of the paretic leg [39]. These factors presumably would reduce the amount of mechanical loading on the paretic leg and may lead to more bone loss. However, the tonic muscle activity due to spasticity may help preserve muscle mass and provide some mechanical loading to the bone. The complex relationships among spasticity, bone mineral level and muscle mass have been demonstrated in patients with spinal cord injury [63]. Muscle mass was better preserved in spinal cord injured patients with spastic paralysis than those with flaccid paralysis [63]. The lack of a significant relationship between hip BMD and spasticity reported in our study is also consistent with the study by Jorgensen and Jacobsen [7], who found no significant relationship between spasticity and 1 year changes in total leg BMC in individuals with stroke.
Age was not significantly correlated with proximal femur BMD, in contrast to the healthy population [41,44,45]. In addition, post-stroke duration was also not related to proximal femur BMD. These findings indicate that the severity of stroke took precedence over the effects of age and post-stroke duration on proximal femur BMD. It is noteworthy that 20 of our subjects were of Asian ethnicity. However, it should not affect the interpretation of our results. We found that side-to-side differences in BMD and soft tissue composition measures were consistent across ethnicities. Previous studies have shown that most differences in BMC and BMD between Asian and Caucasian populations could be accounted for by body size (i.e. height) rather than true ethnic differences [64,65]. The prevalence of osteoporosis in older Asian women is also comparable to that in the Caucasian reference [66].
Clinical implications
This study has important clinical implications for stroke rehabilitation. First, a substantial proportion of subjects have osteoporosis in the paretic leg. The prevalence of osteopenia also tends to be higher in individuals with chronic stroke than the reference population. Osteopenia itself does not result in fractures [67]. However, the departure from healthy BMD values, when coupled with the presence of physical impairments and high incidence of falls, would increase traumatic fractures [67]. Second, given that improving physical fitness (VO2max) is a strong predictor of paretic proximal femur BMD and lean mass of the paretic leg, physical activities and exercises should be promoted to improve muscle and bone health in the lower extremities. A 6-month resistive training program has been shown to increase muscle mass and improve BMD in the femoral region in healthy young and older adults (Ryan et al. 2004). The beneficial effects of resistive or aerobic exercises on bone health in the femoral region have been reported in other studies [68–72]. So far, no study has been conducted to examine the effects of different forms of exercise training on bone health and soft tissue composition in individuals with stroke. Future research is urgently needed in this important area.
Limitations
DXA was used to measure BMC, BMD and soft tissue composition in this study. This technique has its own limitations. Due to its planar nature, DXA is not able to assess the true composition and geometry of bone. Areal BMD only partially corrects for the bone size and therefore tends to overestimate the sex-specific differences due to the larger skeletons in men [74]. In spite of its limitations, DXA remains the most common method of evaluating bone mineral status. Areal BMD is used to diagnose osteoporosis and to predict fracture risk [42]. Reproducibility of DXA in individuals with stroke has also been demonstrated [75].
Lean mass measured by DXA is sensitive to changes in hydration [76]. Edema in stroke patients, if present, is most commonly seen on the paretic side [77]. Overestimation of muscle mass in the paretic leg may occur as a result. Our data showed that the paretic leg had a significant lower lean mass than the non-paretic leg. Therefore, this potential artifact would not explain our results. Fat mass has also been shown to overestimate BMC [78]. Again, this possible artifact should not affect the interpretation of our results because the paretic leg, with its higher fat mass, still had a lower proximal femur BMC than the non-paretic leg.
Conclusion
This is the first paper to show that many older individuals with chronic stroke have low bone mineral levels in the proximal femur although they were able to ambulate without supervision or physical assistance. Muscle atrophy was also apparent in the paretic leg. We have thus highlighted the potential importance of physical activity and exercise for improving muscle and bone health in the lower extremities of individuals with chronic stroke.
Acknowledgments
MYCP was supported by a post-doctoral fellowship from Natural Sciences and Engineering Research Council of Canada. This study was supported by a grant-in-aid from the Heart Stroke Foundation of British Columbia and Yukon (JJE), the Rx&D Health Research Foundation Special Research Allowance program and from career scientist awards from Canadian Institute of Health Research (JJE) (MSH-63617)) and the Michael Smith Foundation for Health Research (JJE and HAMc).
References
- 1.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic injury. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls. Depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 3.Cheng PT, Liaw MY, Wong MK, Tang FT, Lee MY, Lin PS. The sit-to-stand movement in stroke patients and its correlation with falling. Arch Phys Med Rehabil. 1998;79:1043–1046. doi: 10.1016/s0003-9993(98)90168-x. [DOI] [PubMed] [Google Scholar]
- 4.Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM. Risk factors for falling in home-dwelling older women with stroke. The women’s health and aging study. Stroke. 2003;34:494–501. [PubMed] [Google Scholar]
- 5.Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: factors associated with high risk. Arch Phys Med Rehabil. 2002;83:329–333. doi: 10.1053/apmr.2002.29623. [DOI] [PubMed] [Google Scholar]
- 6.Iversen E, Hassager C, Christiansen C. The effect of hemiplegia on bone mass and soft tissue body composition. Acta Neurol Scand. 1989;79:155–159. doi: 10.1111/j.1600-0404.1989.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen L, Jacobsen BK. Changes in muscle mass, fat mass and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001;28:655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 8.Poole KE, Reeve J, Warburton EA. Falls, fractures and osteoporosis after stroke: time to think about protection? Stroke. 2002;33:1432–1436. doi: 10.1161/01.str.0000014510.48897.7d. [DOI] [PubMed] [Google Scholar]
- 9.Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999;30:755–760. doi: 10.1161/01.str.30.4.755. [DOI] [PubMed] [Google Scholar]
- 10.Ramnemark A, Nyberg L, Borssen B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int. 1998;8:92–95. doi: 10.1007/s001980050053. [DOI] [PubMed] [Google Scholar]
- 11.Ramnemark A, Nilsson M, Borssen B, Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fractures. Stroke. 2000;31:1572–1577. doi: 10.1161/01.str.31.7.1572. [DOI] [PubMed] [Google Scholar]
- 12.Prince RL, Price RI, Ho S. Forearm bone loss in hemiplegia: a model for the study of immobilization osteoporosis. J Bone Miner Res. 1988;3:305–310. doi: 10.1002/jbmr.5650030309. [DOI] [PubMed] [Google Scholar]
- 13.Giangregorio L, Blimkie CJR. Skeletal adaptations to alterations in weight-bearing activity. A comparison of models of disuse osteoporosis. Sports Med. 2002;32:459–476. doi: 10.2165/00007256-200232070-00005. [DOI] [PubMed] [Google Scholar]
- 14.Frost HM, Ferretti JL, Jee WSS. Perspectives: some roles of mechanical usage, muscle strength, and the mechanostat in skeletal physiology, disease, and research. Calcif Tissue Int. 1998;62:1–7. doi: 10.1007/s002239900384. [DOI] [PubMed] [Google Scholar]
- 15.Del Puente A, Pappone N, Mandes MG, Mantova D, Scarpa R, Oriente P. Determinants of bone mineral density in immobilization: a study on hemiplegic patients. Osteoporos Int. 1996;6:50–54. doi: 10.1007/BF01626538. [DOI] [PubMed] [Google Scholar]
- 16.Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int. 1999;9:269–275. doi: 10.1007/s001980050147. [DOI] [PubMed] [Google Scholar]
- 17.Gresham GE, Fitzpatrick TE, Wolf PA, McNamara PM, Kannel WB, Dawber TR. Residual disability in survivors of stroke – The Framingham Study. N Eng J Med. 1975;293:954–956. doi: 10.1056/NEJM197511062931903. [DOI] [PubMed] [Google Scholar]
- 18.Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165–170. doi: 10.1053/apmr.2002.28030. [DOI] [PubMed] [Google Scholar]
- 19.Nyberg L, Gustafson Y. Fall prediction index for patients in stroke rehabilitation. Stroke. 1997;28:716–721. doi: 10.1161/01.str.28.4.716. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000;11:381–387. doi: 10.1007/s001980070103. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto J, Tsukimura T, Takeda T. Bone mineral density of metatarsus in hemiplegic subjects. Am J Phys Med Rehabil. 1999;78:202–207. doi: 10.1097/00002060-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sahin L, Ozoran K, Gunduz OH, Ucan H, Yucel M. Bone mineral density in patients with stroke. Am J Phys Med Rehabil. 2001;80:592–596. doi: 10.1097/00002060-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Takamoto A, Masuyama T, Nakajima M, Sekiya K, Kosaka H, Morimoto S, Ogihara T, Onishi T. Alterations of bone mineral density of the femurs in hemiplegia. Calcif Tissue Int. 1995;56:259–262. doi: 10.1007/BF00318043. [DOI] [PubMed] [Google Scholar]
- 24.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Nevitt M. Bone mineral density predicts non-spine fractures in very elderly women. Osteoporos Int. 1994;4:235–241. doi: 10.1007/BF01622192. [DOI] [PubMed] [Google Scholar]
- 26.Schott AM, Cormier C, Hans D, Favier F, Hausherr E, Dargent-Molina P, Delmas PD, Ribot C, Sebert JL, Breart G, Meunier PJ. How hip and whole body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int. 1998;8:247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 27.Fraul F, Erdfelder E. G POWER: a priori, post-hoc and compromise analysis: for MS-DOS [computer program] Department of Psychology, Bonn University; Bonn, FRG: 1992. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke. Relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–761. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 31.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83:49–57. [PubMed] [Google Scholar]
- 32.Bohannon RW. Measurement and nature of muscle strength in patients with stroke. J Neuro Rehabil. 1997;11:115–25. [Google Scholar]
- 33.Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res. 2000;15:322–331. doi: 10.1359/jbmr.2000.15.2.322. [DOI] [PubMed] [Google Scholar]
- 34.Uusi-Rasi K, Sievanen H, Pasanen M, Oja P, Vuori I. Maintenance of body weight, physical activity and calcium intake helps preserve bone mass in elderly women. Osteoporos Int. 2001;12:373–379. doi: 10.1007/s001980170105. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 36.Berthouze SE, Minaire PM, Castells J, Busso T, Vico L, Lacour J-R. Relationship between mean habitual daily energy expenditure and maximal oxygen uptake. Med Sci Sports Exerc. 1995;27:1170–1179. [PubMed] [Google Scholar]
- 37.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 38.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 39.Hsu A-L, Tang P-F, Jan M-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild and moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 40.Bohannon BW, Smith MB. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 41.Looker AC, Orwall ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low bone density in older US adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Gluer C-C. An update on the diagnosis and assessment of osteoporosis with densitometry. Osteoporos Int. 2000;11:192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 43.Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7:1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 44.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 45.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 46.Kim CM, Eng JJ. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture. 2003;8:23–28. doi: 10.1016/s0966-6362(02)00122-4. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading. Bone. 2000;27:701–707. doi: 10.1016/s8756-3282(00)00374-4. [DOI] [PubMed] [Google Scholar]
- 48.De Laet CEDH, van der Klift M, Hofman A, Pols HAP. Osteoporosis in men and women: a story about bone mineral density thresholds and hip fracture risk. J Bone Miner Res. 2002;17:2231–2236. doi: 10.1359/jbmr.2002.17.12.2231. [DOI] [PubMed] [Google Scholar]
- 49.White HC. Post-stroke hip fractures. Arch Orthop Trauma Surg. 1988;107:345–347. doi: 10.1007/BF00381059. [DOI] [PubMed] [Google Scholar]
- 50.Hamdy RC, Krishnaswamy G, Cancellaro V, Whalen K, Harvill L. Changes in bone mineral content and density after stroke. Am J Phys Med Rehabil. 1993;72:188–191. doi: 10.1097/00002060-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Liu M, Tsuji T, Higuchi Y, Domen K, Tsujiuchi K, Chino N. Osteoporosis in hemiplegic stroke patients as studied with dual-energy X-ray absorptiometry. Arch Phys Med Rehabil. 1999;80:1219–1226. doi: 10.1016/s0003-9993(99)90019-9. [DOI] [PubMed] [Google Scholar]
- 52.Yavuzer G, Ataman S, Sulder N, Mesut A. Bone mineral density in patients with stroke. Int J Rehabil Res. 2002;25:235–239. doi: 10.1097/00004356-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Faulkner H, Munz M, Scherrer M. Bone mineral density of opposing hips using dual energy X-ray absorptiometry in single-beam and fan-beam design. Calcif Tissue Int. 1997;61:445–447. doi: 10.1007/s002239900365. [DOI] [PubMed] [Google Scholar]
- 54.Mazess RB, Nord RH, Hanson JA, Barden HS. Bilateral measurement of femoral bone mineral density. J Clin Densitom. 2000;3:133–140. doi: 10.1385/jcd:3:2:133. [DOI] [PubMed] [Google Scholar]
- 55.Ryan AS, Dobrovolny L, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12:144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 57.Van Langendonck L, Claessens AL, Lefevre J, Thomis M, Philippaerts R, Delvaux K, Lysens R, Vanden Eynde B, Beunen G. Association between bone mineral density (DXA), body structure, and body composition in middle-aged men. Am J Human Biol. 2002;14:735–742. doi: 10.1002/ajhb.10090. [DOI] [PubMed] [Google Scholar]
- 58.Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C. Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years or older. Gerontology. 2001;47:207–212. doi: 10.1159/000052800. [DOI] [PubMed] [Google Scholar]
- 59.Pluijim SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res. 2001;16:2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 60.Vico L, Pouget JF, Calmels P, Chatard JC, Rehailia M, Minaire P, Geyssant A, Alexandre C. The relations between physical ability and bone mass in women aged over 65 years. J Bone Miner Res. 1995;10:374–383. doi: 10.1002/jbmr.5650100307. [DOI] [PubMed] [Google Scholar]
- 61.Hughes VA, Frontera WR, Dallal GE, Lutz KJ, Fisher EC, Evans WJ. Muscle strength and body composition associations with bone density in older subjects. Med Sci Sports Exerc. 1995;27:967–974. doi: 10.1249/00005768-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Winter DA. Biomechanics and Motor Control of Human Gait: Normal, elderly and Pathological. 2. Waterloo Biomechanics; Waterloo, Canada: 1991. [Google Scholar]
- 63.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 64.Ross PD, He Y-F, Yates AJ, Coupland C, Ravn P, McClung M, Thompson D, Wasnich RD. Body size accounts for most differences in bone density between Asian and Caucasian women. Calcif Tissue Int. 1996;59:339–343. doi: 10.1007/s002239900137. [DOI] [PubMed] [Google Scholar]
- 65.Russell-Aulet M, Wang J, Thornton J, Colt EWD, Pierson RN. Bone mineral density and mass by total-body dual-photon absorptiometry in normal white and Asian men. J Bone Miner Res. 1991;10:1109–1113. doi: 10.1002/jbmr.5650061012. [DOI] [PubMed] [Google Scholar]
- 66.Wu XP, Liao EY, Huang G, Dai RC, Zhang H. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif Tissue Int. 2003;73:122–132. doi: 10.1007/s00223-002-1069-7. [DOI] [PubMed] [Google Scholar]
- 67.Frost HM. Absorptiometry and “osteoporosis”: problems. J Bone Miner Metab. 2003;21:255–260. doi: 10.1007/s00774-003-0418-6. [DOI] [PubMed] [Google Scholar]
- 68.Ryan AS, Ivey FM, Hurlbut DE, Martel GF, Lemmer JT, Sorkin JD, Metter EJ, Fleg JL, Hurley BF. Regional bone mineral density after resistive training in young and older men and women. Scand J Med Sci Sports. 2004;14:16–23. doi: 10.1111/j.1600-0838.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 69.Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int. 2000;67:443–448. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 70.Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women; results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS) Arch Int Med. 2004;164:1084–1091. doi: 10.1001/archinte.164.10.1084. [DOI] [PubMed] [Google Scholar]
- 71.Kerr D, Ackland T, Maslen B, Morton A, Prince R. Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J Bone Miner Res. 2001;16:175–181. doi: 10.1359/jbmr.2001.16.1.175. [DOI] [PubMed] [Google Scholar]
- 72.Ryan AS, Treuth MS, Hunter GR, Elahi D. Positive training maintains bone mineral density in postmenopausal women. Calcif Tissue Int. 1998;62:295–299. doi: 10.1007/s002239900434. [DOI] [PubMed] [Google Scholar]
- 73.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34:17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Melton LJ., III The prevalence of osteoporosis: gender and racial comparison. Calcif Tissu Int. 2001;69:179–181. doi: 10.1007/s00223-001-1043-9. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka N, Sonoda S, Kondo K, Chino N. Reproducibility of dual-energy X-ray absorptiometry in the upper extremities in stroke patients. Disabil Rehabil. 1997;19:523–527. doi: 10.3109/09638289709166045. [DOI] [PubMed] [Google Scholar]
- 76.Going SB, Massett MP, Hall MC, Bare LA, Root PA, Williams DP, Lohman TG. Detection of small changes in body composition by dual-energy x-ray absorptiometry. Am J Clin Nutr. 1993;57:845–850. doi: 10.1093/ajcn/57.6.845. [DOI] [PubMed] [Google Scholar]
- 77.Gibberd FB, Gould SR, Marks P. Incidence of deep vein thrombosis and leg oedema in patients with strokes. J Neurol Neurosurg Psychiatry. 1976;39:1222–1225. doi: 10.1136/jnnp.39.12.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tothill P, Laskey MA, Orphanidou CI, van Wijk M. Anomalies in dual-energy X-ray absorptiometry measurements of total-body mineral during weight change using Lunar, Hologic and Norland instruments. Br J Radiol. 1999;72:661–669. doi: 10.1259/bjr.72.859.10624323. [DOI] [PubMed] [Google Scholar]