Abstract
Inflammasomes are intracellular multiprotein signaling complexes that activate Caspase-1, leading to the cleavage and secretion of IL-1β and IL-18, and ultimately host cell death. Inflammasome activation is a common cellular response to infection; however, the consequences of inflammasome activation during acute infection and in the development of long-term protective immunity is not well understood. To investigate the role of the inflammasome in vivo, we engineered a strain of Listeria monocytogenes that ectopically expresses Legionella pneumophila flagellin, a potent activator of the Nlrc4 inflammasome. Compared with wild-type L. monocytogenes, strains that ectopically secreted flagellin induced robust host cell death and IL-1β secretion. These strains were highly attenuated both in bone marrow-derived macrophages and in vivo compared with wild-type L. monocytogenes. Attenuation in vivo was dependent on Nlrc4, but independent of IL-1β/IL-18 or neutrophil activity. L. monocytogenes strains that activated the inflammasome generated significantly less protective immunity, a phenotype that correlated with decreased induction of antigen-specific T cells. Our data suggest that avoidance of inflammasome activation is a critical virulence strategy for intracellular pathogens, and that activation of the inflammasome leads to decreased long-term protective immunity and diminished T-cell responses.
Keywords: cell-mediated immunity, innate immunity, pathogenesis, CD8+ T cells, vaccine
The innate immune system functions to detect invading microbes, eliminate or contain infections, and orchestrate the development of adaptive immune responses (1). One innate immune pathway triggered by infection is inflammasome activation. Inflammasomes are multiprotein complexes that activate proinflammatory caspases and subsequently lead to cytokine secretion. Detection of pathogen-associated molecular patterns (PAMPs) by cytosolic pattern recognition receptors (PRRs) leads to inflammasome complex formation and activation of Caspase-1. Active Caspase-1 cleaves and activates the proinflammatory cytokines IL-1β and IL-18, leading to their secretion. Concomitant with IL-1β and IL-18 secretion is Caspase-1–dependent cell death, known as pyroptosis (2).
Multiple cytoplasmic PRRs can trigger inflammasome activation, each responding to a different ligand or stimulus (2). One of the most well-characterized PRRs leading to inflammasome activation is Nlrc4 (Ipaf) (3–5). Nlrc4 activates Caspase-1 in response to contamination of the cytosol with either bacterial flagellin or type III secretion inner-rod proteins (3, 4, 6). For example, infection with Legionella pneumophila robustly activates the Nlrc4/Naip5 inflammasome in a process that is dependent on both bacterial flagellin and a type IV secretion system thought to mediate delivery of the flagellin to the cytosol (5, 7).
Numerous microbes trigger Caspase-1 activation in vitro, and in a few cases Caspase-1–deficient mice are more susceptible to infection, implying that pyroptosis can be a host innate immune defense mechanism (8, 9). Not surprisingly, pathogens have evolved mechanisms to avoid inflammasome activation, either by direct inhibition of Caspase-1 activation or by regulating PAMPs expression (10). In addition to its potential role in innate immune defense, inflammasome activation has been implicated in the development of adaptive immunity to influenza virus, fungal β-glucan, and that mediated by the adjuvant alum (11–13).
Listeria monocytogenes is a Gram-positive, facultative intracellular pathogen that has been extensively used as a model to study cell biology, bacterial pathogenesis, and innate and adaptive immunity. Following internalization by a host cell, L. monocytogenes uses a cholesterol-dependent cytolysin, listeriolysin O (LLO encoded by the gene hly), to break out of a phagosome and enter the host cytosol (14). Once in the cytosol, L. monocytogenes synthesizes and secretes ActA to hijack the host actin machinery and spread to neighboring cells (15). Maintenance of its intracellular replication niche is essential to L. monocytogenes virulence as strains that fail to compartmentalize LLO activity to the phagosome are cytotoxic and highly attenuated (16). Nevertheless, there have been numerous reports that L. monocytogenes infection leads to activation of multiple inflammasomes in vitro, including the Nlrp3, Nlrc4, and AIM2 inflammasomes (17–23). Although these responses can be detected in vitro, the role of inflammasome activation and pyroptosis during in vivo infections is not appreciated. Therefore, to address the role of the inflammasome in vivo, we used L. monocytogenes as a model pathogen and compared wild-type bacteria to a strain engineered to activate the Nlrc4 inflammasome. We found that activation of the inflammasome not only attenuated virulence, but also inhibited the development of long-term protective immunity.
Results
L. monocytogenes Infection Triggers Negligible Inflammasome Activation.
L. monocytogenes has evolved multiple mechanisms to maintain its intracellular niche (16). Nevertheless, there are numerous reports that L. monocytogenes infection triggers inflammasome activation in vitro (17–23). To reexamine the degree of inflammasome activation upon L. monocytogenes infection, we measured cell death and IL-1β secretion induced by L. monocytogenes compared with L. pneumophila, a robust activator of the Nlrc4 inflammasome. Following infection of bone marrow-derived macrophages at low multiplicities of infection (MOI = 5), L. monocytogenes induced significantly less lactate dehydrogenase release (10% vs. 89%) and IL-1β secretion (∼60-fold less) than L. pneumophila (Fig. S1). Consistent with previous reports (20), the low levels of cell death and IL-1β secretion induced by L. monocytogenes were dependent on bacterial access to the cytosol, as infection with Δhly L. monocytogenes led to almost no cell death or IL-1β secretion (Fig. S1).
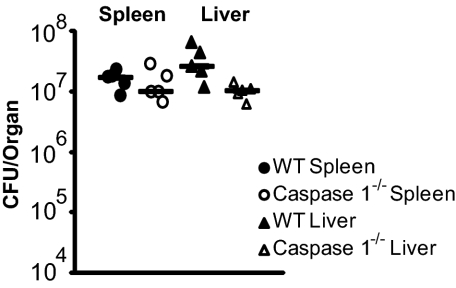
Because Caspase-1 is required for inflammasome-mediated cell death and IL-1β secretion, we used Caspase-1−/− mice to evaluate the role of the inflammasome during primary listeriosis. Wild-type and Caspase-1−/− mice were equally susceptible to infection as monitored by bacterial load in the liver and spleen 48 h postinfection (Fig. 1). In fact, Caspase-1−/− mice were slightly more resistant to L. monocytogenes infection at both 2 and 5 d postinfection (Fig. 1 and Fig. S2). The observation that wild-type L. monocytogenes induced only low levels of pyroptosis, as well as the observation that Caspase-1−/− mice are not hypersusceptible to infection, suggested that the low levels of inflammasome activation observed in vitro play at most a minor role in host defense during primary listeriosis.
Fig. 1.
L. monocytogenes minimally activates the inflammasome. Wild-type (closed symbols) or Caspase-1−/− (open symbols) mice were infected with 1 × 105 wild-type L. monocytogenes and CFU per organ were determined 48 h postinfection. Data are representative of at least two independent experiments.
L. monocytogenes Ectopically Expressing Flagellin Hyperactivate Pyroptosis.
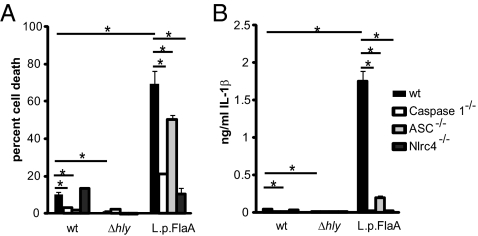
To further evaluate the potential impact of inflammasome activation in vivo, we engineered a L. monocytogenes strain, referred to as L. monocytogenes-L.p.FlaA, that ectopically secreted L. pneumophila flagellin, a potent activator of the Nlrc4/Naip5 inflammasome (3–5). Flagellin was fused to the N-terminal 100 amino acids of ActA to facilitate secretion, and was expressed under control of the actA promoter to restrict expression to cytosolic bacteria. L. monocytogenes-L.p.FlaA induced host cell death 6 h postinfection, killing 70% of infected cells compared with 10% killing by wild-type L. monocytogenes (Fig. 2A). As expected, L. monocytogenes-L.p.FlaA–induced host cell death was largely dependent on Nlrc4 (Fig. 2A). The majority of cell death induced by L. monocytogenes-L.p.FlaA was also independent of ASC (apoptosis-associated speck-like protein containing a CARD). The small but statistically significant portion of cell death that was dependent on ASC was likely caused by background induction of AIM2-dependent cell death normally induced by wild-type L. monocytogenes (Fig. 2A) (18).
Fig. 2.
L. monocytogenes strains that ectopically secrete flagellin hyperinduce host cell death and IL-1β secretion. Cell death (A) and IL-1β (B) secretion were measured following 6 h of infection at an MOI of 5 in wild-type, Caspase-1−/−, ASC−/−, or Nlrc4−/− bone marrow-derived macrophages. Data are representative of at least three independent experiments. *P < 0.05 by Student's t-test.
Concomitant with cell death, L. monocytogenes-L.p.FlaA induced ∼35-fold more IL-1β secretion than wild type L. monocytogenes infection (Fig. 2B). As expected, the secretion of IL-1β by L. monocytogenes-L.p.FlaA was dependent on both Nlrc4 and the adaptor ASC. Hyper-induction of host cell death and IL-1β secretion was not unique to expression of L. pneumophila flagellin as ectopic expression of another Nlrc4 agonist, Salmonella typhimurium PrgJ (6), also resulted in hyperinduction of pyroptosis (Fig. S3).
L. monocytogenes-L.p.FlaA Are Highly Attenuated in Vitro and in Vivo.
To determine if inflammasome activation affects the virulence of intracellular pathogens, we analyzed intracellular growth of wild-type bacteria compared with L. monocytogenes-L.p.FlaA. Growth of L. monocytogenes-L.p.FlaA in wild-type bone marrow-derived macrophages was severely attenuated compared with growth of wild-type L. monocytogenes (Fig. S4A). The growth defect was rescued in Nlrc4−/− bone marrow-derived macrophages, suggesting that the defect was a result of inflammasome activation (Fig. S4B).
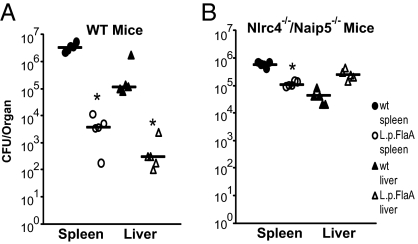
To examine the effect of inflammasome activation on virulence in vivo, we infected wild-type mice intravenously with wild-type or L. monocytogenes-L.p.FlaA. L. monocytogenes-L.p.FlaA were severely attenuated compared with wild-type L. monocytogenes, as indicated by fewer colony forming units (CFU) in both the spleen (∼900-fold) and the liver (∼400-fold) of L. monocytogenes-L.p.FlaA–infected mice 48 h postinfection (Fig. 3A). The severe virulence defect was largely rescued (>99% in the spleen and 100% in the liver) in mice deficient for the cytosolic flagellin detection system (Naip5−/−/Nlrc4−/−) (Fig. 3B). In addition, the LD50 of the L. monocytogenes-L.p.FlaA strain was 7.5 × 106, ∼75-fold higher than the LD50 of the wild-type L. monocytogenes strain in wild-type mice. Taken together, these data suggested that robust inflammasome activation can help control infections by intracellular pathogens in vitro and in vivo.
Fig. 3.
Inflammasome activation attenuates L. monocytogenes virulence. Wild-type (A) or Naip5−/−/Nlrc4−/− (B) mice were infected with 1 × 104 wild type (closed symbols) or L. monocytogenes-L.p.FlaA (open symbols) and CFU was determined 48 h postinfection in the spleen (circles) and liver (triangles). Data are representative of at least two independent experiments. *P < 0.05 by Mann-Whitney test.
L. monocytogenes-LpFlaA Is Attenuated in Vivo Independent of Neutrophil Activity and the Adaptor ASC.
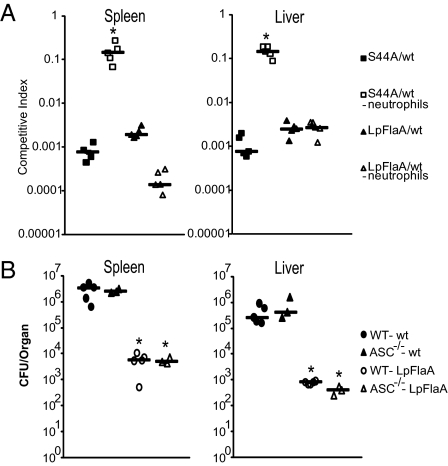
L. monocytogenes strains (e.g., LLOS44A) that induce inflammasome-independent cell death are highly attenuated in vivo. However, depletion of neutrophils rescues the virulence defect, suggesting that neutrophils kill bacteria released by dying cells (16). To determine if neutrophils were responsible for controlling infection of L. monocytogenes-L.p.FlaA strains, we performed competitive index assays in wild-type mice and mice rendered neutropenic by depletion of neutrophils using an anti-Gr1 antibody (24). As previously shown, virulence of L. monocytogenes-LLOS44A was rescued by depletion of neutrophils (>99% spleen and liver) (Fig. 4A). In contrast, the virulence defect of L. monocytogenes-L.p.FlaA was not rescued by depletion of neutrophils as L. monocytogenes-L.p.FlaA was still outcompeted by wild-type bacteria more than 500-fold in both the spleen and liver (Fig. 4A).
Fig. 4.
Neither neutrophil depletion or ASC deficiency rescue the L. monocytogenes-L.p.FlaA virulence defect. (A) Wild-type mice were mock treated (closed symbols) or treated with 10 μg/mL of RB6-8C5 anti-GR1 antibody (open symbols) and infected with either 5 × 103 wild-type + 5 × 103 LLOS44A mutants (squares) or 5 × 103 wild-type + 5 × 103 L. monocytogenes-L.p.FlaA (triangles) and competitive index was determined 48 h postinfection. Data are representative of at least two experiments. (B) Wild-type (circles) or ASC−/− (triangles) mice were infected with 1 × 104 wild-type (closed symbols) or L. monocytogenes-L.p.FlaA (open symbols) and CFU were determined 48 h postinfection. Data are representative of at least two independent experiments. *P < 0.05 by Mann-Whitney test.
It was previously shown that inflammasome-mediated control of infection by influenza virus is partially mediated by IL-1β (25). Additionally, IL-18 has been shown to mediate resistance to variety of pathogens, including Mycobacterium tuberculosis and Shigella flexneri (26, 27). Although ASC is required for activation and secretion of IL-1β and IL-18 downstream of the Nlrc4 inflammasome, it is dispensible for activation of host cell death (Fig. 2B) (28). Therefore, to determine if IL-1β and IL-18 were required for attenuation of L. monocytogenes-L.p.FlaA, we assayed virulence in ASC−/− mice. L. monocytogenes-L.p.FlaA was still highly attenuated, greater than 500-fold in both the spleen and liver, in ASC-deficient mice (Fig. 4B), indicating that activation of IL-1β and IL-18 did not mediate attenuation. Taken together, these data suggested that attenuation of L. monocytogenes-L.p.FlaA infection was not caused by clearance by neutrophils or inflammatory cytokine activation.
Immunization with L. monocytogenes-L.p.FlaA Results in Decreased Protection to Subsequent L. monocytogenes Challenge.
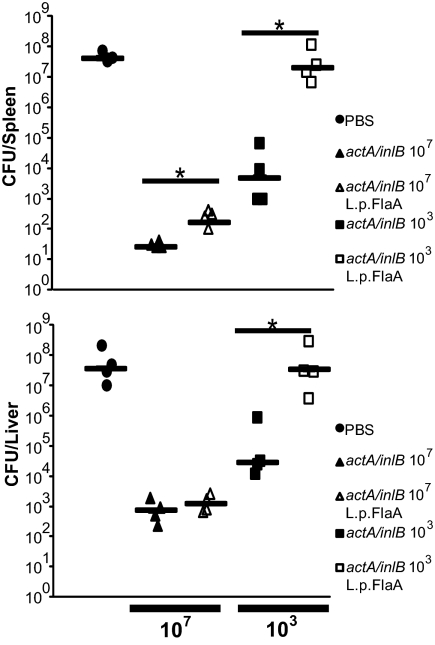
In addition to its role in host defense, inflammasome activation may play a role in the development of adaptive immunity (11, 13). To determine if inflammasome activation, in the context of a L. monocytogenes infection, had an effect on the development of adaptive immunity, we immunized mice with 0.1LD50s of either ΔactA/ΔinlB bacteria or ΔactA/ΔinlB bacteria expressing L. pneumophila flagellin. The ΔactA/ΔinlB background was used to minimize differences in CFUs as the LD50s of ΔactA/ΔinlB and ΔactA/ΔinlB L. monocytogenes-L.p.FlaA were identical. Thirty days postimmunization, we challenged mice with 2 LD50s (2 × 105) of wild-type L. monocytogenes. Seventy-two hours following challenge, ΔactA/ΔinlB L. monocytogenes-L.p.FlaA–immunized mice had slightly higher bacterial burdens in both the spleen (10-fold) and the liver (twofold) compared with ΔactA/ΔinlB-immunized mice (Fig. 5). Similarly, mice challenged 90 d postimmunization with ΔactA/inlB were more protected from subsequent lethal challenge than mice immunized with ΔactA/ΔinlB L. monocytogenes-L.p.FlaA (Fig. S5).
Fig. 5.
Immunization with inflammasome activating L. monocytogenes results in a loss of protective immunity. Wild-type mice were immunized with1 × 107 (triangles) or 1 × 103 (squares) ΔactA/inlB (closed symbols) or ΔactA/inlB L. monocytogenes-L.p.FlaA (open symbols) bacteria. Thirty days postimmunization, mice were challenged with 2 × 105 wild-type bacteria and CFU per organ were analyzed 68 to 72 h postchallenge. Data are representative of at least two independent experiments. *P < 0.05 by Mann-Whitney test.
ΔactA/ΔinlB L. monocytogenes induce potent protective immunity even at low doses, therefore, to further investigate the role of the inflammasome on the development of cell-mediated immunity, we immunized mice with 0.00001 LD50 (103 CFU) and returned 30 d later with a 2× LD50 challenge. At an immunizing dose of 103, there were identical CFU of ΔactA/ΔinlB and ΔactA/ΔinlB L. monocytogenes-L.p.FlaA 24 and 48 h postimmunization (Fig. S6). Mice immunized with 103 ΔactA/ΔinlB displayed ∼3 to 4 logs of protection in both the spleen and the liver following challenge with 2 LD50s of wild-type L. monocytogenes (Fig. 5). In contrast, mice immunized with ΔactA/ΔinlB L. monocytogenes-L.p.FlaA were completely unprotected from challenge, harboring bacterial loads similar to naive mice (Fig. 5). Importantly, ΔactA/ΔinlB L. monocytogenes-L.p.FlaA were capable of inducing protective immunity in Caspase-1−/− mice (Fig. S5), suggesting that activation of the inflammasome during immunization inhibited the development of protective immunity.
L. monocytogenes-L.p.FlaA Induced Defective T-Cell Responses Following Immunization.
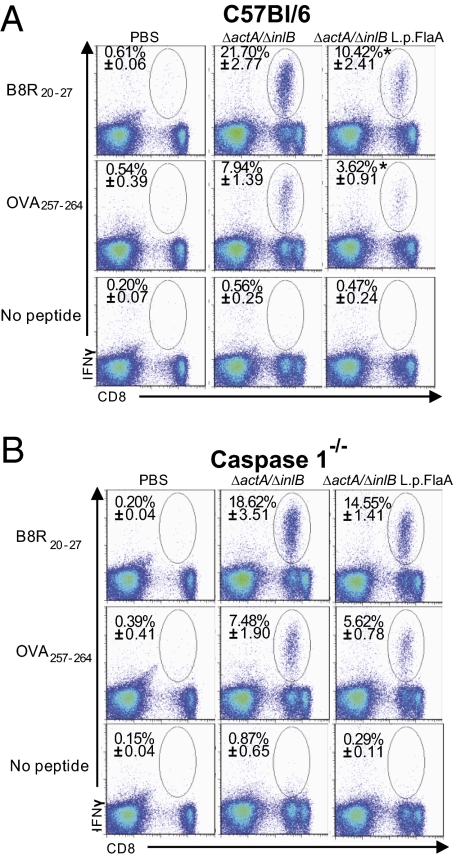
Immune protection against L. monocytogenes is largely mediated by CD8+ T cells (29). L. monocytogenes stimulates only modest antibody responses and antibodies do not provide protection from subsequent challenge with L. monocytogenes (30). Our previous results demonstrated that activation of the inflammasome during immunization resulted in failure to develop long-term protective immunity to L. monocytogenes. Therefore, to address whether inflammasome activation specifically resulted in defects in T-cell development, we analyzed antigen specific CD8+ T-cell responses to L. monocytogenes strains that secreted ovalbumen (OVA) and B8R, two well-characterized CD8+ T-cell antigens. Mice immunized with ΔactA/ΔinlB L. monocytogenes-L.p.FlaA had approximately half as many antigen-specific OVA and B8R CD8+ T cells 7 d postimmunization compared with ΔactA/ΔinlB-immunized mice. This phenotype was observed by both ex vivo peptide stimulation of splenocytes (Fig. 6A) and by direct tetramer staining of OVA-specific CD8+ T cells (Fig. S7). Similarly, LLO190–201 specific CD4+, IFN-γ–producing T cells were reduced in ΔactA/inlB L. monocytogenes-L.p.FlaA–immunized mice compared with ΔactA/inlB-immunized mice (Fig. S8). Importantly, the defect in development of antigen-specific T cells following immunization with ΔactA/inlB L. monocytogenes-L.p.FlaA was rescued in Caspase-1–deficient mice, suggesting that the T-cell development defect was correlated with inflammasome activation (Fig. 6B and Fig. S7).
Fig. 6.
L. monocytogenes that activates the Nlrc4 inflammasome impairs the primary specific CD8+ T-cell response. C57BL/6 (A) or Caspase1−/− (B) mice were injected with 1 × 107 CFU of ΔactA/inlB or ΔactA/inlB L. monocytogenes-L.p.FlaA–expressing OVA and B8R epitope. Seven days postimmunization, the percentage of antigen-specific IFN-γ+ CD8+ T cells was determined using intracellular cytokine staining after in vitro restimulation with the indicated peptide. Values in each plot represent the mean ± SD of antigen-specific cells within a CD8+ cell gate among splenocytes from four to five animals per groups. One representative experiment of two to four is shown.
In addition to abrogated primary T-cell responses, we saw fewer antigen-specific, CD8+ memory T cells 35 d postimmunization in mice immunized with ΔactA/ΔinlB L. monocytogenes-L.p.FlaA compared with ΔactA/ΔinlB (Fig. S9A). However, the smaller memory cell population did not result in a differential number of antigen-specific CD8+ T cells upon secondary challenge. There were equal numbers of IFN-γ–producing antigen-specific CD8+ T cells 5 d after challenge of mice immunized with either ΔactA/inlB or the isogenic strain L. monocytogenes-L.p.FlaA (Fig. S9B). Taken together, these data suggested that inflammasome activation in the context of L. monocytogenes immunization resulted in a defect in both the primary CD8+ T-cell response and in the maintenance of long-term memory CD8+ T cells.
Discussion
The goal of this study was to determine the role of inflammasome activation during infection and immunity using L. monocytogenes as a model pathogen. We determined that wild-type L. monocytogenes infection triggered low levels of inflammasome activation and that this response played a negligible role in the host defense against wild-type L. monocytogenes in vivo. L. monocytogenes-L.p.FlaA, engineered to activate the inflammasome via ectopic expression of flagellin, were severely attenuated in vitro and in vivo. In addition, inflammasome activation resulted in decreased long-term protection from subsequent lethal challenge and diminished antigen-specific T-cell responses.
Although many pathogens activate the inflammasome in vitro, the role of inflammasome activation in vivo is less appreciated. We propose that many intracellular pathogens either inhibit or avoid inflammasome activation to promote their virulence (10). For example, L. monocytogenes has a variety of regulatory mechanisms to avoid activation of host cell death, including regulation of LLO compartmentalization and lack of flagellin expression in vivo (3, 4, 17, 18, 22). Indeed, when LLO or flagellin regulation is disrupted, L. monocytogenes are rendered less virulent (16, 17, 31). As another example, Miao et al. recently reported that ectopic expression of flagellin by S. typhimurium attenuates virulence in an inflammasome-dependent manner (32), further supporting the hypothesis that some intracellular pathogens avoid activation of the inflammasome to promote their virulence.
We previously reported that wild-type L. monocytogenes activates low levels of AIM2-dependent inflammasome activation caused by infrequent bacteriolysis in the cytosol during infection (18). In the present work, we also observed very low levels of inflammasome activation by L. monocytogenes infection. In contrast, other studies have reported substantial activation of the inflammasome by L. monocytogenes. These studies frequently used nonphysiologic MOIs (20, 21), extended infection times (19, 22), or bacterial mutants (17, 18) to study inflammasome activation in vitro. Although these studies are useful for understanding mechanisms of inflammasome activation, they did not address the role of the inflammasome during L. monocytogenes infection in vivo. We propose that the level of inflammasome activation triggered by wild-type L. monocytogenes under normal physiological conditions is so low that it plays a negligible role in vivo. Our observation that Caspase-1 mice are not highly susceptible to infection supports this model, but contradicts previously published reports (33, 34). Differences in infectious dose (1 LD50 vs. 5 LD50), as well as bacterial and mouse genetic backgrounds, likely explain the differences between our results. However, the very low level of inflammasome activation that we see induced by wild-type L. monocytogenes in vitro is consistent with our finding that Caspase-1 mice are not highly susceptible to infection. Furthermore, the severe attenuation of L. monocytogenes-L.p.FlaA in vivo (Fig. 3) illustrates that the inflammasome can exert a strong selective pressure that pathogens, including L. monocytogenes, must avoid to promote their virulence.
Miao et al. recently demonstrated that inflammasome-mediated attenuation of S. typhimurium was independent of IL-1β and IL-18 but dependent on the phagocyte oxidase component p47, suggesting that neutrophil oxidative burst was responsible for controlling infection in the context of inflammasome activation (32). In the present study, neutrophil depletion did not rescue the virulence of L. monocytogenes-L.p.FlaA. Both the S. typhimurium study and the present study find attenuation of intracellular bacteria caused by activation of the Nlrc4 inflammasome; however, we find differences in the role that neutrophils play in this process. In agreement with our findings, Warren et al. recently reported that L. monocytogenes that hyperactivate the Nlrc4 inflammasome were highly attenuated in vivo by a mechanism independent of IL-1β and IL-18 (35). Understanding how inflammasome activation mediates host defense and how pathogens have overcome these defenses is central to our broader understanding of both mechanisms of pathogenesis and the function of the innate immune system.
The inflammasome may also play a role in the development of adaptive immune responses (11–13). L. monocytogenes has been used for almost 50 years as a model to study basic aspects of cell-mediated immunity. The hallmark of this model is the robust induction of antigen-specific CD8+ T cells, but the factors leading to the development of such potent cell-mediated immunity remain an important topic of investigation. In this study we examined the role of inflammasome activation in the development of protective immunity to L. monocytogenes infection. Warren et al. recently reported that L. monocytogenes strains engineered to activate the Nlrc4 inflammasome were capable of inducing protective immunity at high immunizing doses (35). In agreement with these findings, we also found that immunization with high doses of L. monocytogenes-L.p.FlaA induced protection from subsequent challenge. However, when analyzing antigen-specific T-cell responses or analyzing protection at low immunizing doses, we found that there was an inverse correlation between inflammasome activation and induction of protective immunity: that is, strains engineered to activate the Nlrc4-dependent inflammasome failed to induce wild-type levels of immunity.
The simplest explanation is that the failure of the attenuated strain to replicate in the tissues resulted in decreased antigen load. However, using a ΔactA/ΔinlB background strain, the number of CFUs in the liver and spleen were identical between ΔactA/ΔinlB L. monocytogenes-L.p.FlaA and the ΔactA/ΔinlB strain (Fig. S6). Another possibility is that ΔactA/ΔinlB L. monocytogenes-L.p.FlaA kills antigen-presenting cells. Indeed, the majority of splenic L. monocytogenes are found within dendritic cells (DCs) rapidly after infection (36, 37). However, we saw no significant difference in the total number of DCs or the number of dead DCs between ΔactA/ΔinlB L. monocytogenes-L.p.FlaA and the ΔactA/ΔinlB infections, with the exception being the slightly higher number of both total and dead DC in ΔactA/ΔinlB L. monocytogenes-L.p.FlaA–infected mice 24 h postinfection (Fig. S10). Although there are not striking differences in the number of DC or the amount of DC death, because DCs are critical for the induction of acquired immunity to L. monocytogenes (38), even if the antigen load and number of DCs are the same, specific death of infected DCs could have a detrimental impact on antigen presentation and the development of protective immunity. A third possibility is that cytokines released by pyroptotic cells, IL-1β and IL-18, alter the adaptive immune response. IL-1β and IL-18 exert highly pleiotropic effects that can promote TH1, TH2, and TH17 responses (39). Furthermore, acute induction of these cytokines likely alters levels of other cytokines, leading to changes in the inflammatory milieu that may affect development of cell-mediated immunity. Thus, inflammasome activation results in a complex set of signals and dissecting which signal(s) affect the development of cell-mediated immunity, although complicated, is of central importance for understanding the development of long-term protective immunity.
We have shown that, for L. monocytogenes, avoiding activation of the inflammasome is critical not only to its pathogenesis, but also to its ability to stimulate adaptive immunity. How inflammasome activation attenuates virulence and how pathogens have evolved to circumvent this host response remain open questions. In addition, the surprising finding that inflammasome activation is detrimental to the development of cell-mediated immunity leaves many unanswered questions, especially as it pertains to the mechanism of adjuvant activity. This work highlights the importance of studying innate immune responses in the context of infection. Understanding the role of inflammasome activation during acute infections and in the development of immunity will be critical in the rational design of potent vaccines.
Materials and Methods
Bacterial Strains and Construction.
All L. monocytogenes strains used in this study were in the 10403s background. L. monocytogenes were cultured in brain heart infusion (BHI) media, as indicated below for different infection models.
Mouse Strains and Macrophages.
Six- to 8-wk-old C57BL/6 female were purchased from The Jackson Laboratory. Caspase-1−/−, Nlrc4−/−, and Naip5/Nlrc4−/− mice were a kind gift from Russell Vance (University of California, Berkeley, CA) and ASC−/− mice were a kind gift from Vishva Dixit (Genentech Inc., South San Francisco, CA).
Six-to 8-wk-old wild-type C57BL/6J, ASC−/−, Caspase1−/−, and Nlrc4−/−mice were used to make bone marrow-derived macrophages as previously described (40). All macrophages used in this study were cultured in the presence of 100 ng/mL Pam3CSK4 (Invivogen) for 12 to 16 h before infection. All protocols were reviewed and approved by the Animal Care and Use Committee at the University of California, Berkeley.
Cell Death and IL-1β Release Assays.
Bone marrow-derived macrophages were plated in 24-well plates at 5 × 105 macrophagesper well in 500 μL DMEM (Invitrogen) overnight before infection. L. monocytogenes cultures were grown to stationary phase overnight in BHI at 30 °C, nonshaking before infection, and macrophages were infected at an MOI of five bacteria per cell. Following 30 min of infection, media was aspirated from cells and replaced with 500 μL containing 100 ng/mL fresh Pam3CSK4 and 50 μg/mL gentamicin for an additional 5.5 h. Following 6 h total incubation, supernatants were collected and assayed for lactate dehydrogenase release, as previously described (18). Supernatants were also collected and assayed for IL-1β using the ready-set-go mouse IL-1β ELISA (eBioscience).
Intracellular Growth Curves.
Pam3CSK4 treated bone marrow-derived macrophages were infected at an MOI of one bacterium per macrophage and CFU were enumerated as previously described (41).
In Vivo Infections.
Six- to 8-wk-old female mice were infected intravenously with either 1 (1 × 105) or 0.1 LD50s (1 × 104) of logarithmic phase L. monocytogenes (OD600 0.5) subcultured in BHI at 37 °C shaking for ∼2 h. Forty-eight hours postinfection, livers and spleens from infected mice were harvested and homogenized in 0.1% Nonidet P-40 and plated on BHI plates to enumerate CFU.
T-Cell Analysis.
For analysis of primary CD8+ T-cell responses, mice were infected with 0.1 LD50 (1 × 107 CFU) of either ΔactA/inlB or ΔactA/inlB L. monocytogenes-L.p.FlaA (both expressing full length OVA and B8R20–27 epitope) and spleens were harvested on day 7. Spleens were dissociated and red blood cells removed using red blood cell lysing buffer (Sigma). A total of 1.4 × 106 splenocytes were stimulated for 5 h with 2 μM OVA257–264 (SIINFEKL), B8R20–27 (TSYKFESV), or LLO190–201 (NEKYAQAYPNVS) peptide in the presence of brefeldin A (GolgiPlug reagent; BD Biosciences). Stimulated cells were surface stained with anti-CD4 (clone GK1.5; eBioscience) and anti-CD8α (clone 53–6.7; eBioscience), fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences), then stained for intracellular IFN-γ with anti-IFN-γ (clone XMG1.2; eBioscience).
Samples were acquired using a LSRII flow cytometer (BD Biosciences) with DIVA 6 software (BD Biosciences). Data were analyzed using FlowJo software (Treestar).
Protection Assays.
Six- to 8-wk-old female mice were immunized intravenously with either 0.1 (1 × 107) or 0.00001 (1 × 103) LD50s of ΔactA/inlB or ΔactA/inlB L. monocytogenes-L.p.FlaA (both expressing OVA and B8R) grown as described above. Thirty or 90 d postimmunization, mice were challenged with 2× LD50s (2 × 105) of wild type L. monocytogenes grown as described above. Sixty-eight to 72 h postchallenge, livers and spleens were harvested and analyzed for CFU, as described above.
Statistics.
Student's t-test or Mann-Whitney test statistical analysis was performed using Analyze-It software, as indicated in each figure legend. Asterisk indicates P-value less than 0.05.
Supplementary Material
Acknowledgments
We thank Drs. Lee Shaughnessy, Greg Barton, and Russell Vance for helpful discussions and critiques. This work was supported by National Institutes of Health Grant P01 A1063302 (to D.A.P.), and National Institutes of Health Grant AI27655 and American Cancer Society Postdoctoral Fellowship PF-07-066-01-LIB (to J.-D.S.).
Footnotes
Conflict of interest statement: D.A.P. has a consulting relationship with and a financial interest in Aduro Biotech. P.L. and B.H. are employees of Aduro Biotech.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019041108/-/DCSupplemental.
References
- 1.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 3.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 4.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 5.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayeri M, et al. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taxman DJ, Huang MT, Ting JP. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe. 2010;8(1):7–11. doi: 10.1016/j.chom.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar H, et al. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect Immun. 2003;71:6754–6765. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer JD, et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 21.Ozören N, et al. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 22.Meixenberger K, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 23.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sansonetti PJ, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara I, et al. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 29.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 30.Miki K, MacKaness GB. The passive transfer of acquired resistance to Listeria monocytogenes. J Exp Med. 1964;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gründling A, Burrack LS, Bouwer HG, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc Natl Acad Sci USA. 2004;101:12318–12323. doi: 10.1073/pnas.0404924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji NM, et al. Roles of caspase-1 in Listeria infection in mice. Int Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 34.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: No role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 35.Warren SE, et al. Generation of a Listeria vaccine strain by enhanced Caspase-1 activation. Eur J Immunol. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuenhahn M, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Aoshi T, et al. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 40.Jones S, Portnoy DA. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.