Abstract
Recent evidence has unveiled the critical role of tumor cells with stem cell activities in tumorigenicity and drug resistance, but how tumor microenvironments regulate cancer stem/initiating cells (CSCs) remains unknown. We clarified the role of tumor-associated macrophages (TAMs) and their downstream factor milk-fat globule-epidermal growth factor-VIII (MFG-E8) in the regulation of CSC activities. Bone marrow chimeric systems and adoptive cell transfers elucidated the importance of MFG-E8 from TAMs in conferring to CSCs with the ability to promote tumorigenicity and anticancer drug resistance. MFG-E8 mainly activates signal transducer and activator of transcription-3 (Stat3) and Sonic Hedgehog pathways in CSCs and further amplifies their anticancer drug resistance in cooperation with IL-6. Thus, the pharmacological targeting of key factors derived from tumor-associated inflammation provides a unique strategy to eradicate therapy-resistant tumors by manipulating CSC activities.
Keywords: chemoresistance, tumor progression
Because tumor cells acquire multiple layers of anticancer drug resistance through the alteration of genetic and epigenetic profiles (1, 2) and activation of multidrug resistance transporters (3), it is extremely difficult to treat tumors in which multiple drug resistance is achieved systematically. Recent evidence has clarified the critical role of rare tumor cell populations, termed cancer stem/initiating cells (CSCs), in restraining the drug sensitivities of tumor cells (4). However, the molecular mechanisms whereby CSCs acquire tumorigenicity and drug-resistant machineries remain largely unknown.
Besides intrinsic genetic and epigenetic signatures in tumor cells, the tumorigenicity is regulated by extrinsic signals delivered from microenvironments or niches, which are composed of endothelial cells, stromal fibroblasts, and inflammatory cells (5–7). In addition, accumulating evidence has validated the critical role of tumor-associated myeloid cells in tumor progression and metastasis (5, 6). Thus, the molecular events linking intrinsic oncogenic signals with tumor-associated microenvironments may play an important role in rendering CSCs with the ability to modulate tumorigenicity and drug responses.
Milk-fat globule EGF-8 (MFG-E8) has been identified as a growth factor involving phagocytosis, angiogenesis, and immune tolerance (8–10). MFG-E8 was also highly produced from tumor-associated macrophages (TAMs) (11), but it remains largely unknown whether TAM-derived MFG-E8 regulates CSC activities. Here we found that TAMs produced large amounts of MFG-E8 in stimulation with CSCs. The MFG-E8 increased tumorigenicity and anticancer drug resistance in CSCs derived from murine and human tumor cells and primary tumor samples. MFG-E8 triggered anticancer drug resistance through the coordinated activation of Stat3 and Sonic Hedgehog signals in CSC populations. Furthermore, MFG-E8 and IL-6 from TAMs synergistically mediate tumorigenicity and drug resistance in subsets of CSCs including primary human tumors. These findings provide evidence that TAM serves as a source of key components in inflammatory microenvironments, such as MFG-E8 and IL-6, which trigger tumorigenicity and resistance to anticancer therapeutics by regulating CSC activities.
Results
Tumor-Infiltrating Macrophages Produce Large Amounts of MFG-E8.
MFG-E8 is produced in large amounts by myeloid cells such as follicular dendritic cells and tangible macrophages and tumor-infiltrating myeloid cells (11, 12). However, it remains largely unknown which tumor cell subset is responsible for inducing MFG-E8 in tumor microenvironments. Because accumulating evidence demonstrates that CSCs are responsible for rendering tumor cells with the ability to promote tumorigenicity and drug resistance (4, 13–16), we examined the role of CSCs for the MFG-E8 expression in tumor microenvironments.
We inoculated CD44+ALDH1+ colon tumor cells (MC38-CSCs) and CD133+ALDH1+ lung cancer cells (3LL-CSCs), which have been validated as CSCs by the in vivo serial transplantation procedures (Fig. S1) and their nonstem cell counterpart (non-CSCs) into C57BL/6 mice. Various cell types were isolated from established tumors and splenocytes at the time when each tumor reached 100 mm2, and the MFG-E8 expression was quantified by RT-PCR. MFG-E8 expression was largely confined into CD11b+ and F4/80+ populations in MC38-CSC–challenged sites but not spleen, and other populations did not express MFG-E8 (Fig. S2). Furthermore, MFG-E8+ populations were enriched in F4/80+CD11b+ macrophages derived from MC38- or 3LL-CSCs–derived tumors but not those from their non-CSC counterparts, tumor-draining lymph node (TDL), or splenocytes (Fig. 1 A and B and Fig. S3). MFG-E8 proteins were detected at much higher levels in TAM than splenic macrophages isolated from MC38-CSCs bearing wild-type mice, as quantified by ELISA (Fig. S4A). Furthermore, F4/80+ splenic macrophages expressed MFG-E8 when directly cocultured with MC38-CSCs in vitro, whereas those cultured with MC38–non-CSCs, the macrophages, or MC38 alone did not trigger MFG-E8 induction (Fig. 1C). The supernatants from CSCs, but not other tumor cells, were sufficient for MFG-E8 expression in splenic macrophages, suggesting that soluble factors specifically released from CSCs are responsible for MFG-E8 induction (Fig. S4B). Furthermore, the inhibitors for IL-4, IL-10, TGF-β, CCL-2, and arginase-I, which are critical for M2 macrophage differentiation and activities (17), and GM-CSC, which is responsible for MFG-E8 production by peritoneal macrophages (9), had little effect on CSC-mediated MFG-E8 induction by TAM (Fig. S4B). Together, these results validate the CSC-specific role in MFG-E8 induction of macrophages.
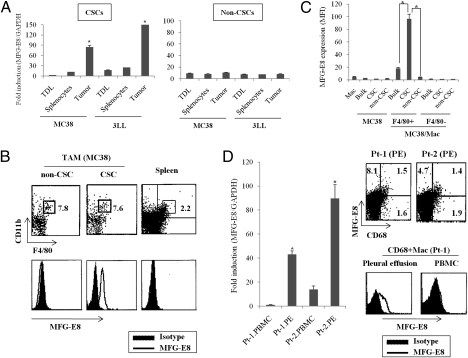
Fig. 1.
CSCs induce MFG-E8 expression from TAM. (A) F4/80+CD11b+ macrophages were isolated from tumors, tumor-draining lymph nodes (TDLs), and splenocytes in mice bearing CD44+ALDEFLOUR+ MC38-CSCs, or CD133+ALDEFLOUR+ 3LL-CSCs, or their non-CSC counterparts (n = 3 per group). Murine MFG-E8 mRNA was quantified by RT-PCR. The results are shown as fold induction of target genes relative to a reference gene (GAPDH). (B) MFG-E8 expression in F4/80+CD11b+ macrophages isolated from MC38-CSCs or non-CSCs was evaluated by intracellular flow cytometry. The splenic macrophages from CSC-bearing mice (spleen) serve as negative control. (C) MC38-CSCs or non-CSCs, bulk MC38 cells were cultured with F4/80+ splenic macrophages (Mac) for 24 h, and MFG-E8 in macrophages (F4/80+) or MC38 tumor cells (F4/80−) were quantified by flow cytometry. Splenic macrophages or MC38 tumor cells without coculture served as a negative control. The expression was shown as mean fluorescence intensity (MFI). (D) MFG-E8 in CD68+ macrophages (Mac) isolated from pleural effusion (PE) or peripheral blood (PBMC) of two nonsmall cell lung cancer patients (Pt-1 and Pt-2). The MFG-E8 mRNA (Left) or protein (Right) levels were analyzed by RT-PCR or flow cytometry, respectively. Data are representative of three independent experiments.
To evaluate MFG-E8 expression in primary human tumor samples, we used pleural effusion cells isolated from stage IV nonsmall cell lung cancer (NSCLC) patients. Notably, most of the EpCAM+ epithelial cells in pleural effusions represent CD133+ALDH1+ populations (∼90%) (18), indicating that CSC-enriched conditions have been established in the pleural environments of advanced NSCLC (Fig. S5). MFG-E8 was highly detected in CD68+ human macrophages isolated from pleural effusion at much higher levels than EpCAM+ tumor cells or CD68+ macrophages from peripheral blood mononuclear leukocytes (PBMC) of the same donors (Fig. 1D and Fig. S5).
TAMs expressed genes characteristic of tumor-promoting functions, such as hypoxia-inducible factor-1α, arginase-II, and Ets-2 (17, 19, 20). However, CSC-derived TAMs expressed these effectors at levels similar to those from tumors depleted of CSCs (Fig. S6). In addition, the macrophage mannose receptor (MMR) and TIE-2, which served as a marker for alternative (M2) and angiogenic subsets of TAMs (21, 22), was expressed on TAMs from wild-type and MFG-E8–defieicent mice at similar levels. However, MFG-E8 was highly detected in TAM expressing MMR or TIE-2 (Fig. S7), indicating that tumorigenic macrophages characterized by M2 and angiogenic profiles may regulate CSC activities in an MFG-E8–dependent manner. Collectively, these results demonstrate that CSCs are responsible for triggering MFG-E8 induction from macrophages.
TAM-Specific MFG-E8 Renders CSCs with the Ability to Promote Chemoresistance.
Although MFG-E8 has been reported to accelerate tumorigenicity of certain spontaneously arising tumors (23), it remains unknown whether MFG-E8 modulates CSC functions. Thus, MC38-CSCs or 3LL-CSCs were inoculated into MFG-E8–deficient mice or their wild-type counterparts, and the CSC frequencies in established tumors were evaluated by measuring CSC-specific marker expression 1 mo after in vivo tumor challenge. The CSC markers in established MC38-CSCs and 3LL-CSCs were largely lost but still detectable 1 mo after in vivo inoculation, consistent with previous finding that CSCs differentiate into heterogeneous cell populations (4). In contrast, the frequencies of original CSC populations were largely undetectable in tumors grown into MFG-E8–deficient mice (Fig. 2A). The CSC growth in MFG-E8–deficient mice was compatible to that in wild-type mice, excluding the possibility that the different growth kinetics in MFG-E8–deficient and wild-type mice have an impact on the CSC frequencies (Fig. S8).
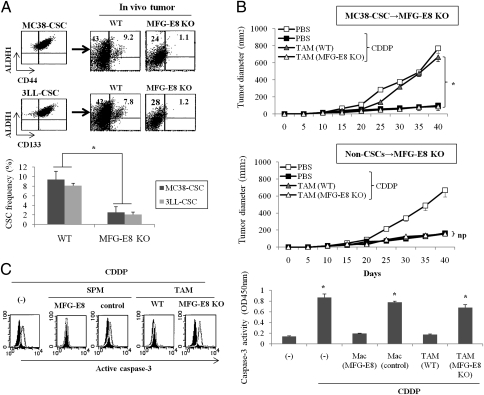
Fig. 2.
TAMs contribute to triggering tumorigenicity and anticancer drug resistance in an MFG-E8–dependent manner. (A) MC38-CSCs or 3LL-CSCs were inoculated into wild-type (WT) or MFG-E8–deficient (MFG-E8 KO) mice for 1 mo, and the frequencies of CSCs were determined in established tumors by quantifying each CSC marker. Representative dot plots (Upper) and data from three experiments (Lower) are shown. (B) TAMs were isolated from tumors of wild-type (WT) or MFG-E8–deficient CD45.1+ mice (MFG-E8 KO). MC38-CSCs or non-CSCs were injected into CD45.2+ MFG-E8–deficient mice (CSCs or non-CSCs→MFG-E8 KO; n = 4 per group) either alone or with TAM, treated with CDDP after tumor inoculation, and tumor growth was measured on the indicated days. (C) CD44+ALDH1+ MC38-CSCs were treated with CDDP in the presence or absence of TAM supernatant (1:10 dilution) from wild-type (WT) or MFG-E8–deficient (MFG-E8 KO) mice, or splenic macrophages infected with control, or MFG-E8 retrovirus. After 24 h of treatment, cell viability was quantified by cleaved caspase-3 intensities by colorimetric assay. Data are representative of three independent experiments.
To further define whether TAM influences CSC activities in an MFG-E8–dependent manner, we evaluated the role of TAM-derived MFG-E8 in modulating anticancer drug sensitivities. To do so, CD11b+F4/80+ macrophages were isolated from established tumors from wild-type or MFG-E8–deficient CD45.1+ mice and were injected along with MC38-CSCs or their non-CSC counterparts into CD45.2+ MFG-E8–deficient mice. We first confirmed that there were little differences between wild-type and MFG-E8–deficient TAMs in the recruitment in tumor tissues (Fig. S9). Transfer of wild-type TAMs resulted in the impaired antitumor effect of chemotherapeutic agent cisplatin (CDDP) against MC38-CSCs, but the same regimens regressed tumor growth when MFG-E8–deficient TAM was transferred with MC38-CSCs (Fig. 2B). In contrast, neither wild-type nor MFG-E8–deficient TAMs had any effect on the drug sensitivities of non-CSC–derived tumors (Fig. 2B).
We next evaluated the role of TAM-derived MFG-E8 in the in vitro drug-induced apoptosis. The supernatant of wild-type TAM or splenic macrophages infected with MFG-E8 retrovirus suppressed CDDP-induced caspase-3 activation in MC38-CSCs, but that of MFG-E8–deficient TAM or splenic macrophages infected with control retrovirus sensitized MC38-CSCs to apoptotic cell death by CDDP treatment (Fig. 2C). The role of MFG-E8 in anticancer drug resistance was also confirmed in other human tumor cells (melanomas, breast carcinomas, and NSCLC cells) expressing CSC markers, whereas MFG-E8 has little effect with chemotherapy on their bulk populations (Fig. S10).
Together, these results demonstrated that TAM-derived MFG-E8 plays an indispensable role in rendering CSCs with the ability to restrict anticancer drug responses.
TAM-Specific MFG-E8 Renders CSCs with the Ability to Promote Tumorigenicity.
Because long-term sphere-forming capacity is a common characteristic of CSCs, we evaluated the role of MFG-E8 in tumor sphere-forming activities. The supernatant of wild-type, but not MFG-E8–deficient TAM increased sphere numbers and diameters in bulk MC38 cells. In addition, the recombinant murine MFG-E8 protein promoted sphere formation in a concentration-dependent manner (Fig. 3A). The addition of human MFG-E8 protein also accelerated sphere formation of human colon cancer cells (HCT116) (Fig. S11). MFG-E8 also regulates the PKH-26 dye-retaining ability of HCT116 cells, suggesting that MFG-E8 maintains CSCs as quiescent-state populations linking with their chemoresistant phenotype (24–26) (Fig. S12).
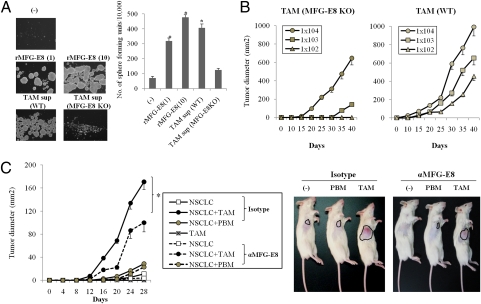
Fig. 3.
MFG-E8 plays a critical role in tumor self-renewal. (A) MC38 cells were treated with TAM supernatant (1:10 dilution) from wild-type (WT) or MFG-E8–deficient (MFG-E8 KO) mice or recombinant murine MFG-E8 protein (1 or 10 μg/mL) in ultra-low attachment plates. The cells were then cultured for three passages, and the numbers of formed spheres generated per 10,000 cells were determined. (B) In vivo serial tumor passages were performed to evaluate the frequency of CSCs. TAM from wild-type or MFG-E8–deficient mice was injected along with MC38-CSCs s.c. into MFG-E8–deficient mice (n = 4 per group) at 1 × 105 per mouse. After 30 d, single cell suspensions were prepared from growing tumors and further transplanted with each TAM into tumor-free MFG-E8–deficient mice using the indicated number of cells. Tumor growth was measured on the indicated days. (C) EpCAM+CD133+ tumor cells obtained from patients with advanced NSCLC (1 × 102 per mouse) were inoculated into clodronate-pretreated NOD-SCID mice (n = 4 per group) with or without autologous TAM or peripheral blood macrophages (PBMs) in the presence of isotype control Ig or antihuman MFG-E8 Ab. The growth curves of each tumors (Left) and representative results (Right) are shown. Similar results were obtained in three independent experiments.
To further define the contribution of TAM-derived MFG-E8 in self-renewal in vivo, TAM isolated from wild-type or MFG-E8–deficient mice were injected along with MC38-CSCs into MFG-E8–deficient mice, and the isolated tumor cells were serially transplanted with wild-type or MFG-E8–deficient TAM into tumor-free MFG-E8–deficient mice. TAMs from wild-type mice accelerated tumor formation with high potency even when small amounts of tumor cells (1 × 102 per mouse) were inoculated, whereas TAMs from MFG-E8–deficient mice could not stimulate tumorigenicity even at more than 1 × 103 cells inoculated (Fig. 3B).
The tumorigenic activities induced by TAM-derived MFG-E8 were also observed in primary human tumors because CD68+ TAMs isolated from NSCLC patients stimulate sphere-forming activities of autologous CD133+EpCAM+ CSCs in an MFG-E8–dependent manner (Fig. S13). To further evaluate whether TAM-derived MFG-E8 plays a critical role in accelerating tumorigenic activities of CSCs in clinically relevant settings, EpCAM+CD133+ primary NSCLC-CSCs were injected s.c. into NOD-SCID mice at small doses (1 × 102 per mouse) in conjunction with autologous CD68+ macrophages isolated from pleural effusions (TAMs) or peripheral blood (PBM), and the tumor formations were evaluated in vivo. In this experiment, NOD-SCID mice were pretreated with clodronate liposome to remove endogenous macrophages. TAMs elicited large tumor formation in NOD-SCID mice, whereas EpCAM+CD133+ CSCs alone or that inoculated with peripheral blood-derived macrophages (PBM) formed small tumors at final evaluation periods (28 d). Importantly, TAM-mediated CSC tumorigenesis was suppressed by the human MFG-E8 blocking Ab (Fig. 3C). Overall, these results demonstrate that TAM-derived MFG-E8 may be responsible for regulating self-renewal and tumorigenic activities of CSCs of mice and human origins.
MFG-E8 Mediates Tumor Drug Resistance by Activating Stat3 and Hedgehog Signals.
We next evaluated how MFG-E8 modulates oncogenic signals and triggers chemoresistance in CSCs. We found that MFG-E8 induced Stat3 phosphorylation and smoothened (SMO) expression, the downstream regulator of Sonic Hedgehog (shh) pathways, to a greater extent in CSCs than non-CSCs in stimulation with supernatant of wild-type but not MFG-E8–deficient TAMs (Fig. 4A). The up-regulation of Stat3 and shh activities was also confirmed in CSCs stimulated with supernatant of wild-type TAM by flow cytometry, but MFG-E8–deficient TAM or splenic macrophage (SPM) had little effect on Stat3 and shh activation in CSCs (Fig. 4B). Moreover, the target gene expression for Stat3 (SOCS3, myeloid cell leukemia protein, and vascular endothelial growth factor-A) and shh (GLi1, PTCH1, and GAS1) were up-regulated in CSCs stimulated with wild-type, but not MFG-E8–deficient, TAM (Fig. S14). These results demonstrate that TAM-derived MFG-E8 plays an important role in Stat3 and shh activation of CSCs.
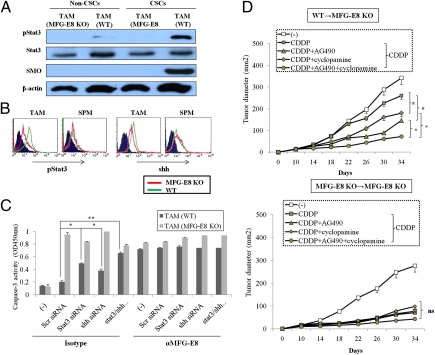
Fig. 4.
Oncogenic signals induced by MFG-E8. (A) Immunoblot for pSTAT3, STAT3, and SMO on lysates from CD44+ALDH1+ MC38 (CSC) or their non-CSC counterparts 2 h after stimulation with wild-type TAM supernatant (TAM) or not (−). (B) MC38-CSCs were treated with TAM supernatant or splenic macrophages (SPMs) from wild-type (WT) or MFG-E8–deficient (MFG-E8 KO) mice for 6 h. The expression of phospho-stat3 and shh were evaluated by flow cytometry. (C) Target gene expression for stat3 (SOCS3, MCL, and VEGFA) and shh (GLi1, PRCH1, and GAS1) in CSCs stimulated with TAM supernatant from wild-type (WT) or MFG-E8–deficient mice (MFG-E8 KO). (D) MC38-CSCs were infected with siRNA specific for Stat3, Sonic Hedgehog (shh), or both and treated with WT or MFG-E8–deficient TAM supernatant (1:10 dilution) in the presence of anti–MFG-E8 or isotype-matched control IgG for 24 h. The cell viability was quantified by cleaved caspase-3 intensities with colorimetric assay. *P < 0.05 compared with scrambled siRNA. (E) The chimeric mice reconstituted with wild-type (WT→MFG-E8 KO) or MFG-E8 KO (MFG-E8 KO→MFG-E8 KO) bone marrow into MFG-E8-deficient host (n = 4 per group) were generated. Eight weeks after transplantation, MC38-CSCs were inoculated s.c. into each chimeric mice, and mice were then treated with CDDP with or without AG490, cyclopamine, or both on days 10, 12, 14, and 16 after tumor challenge. Tumor growth was measured on the indicated days. Similar results were obtained in two independent experiments, and representative results are shown.
We next examined whether Stat3 and shh signals are responsible for regulating CSC activities in TAM-derived MFG-E8–dependent manner. The importance of Stat3-mediated signals in MFG-E8–mediated tumorigenic activities have been validated by the observation that a constitutively active form of Stat3 (Stat3C) abrogated the tumor suppressive effects of anti–MFG-E8 Ab in MC38-CSCs stimulated with wild-type TAMs (Fig S15). The transcriptional activation of the Hedgehog effector Gli-1 was also increased in MC38-CSCs treated with wild-type TAM supernatant, which was abrogated by anti–MFG-E8 blocking Ab (Fig. S16).
To further evaluate the functional relevance of MFG-E8–mediated stat3 and shh regulation, we used siRNA knockdown for each signal and assessed cell viability of MC38-CSCs after treatment with TAM supernatant and CDDP. The combined inhibition of Stat3 and shh pathways substantially increased CDDP-mediated CSC apoptosis even in the presence of wild-type TAM (Fig. 4C). In contrast, CDDP alone was sufficient to reduce CSC viability irrespective of the Stat3 and/or shh inhibition when either MFG-E8–deficient TAM or anti–MFG-E8 blocking Ab was added during the in vitro cultures (Fig. 4D).
To define the contribution of TAM-derived MFG-E8 in activating Stat3 and shh pathways in vivo, lineage negative and c-Kit/Sca-1+ hematopoietic stem cells (HSC) generated from wild-type or MFG-E8–deficient mice were used to reconstitute lethally irradiated MFG-E8-KO recipients. Two months after transplantation, mice were challenged with MC38-CSC and treated with CDDP in the presence or absence of JAK2 inhibitor AG490 and/or shh inhibitor cyclopamine. The combined treatments of CDDP with cyclopamine and/or AG490 had greater antitumor effects than CDDP alone on MC38-CSC tumor growth in wild-type bone marrow (BM) chimeric mice. In contrast, CDDP alone was sufficient to reduce tumor burden in MFG-E8–deficient BM chimeric mice independently of AG490 and cyclopamine treatments (Fig. 4D).
Together, these findings highlight the role of distinct oncogenic pathways mediated by Stat3 and shh in orchestrating the TAM-dependent CSC activities.
IL-6 Plays a Critical Role in Amplifying MFG-E8–Mediated Tumorigenicity of Human CSCs.
TAMs secrete large amounts of IL-6, which serves as a key player to activate Stat3 and promote tumorigenesis in cooperation with inflammatory signals (27–29).
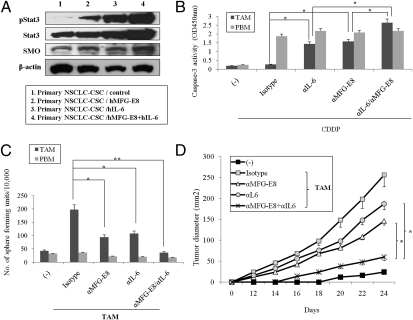
We therefore addressed the involvement of TAM-derived IL-6 in modulating CSC activities. IL-6 coordinates with MFG-E8 to further increase Stat3 activation and SMO expression in human primary NSCLC-CSCs (Fig. 5A). However, the MFG-E8 blockade did not influence the IL-6 expression in primary human TAM and vice versa, indicating that MFG-E8 and IL-6 does not regulate each other (Fig. S16).
Fig. 5.
MFG-E8 and IL-6 coordinately trigger CSC chemoresistance in human CSCs. (A) Primary NSCLC-CSCs were treated with recombinant MFG-E8 (50 ng/mL), IL-6 (10 ng/mL), or both for 16 h. Immunoblotting for Stst3, pStat3, and SMO proteins was performed on the cell lysates. (B) Primary NSCLC-CSCs were treated with TAM or PBM supernatant (1:10 dilution) in the presence of anti–MFG-E8 Ab and/or anti–IL-6 mAb in ultra-low attachment plates. The cells were then cultured for three passages, and the numbers of formed spheres generated per 10,000 cells were determined. (C) Primary NSCLC-CSCs were treated with CDDP and the TAM or PBM supernatant (1:10 dilution) in the presence or absence of anti–MFG-E8 Ab or anti–IL-6 mAb for 24 h, and the cell viability was quantified by cleaved caspase-3 intensities with colorimetric assay. (D) Primary NSCLC-CSCs were inoculated into clodronate-pretreated NOD-SCID mice (n = 4 per group) with CD68+ macrophages isolated from primary tumors (TAM), treated with isotype-matched IgG (Isotype), anti–MFG-E8 Ab and/or anti–IL-6 mAb, or both, and tumor growth was measured on the indicated days. The NSCLC-CSCs without TAM serve as a negative control (−). The differences in tumor growth were compared between monotherapy (either anti–MFG-E8 or anti–IL-6 alone) and combined regimens. Data are representative of three independent experiments.
We found that the combined inhibition of MFG-E8 and IL-6 increased CDDP-induced apoptosis (Fig. 5B) and suppressed sphere-forming activities (Fig. 5C and Fig. S18) at greater degrees than anti–MFG-E8 Ab or anti–IL-6 Ab alone in primary NSCLC-CSCs stimulated with TAM supernatant but not PBM. In contrast, MFG-E8 serves as a main factor for tumorigenic activities of murine MC38-CSCs (Figs. 2D and 4D). These results indicate that coregulation of MFG-E8 and IL-6 may be required for the tumorigenicity and drug resistance in subsets of CSCs including primary NSCLCs.
We finally examined the interplay between MFG-E8 and IL-6 in regulating CSC tumorigenic activities in vivo. The combined blockade of MFG-E8 and IL-6 markedly suppressed primary NSCLC-CSC–derived tumor growth in coinjection with autologous TAM, whereas the anti–MFG-E8 Ab or anti–IL-6 Ab alone had partial antitumor effects (Fig. 5D). These results demonstrate that IL-6 amplifies MFG-E8–mediated activities in increasing tumorigenic activities in subsets of CSCs including primary human tumors.
Discussion
Recent evidence has revealed that tumorigenic cells are infrequent and heterogeneous populations as measured by CSC marker expression (30). However, the identification of tumorigenic cells, including CSCs, has been largely based on the tumor formation of purified patient-derived cell suspensions in immunodeficient animals, and it is difficult to clarify the role of environmental differences between tumors in modulating tumorigenicity and anticancer drug sensitivities. Therefore, it is urgent to elucidate the possibility that extrinsic signals delivered by distinct microenvironments may regulate the plasticity of CSC phenotypes and functions.
Because inflammatory cells in tumor microenvironments play an important role in affecting tumor progression via inflammatory and angiogenic signals (5, 6, 24), they may have a role in modulating tumorigenicity and stem cell activities.
MFG-E8 has been identified as a growth factor that signals through integrin-αvβ3 and αvβ5. Although MFG-E8 exerts various physiological processes, such as apoptotic cell phagocytosis and angiogenesis (8, 9, 31), it also plays a critical role for tumor progression through coordinated interplay of oncogenic and immune-dependent mechanisms (10, 11, 23). In this study, we have identified that MFG-E8, mainly derived from CSC-associated macrophages, is a major contributor in triggering the tumorigenicity and resistance to anticancer drugs of CSCs. Interestingly, αv-integrins, which serve as MFG-E8 receptors, were expressed on CSCs at higher levels than non-CSCs (Fig. S19), implying that MFG-E8 interaction with αv-integrins may be critical for triggering tumorigenic activities and drug resistance in a CSC-specific manner. Thus, it is of great interest to clarify whether αv-integrins serve as functional markers of particular CSC subsets that specifically communicate with tumor-associated myeloid cells.
We also demonstrated that IL-6 coordinates with MFG-E8 in further amplifying anticancer drug resistance by boosting specific oncogenic signals, the Stat3 and Hedgehog pathways, in subsets of CSCs including primary NSCLCs. Together, these findings clarified the indispensable role of inflammatory signals in tumor microenvironments to determine the clinical efficacy of various anticancer modalities against therapy-resistant tumor cells (Fig. S20).
There is a growing appreciation that myeloid cells, including macrophages and dendritic cells, are composed of several distinct populations that may influence the quality of tumor microenvironments. Recent genetic profiling and phenotypic analyses have also revealed that tumor cells manipulate tumor-infiltrating macrophages to induce distinct factors, such as versican and IL-13, which differentiate myeloid cells into specialized subsets with tumor-promoting capacities (32, 33). Because MFG-E8 and IL-6 were up-regulated in macrophages infiltrating the microenvironment formed by CSCs, the genetic and phenotypic profiles in CSCs distinct from bulk tumor cells may manipulate macrophages to induce tumorigenesis and drug resistance in a paracrine fashion. Thus, it is of great interest to clarify the molecular pathways and identify the distinct factors induced by CSCs.
Tumorigenic cells possessing anticancer therapy resistance, in particular CSC, have unique characteristics with which to manipulate complex signal cascades leading to oncogenic addiction, stem cell maintenance, and angiogenesis (4). Several oncogenic pathways, including PI3K/Akt, β-catenin, mTOR, TGF-β/FOXO, etc., regulate anticancer drug responsiveness and tumorigenicity in chemotherapy-resistant tumor cell populations (34, 35). Stat3 also plays a critical role in positively regulating self-renewal of embryonic stem cells stimulated with leukemia inhibitory factor (36). Hedgehog signals have been identified as sentinel in linking oncogenic aberration with the developmental programs of normal and cancer stem cells (37). Consistent with the importance of Stat3 and Hedgehog signals in stem cell activities, our findings clarify the coordinated interplay between Stat3 and Hedgehog signals in rendering CSCs tumorigenic and resistant to anticancer drugs. Although the molecular interplay that connects two different signaling pathways in CSCs remains largely unresolved and must necessarily be further clarified, recent studies have unveiled the critical role of epigenetic alterations in generating tumorigenic cells in NF-κB–dependent inflammatory signals (27). Thus, it is possible that tumor-associated inflammation may modify chromatin structure and epigenetic signals, leading to the transcriptional activation of subsets of target genes in CSCs.
In summary, we have unveiled the critical role of MFG-E8 and IL-6 in combination with inflammatory tumor environments in determining the clinical efficacies of anticancer therapeutics against therapy-difficult CSC populations. Recent breakthroughs in comprehensive approaches along with the progress of stem cell biology have led to the identification of CSC-specific markers and multiple genetic pathways suitable for specifically targeting cancer stem cells (38). In addition to the novel therapeutic candidates identified from CSCs, our findings demonstrate that the targeting of components derived from tumor microenvironments may provide new therapeutic strategies, in combination with inhibitors of CSC-specific pathways, to eradicate treatment-difficult tumors across the different genetic and epigenetic alterations.
Materials and Methods
MFG-E8 KO mice, C57BL/6, and NOD-SCID animals were used as hosts of tumor inoculations. Pleural effusion cells and PBMCs were obtained from advanced NSCLC patients according to the protocols approved by the institutional review board (no: 10-0114). Tumor-associated macrophages isolated from tumors inoculated into wild-type or MFG-E8 KO mice, or from pleural effusion of advanced NSCLC patients were used for assessing MFG-E8 expression and role of CSC activities. Detailed information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Shigekazu Nagata (Kyoto University) for providing MFG-E8–deficient mice. We also express appreciation to Dr. Glenn Dranoff (Dana-Farber Cancer Institute) for providing critical comments and carefully evaluating the manuscript. In addition, we extend appreciation to Kyoko Hoshina and Ruriko Miyake for their great assistance with the animal care and Muhammad Baghdadi for cell sorting. This study is partially supported by a Grant-in-Aid for Scientific Research for Young Scientists (A) and Scientific Research on Innovative Areas (to M.J.), Uehara Memorial Research Awards (to M.J.), Takeda Science Foundation (M.J.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (M.J.), Sagawa Foundation for Promotion of Cancer Research (M.J.), and the Grant for Joint Research Program of the Institute for Genetic Medicine, Hokkaido University (to M.J. and H. Yagita.)
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106645108/-/DCSupplemental.
References
- 1.Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: Implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–3234. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 2.Esteller M, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 3.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 9.Jinushi M, et al. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinushi M, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinushi M, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889–8898. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- 12.Kranich J, et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205:1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 15.Schatton T, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eramo A, Haas TL, De Maria R. Lung cancer stem cells: Tools and targets to fight lung cancer. Oncogene. 2010;29:4625–4635. doi: 10.1038/onc.2010.207. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabuawala T, et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagemann T, et al. Ovarian cancer cells polarize macrophages towards a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: Regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 23.Sugano G, et al. Milk fat globule—epidermal growth factor—factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene. 2011;30:642–653. doi: 10.1038/onc.2010.446. [DOI] [PubMed] [Google Scholar]
- 24.Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML) Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- 25.Clarke MF, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 26.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 29.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aspord C, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naka K, et al. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, et al. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:319–328. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.