Abstract
The pituitary gland has long been considered to be a random patchwork of hormone-producing cells. By using pituitary-scale tridimensional imaging for two of the least abundant cell lineages, the corticotropes and gonadotropes, we have now uncovered highly organized and interdigitated cell networks that reflect homotypic and heterotypic interactions between cells. Although newly differentiated corticotrope cells appear on the ventral surface of the gland, they rapidly form homotypic strands of cells that extend from the lateral tips of the anterior pituitary along its ventral surface and into the medial gland. As the corticotrope network is established away from the microvasculature, cell morphology changes from rounded, to polygonal, and finally to cells with long cytoplasmic processes or cytonemes that connect corticotropes to the perivascular space. Gonadotropes differentiate later and are positioned in close proximity to corticotropes and capillaries. Blockade of corticotrope terminal differentiation produced by knockout of the gene encoding the transcription factor Tpit results in smaller gonadotropes within an expanded cell network, particularly in the lateral gland. Thus, pituitary-scale tridimensional imaging reveals highly structured cell networks of unique topology for each pituitary lineage. The sequential development of interdigitated cell networks during organogenesis indicate that extensive cell:cell interactions lead to a highly ordered cell positioning rather than random patchwork.
Keywords: reproduction, stress, systems biology, pro-opiomelanocortin, gonadotropins
Since its discovery and early characterization, the anterior pituitary gland has been described histologically as a patchwork of cells with little evidence of higher-order organization. This tissue is composed in the adult of five secretory cell types, each dedicated to a production of a different hormone, together with support tissue in the form of glial-like cells, the folliculostellate cells, and a capillary bed. All secretory lineages of the anterior pituitary, as well as the intermediate lobe (IL) melanotropes, have a common origin in the oral ectoderm. The IL is thus a much simpler tissue, as it contains only the pro-opiomelanocortin (POMC)-expressing melanotropes that process POMC into the melanotropic hormone α–melanocyte-stimulating hormone. With its five lineages, the developmentally related anterior lobe (AL) is more complex: it also contains a POMC-expressing lineage, the corticotropes that process the same POMC precursor into adrenocorticotropic hormone (ACTH), the gonadotrope cells that produce the gonadotropins luteinizing hormone (LH) and FSH, the thyrotropes that produce TSH, the somatotropes that produce growth hormone (GH), and the lactotropes that produce prolactin. The AL and IL are separated by a cleft that is the remnant of the original lumen of the pituitary primordium, the Rathke pouch. Interestingly, it was recently shown that pituitary stem cells and progenitors are maintained along that cleft in the adult pituitary (1, 2).
The investigation of mechanisms for control of pituitary function led to the concept of “neuroendocrine” interactions (3) and to the discovery of hypothalamic hypophysiotropic hormones that are dedicated to the control of hormone secretion by pituitary cells (4, 5). The hypothalamus thus provides major inputs for control of pituitary function, and this is balanced by feedback regulation exerted by circulating hormones that inform the pituitary of the status of peripheral endocrine glands. In addition to these critical interactions between central and peripheral inputs, a large body of evidence has supported the idea of autocrine and paracrine regulation within the pituitary: the structural basis for intrapituitary regulation has, however, remained unclear. Another conundrum that remained poorly understood until recently is the significant difference in responsiveness observed when comparing in vivo pituitary tissue with isolated pituitary cells. The recent discovery of homotypic GH cell networks extending throughout the pituitary has provided the structural basis to explain these types of interactions and differing responsiveness. Indeed, all GH cells of the pituitary maintain adherent junctions with each other to form an extensive cell network that allows extremely rapid exchange of signals between cells and the production of highly synchronized and high amplitude secretory response (6, 7). The existence of this 3D web-like GH cell network was not suspected before on the basis of traditional of 2D histological analyses, particularly as GH cells account for the bulk of anterior pituitary cells.
We currently have little idea whether and how the less abundant anterior pituitary cell lineages fit within this network and whether they are themselves part of homotypic or heterotypic cell networks. The present work investigated the putative network organization of two less abundant anterior pituitary lineages, the corticotropes and gonadotropes, and defined the relationship between different cell networks and microvasculature. We further revealed an ordered developmental process for establishment of adult cell networks and showed how blockade of corticotroph differentiation affects cells and networks of the gonadotroph lineage. The interdigitated homotypic networks of cells of these two lineages exhibit unique cell morphologies, positions relative to microvasculature and other cells, as well as overall tissue distributions.
Results
Highly Structured Corticotrope Cell Network.
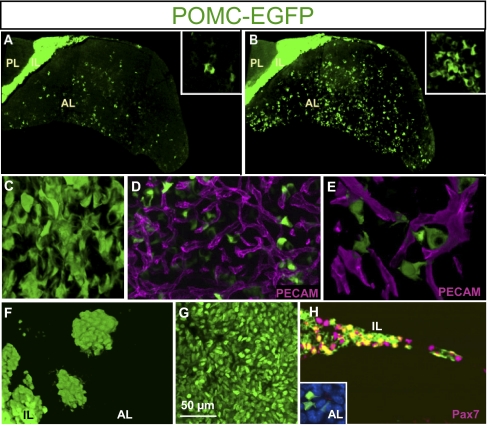
To visualize the organization of pituitary POMC cells, we used a line of POMC–EGFP transgenic mice that was previously characterized (8). These mice express GFP in pituitary POMC lineages and the developmental pattern of EGFP expression follows closely that of the endogenous POMC gene. The analysis of EGFP-positive cells was carried out by using two-photon excitation microscopy and fixed whole pituitaries. Classical coronal pituitary sections stained by immunohistochemistry for ACTH often present AL corticotrope cells as isolated cells with discontinuous fragments of positive cytoplasm; we observed a similar distribution of GFP fluorescence in 5-μm sections (Fig. 1A). Tridimensional (i.e., 3D) reconstructions of similar sections from the same POMC–EGFP adult pituitaries revealed structured networks of closely associated POMC cells (Fig. 1B). These reconstructions revealed strands of POMC cells extending from the ventral surface toward the middle of the AL, without reaching the dorsal side. The dorsal side of the gland exhibits the strongly GFP-positive IL cells together with dispersed islets of similarly strong EGFP-positive cells (Fig. 1B). Throughout the AL, POMC cells appeared highly interconnected, suggestive of homotypic interactions (Fig. 1C and Movie S1). These tight contacts between POMC cells are clearly visible within the strands of POMC–EGFP cells and also in lateral areas where cells are more dispersed. This assessment is a likely underestimate because the EGFP transgene is not fully penetrant (8).
Fig. 1.
Tridimensional organization of POMC cells in adult mouse pituitary. (A) Distribution of POMC–EGFP fluorescence on 5-μm coronal section through an adult (P70) mouse pituitary revealing homogenously stained IL, negative posterior lobe (PL), and discontinuous fragments of fluorescent cytoplasm in AL. The x, y, and z axes are 960, 1,705, and 5 μm, respectively; inset is 100 × 100 × 5 μm. (B) Similar view of POMC–EGFP pituitary following 3D reconstruction (depth, 70 μm) of images obtained by two-photon confocal microscopy. Insets in A and B represent high-magnification views of the same pituitary field in 2D and 3D, respectively. The x, y, and z axes are 960, 1,705, and 70 μm, respectively; inset is 100 × 100 × 70 μm. (C) Higher-magnification 3D reconstruction of POMC–EGFP cell network viewed from ventral AL (Movie S1). The x, y, and z axes are 126, 126, and 57 μm, respectively. (D) Three-dimensional reconstruction of fluorescent coimaging of POMC–EGFP cells (green) with PECAM (CD31) labeling of endothelial cells revealing capillary bed of adult AL. The x, y, and z axes are 156, 100, and 20 μm, respectively. (E) High-magnification 3D reconstruction of capillaries with PECAM (magenta) together with POMC–EGFP fluorescence. The x, y, and z axes are 62, 46, and 16 μm, respectively. (F) Dorsal view of adult mouse pituitary revealing islets of very intense EGFP-positive cells. The x, y, and z axes are 228, 181, and 94 μm, respectively. (G) High-resolution view of tightly packed IL melanotrope cells positive for POMC–EGFP. (H) Colabeling of adult POMC–EGFP pituitary for Pax7 (magenta) and POMC–EGFP. Coexpression (yellow) is observed in IL but not in AL (Inset). The x, y, and z axes are 204, 134, and 4 μm; inset is 34 × 31 × 4 μm.
To situate the POMC cell network relative to the microcirculation, we revealed endothelial cells by immunohistofluorescence labeling of PECAM (CD31). These data showed that POMC–EGFP cell bodies are, for the most part, not juxtaposed to capillaries (Fig. 1D). However, higher-resolution imaging of these preparations revealed numerous cytoplasmic projections or cytonemes that provide cell–cell contacts between different POMC cells and between POMC cells and blood vessels (Fig. 1E). Thus, AL POMC cells form homotypic networks and have direct contacts with the capillary bed.
By contrast, the dorsal AL contains islets of EGFP-positive polygonal cells with little or no cytonemes; these cells do not appear to be in contact with other AL POMC cells (Fig. 1F). The strength of the EGFP signal in these islets and their cell morphology are similar to that of IL melanotropes (Fig. 1G), suggesting that these cells may be of IL origin. To establish the identity of these dorsal POMC–EGFP islets, we used a melanotrope-specific marker, Pax7. Immunohistochemical analyses of Pax7 costaining with POMC–EGFP clearly showed that Pax7 expression is restricted to IL cells and to these dorsal cell islets and that it is absent from AL corticotropes (Fig. 1H).
Developmental Dynamics of POMC Cell Networks.
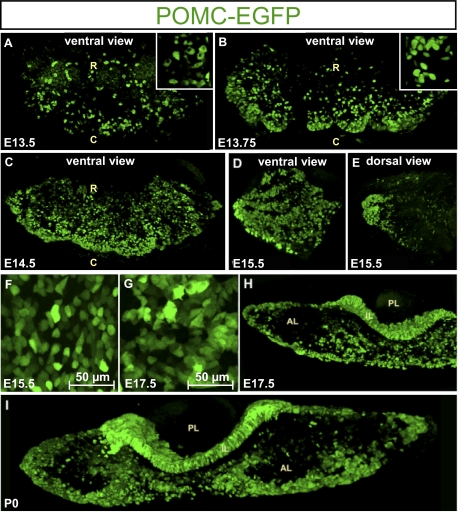
During development, the first POMC–EGFP cells appear as isolated cells on the ventral surface of the gland, suggesting that their differentiation and expansion occur stochastically (Fig. 2A). Indeed, the POMC–EGFP cells that appear at embryonic day (E) 13.5 are small, rounded, and for the most part isolated from each other (Fig. 2A); this is consistent with de novo differentiation rather than clonal expansion of a few differentiated cells (9). Quite rapidly after their initial detection, POMC–EGFP cells appear to cluster together, and they are particularly abundant on the caudal side of the ventral gland surface (Fig. 2B). The rounded cell appearance with clearly visible nuclei initially observed at E13.5 (Fig. 2A, Inset) is rapidly replaced by polygonal cells that exhibit less visible nuclei (E13.75; Fig. 2B, Inset). By E14.5, the number of POMC–EGFP cells increases significantly on the ventral surface, with the highest density on the caudal side (Fig. 2C). Viewed from the ventral surface, the lateral tips of the gland show POMC–EGFP cells organized in strands extending from the ends. This organization is best seen at E15.5 (Fig. 2D). On the dorsal side, these strands come together at the lateral extremities but do not extend dorsally (Fig. 2E). In fact, very few isolated EGFP-positive cells are present on the dorsal surface at this stage. Between E15.5 and E17.5, the most striking difference is a change in cell shape with the appearance of cytoplasmic projections (Fig. 2 F and G). Coronal sections reveal that the ventral strands of POMC–GFP cells penetrate into the AL at the mediolateral level to reach the middle of the developing gland (Fig. 2H). This organization is conserved at P0 (Fig. 2I) and postnatally into adulthood (Fig. 1B).
Fig. 2.
Developmental dynamics of POMC cell morphology and network organization. (A) Ventral view of E13.5 POMC–EGFP mouse pituitary in which the first fluorescent signal appears as small and isolated cells (Inset). R, rostral; C, caudal. The x, y, and z axes are 540, 258, and 199 μm, respectively; inset is 62 × 62 × 199 μm. (B) By E13.75, POMC–EGFP–positive cells appear to regroup on the caudal side of the ventral surface (Inset). The x, y, and z axes are 916, 438, and 151 μm; inset is 62 × 62 × 151 μm. (C) At E14.5, densely packed POMC–EGFP cells are seen on the caudal–ventral side of the AL. The x, y, and z axes are 1,126, 584, and 205 μm, respectively. (D) At E15.5, strands of POMC–EGFP cells extend from the lateral tip of the AL along its ventral surface. The x, y, and z axes are 368, 368, and 107 μm. (E) These strands of POMC–EGFP cells come together at the lateral tip of the AL but do not extend on the dorsal side of the gland. The x, y, and z axes are 368, 368, and 85 μm, respectively. (F) POMC–EGFP cells of E15.5 pituitary have more polygonal appearance than at E13.5. (G) By E17.5, the POMC–EGFP cells extend cytoplasmic projections that maintain contacts between different POMC–EGFP cells. (H) Coronal section through E17.5 POMC–EGFP pituitary shows strands of fluorescent cells extending from ventral surface of the AL into the mediolateral sides of the gland. The x, y, and z axes are 980, 518, and 106 μm, respectively. (I) A similar organization is present in the P0 postnatal pituitary. The x, y, and z axes are 1,788, 453, and 103 μm, respectively.
Interrelated LH Cell Network.
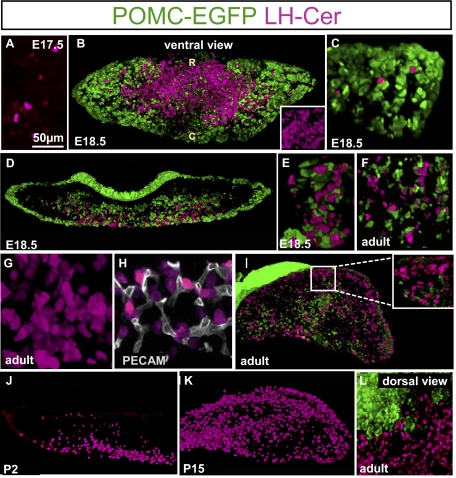
As the POMC lineages were proposed to share a common precursor with gonadotropes (10), it was particularly interesting to assess the organization of gonadotropes and to compare it with that of corticotropes. For this purpose, we generated LH-cerulean transgenic mice that use a bovine β-LH promoter to target expression of a cerulean fluorescent transgene (11) in this lineage (12). Similar to the first POMC cells (Fig. 2A), the earliest (E17.5) LH-cerulean cells appear in the midventral surface of the gland as isolated cells (Fig. 3A). By E18.5, LH-cerulean cells are clustered in the ventral mediolateral AL (Fig. 3B, Inset). These cells are closely packed and always associated with strands of POMC–GFP cells that penetrate the AL (Fig. 3 B, D, and E). In addition, isolated LH-cerulean cells present more laterally also have intimate contact with POMC cells (Fig. 3C). This network is maintained in the adult AL (Fig. 3 F–L). As for POMC cells, the LH cells form homotypic networks within the AL (Fig. 3G and Movie S2). Colabeling with PECAM to reveal capillaries showed that LH cells are in close proximity to the pituitary microvasculature (Fig. 3H), much closer in fact than POMC cells (Fig. 1F).
Fig. 3.
Development of LH cell network during pituitary organogenesis. (A) The first LH-cerulean (LH-Cer, magenta) cells appear at E17.5 as small cells on the ventral surface of the AL. (B) Ventral view of double E18.5 transgenic pituitary for POMC–EGFP (green) and LH-cerulean show initial appearance of LH-cerulean cells in the rostromedial AL, in contrast to POMC–EGFP cells that are more preeminent in the lateral wings. (R, rostral; C, caudal.) The x, y, and z axes are 1,374, 612, and 160 μm, respectively; inset is 124 × 124 × 160 μm. (C) View of lateral wing of E18.5 double transgenic pituitary revealing few scattered LH-cerulean cells in intimate contact with strands of POMC–EGFP cells. The x, y, and z axes are 318, 318, and 160 μm, respectively. (D) Coronal section through E18.5 double transgenic pituitary. Whereas POMC–EGFP cells are mostly present along the edges of the developing AL, the LH-cerulean cells extend from the medioventral surface into the ventral half of the developing AL. The x, y, and z axes are 1,750, 613, and 102 μm, respectively. (E) High-magnification view of E18.5 double transgenic pituitary shows intimate contacts between LH-cerulean cells and strands of POMC–EGFP cells penetrating the AL. The x, y, and z axes are 125, 77, and 102 μm, respectively. (F) Close contacts between LH-cerulean cells and strands of POMC–EGFP cells penetrating the AL are preserved in adulthood (P70 double transgenic male). The x, y, and z axes are 225, 225, and 41 μm, respectively. (G) Higher-magnification 3D reconstruction of LH-cerulean cell network viewed from ventral AL (Movie S2). The x, y, and z axes are 125, 125, and 82 μm, respectively. (H) Colabeling for endothelial PECAM (CD31) and LH-cerulean reveals intimate contacts between gonadotropes and pituitary capillaries (P70). The x, y, and z axes are 111, 106, and 30 μm, respectively. (I) Adult (P70) double transgenic male pituitary revealing the presence of a dorsal population of LH-cerulean cells that form a continuous band of gonadotropes along the dorsal side of the AL. Inset: These dorsal LH-cerulean cells have few contacts with POMC–EGFP cells. The x, y, and z axes are 1,902, 1,000, and 56 μm, respectively; inset is 225 × 225 × 46 μm. (J) At P2, the LH cell network remains concentrated along the medioventral AL and the first LH cells are detected in the dorsal AL. The x, y, and z axes are 1,070, 580, and 127 μm, respectively. (K) Three-dimensional reconstruction of LH-cerulean cells in AL of P15 pituitary reveals intimate contacts between dorsal gonadotropes. The x, y, and z axes are 833, 489, and 135 μm, respectively. (L) Dorsal view of adult (P70) double transgenic pituitary shows high density of LH-cerulean cells that are contiguous with the homogenous POMC–EGFP–positive IL. The x, y, and z axes are 332, 360, and 100 μm, respectively.
The adult pituitary also revealed a dense population of LH-cerulean cells on the dorsal AL surface (Fig. 3I); this streak of LH cells extends under the IL, where very few POMC cells are present (Fig. 3I Inset). These dorsal LH cells appear during the first postnatal week [i.e., postnatal day (P) 2; Fig. 3J] and are fully present in the P15 pituitary (Fig. 3K), but not at E18.5 (Fig. 3D). It is noteworthy that these dorsal LH cells have almost no contact with POMC cells, as clearly visible on dorsal views of the gland (Fig. 3L).
In summary, whereas the POMC cell network appeared to organize itself into strands emerging from the lateral extremities and the caudoventral surface of the gland, the LH cells mostly appear/differentiate in the medioventral surface of the developing gland and develop between the POMC cell and capillary networks. In addition, another population of dorsal LH cells with few contacts with the POMC cell network appears postnatally.
Expanded LH Cell Network in Absence of Corticotropes.
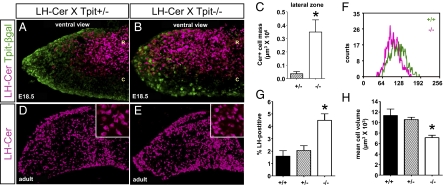
The association of LH cells with the POMC cell network, taken together with their differentiation from a putative common precursor, suggests that development of the LH network may be dependent on the POMC network. We assessed this hypothesis by investigating the LH cells in Tpit−/− pituitaries that are deficient in POMC cell differentiation (10). Ventral views of Tpit−/−;LH-cerulean E18.5 pituitaries showed a marked increase in the number of LH cells, most significantly in lateral wings (Fig. 4B vs. Fig. 4A). These glands were also stained by IHC for the Tpit-lacZ chimera that is expressed in knockout cells that fail to differentiate into either POMC cells (1–2% of cells) or switch into gonadotropes (13). Accordingly, colabeled cells were not observed and the decrease of Tpit-lacZ–positive cells in lateral wings of Tpit−/− pituitaries appeared compensated by LH-cerulean cells (Fig. 4B vs. Fig. 4A). Morphometric quantification of LH cell mass strengthens the proposal that more LH cells are present in the lateral zones of Tpit−/− than Tpit+/− pituitaries (Fig. 4C). In the adult, the increase in LH cells is mostly visible in the lateral wings (Fig. 4E vs. Fig. 4D). To quantitatively assess cell numbers, we performed FACS analysis of freshly dispersed pituitaries of both genotypes (Fig. 4F) and observed significant increases in LH cell numbers (Fig. 4G). In addition, the LH cells from Tpit−/− pituitaries were smaller by approximatey 30% compared with control siblings (Fig. 4 D and E, Insets, and Fig. 4 F and H).
Fig. 4.
Extended LH cell network, but decreased cell volume, in corticotrope-deficient Tpit−/− pituitary. (A and B) LH-cerulean–positive cells (magenta) present in rostromedial AL of control Tpit+/−;LH-cerulean pituitary extend laterally in Tpit−/−;LH-cerulean mice. Immunostaining for the Tpit–β-gal chimeric protein derived from the mutant Tpit allele (green) reveals cells destined for corticotrope lineage. (R, rostral; C, caudal.) The x, y, and z axes in A are 729, 516, and 67 μm, respectively; those in B are 719, 516, and 103 μm, respectively. (C) Morphometric quantitation of LH-cerulean–positive cells in the lateral quarters of the developing AL (mean ± SEM; n = 5). Statistical analysis (Mann–Whitney) indicates statistical difference (P = 0.009). (D and E) Adult LH-cerulean transgenic pituitary reveals increased number of gonadotropes in AL of Tpit−/− compared with Tpit+/− mice. Insets: LH-cerulean–positive cells of Tpit−/− pituitary may be smaller. The x, y, and z axes in D and E are 966, 680, and 105 μm, respectively; inset is 100 × 100 × 69 μm. (F) FACS analysis of LH-cerulean–positive cells from WT (+/+) and Tpit−/− pituitaries revealing smaller cell size in Tpit−/− pituitary (magenta). (G) Quantification by FACS of LH-cerulean–positive cells as percentage of sorted AL cells showing increased number of gonadotrope cells in Tpit−/− pituitaries (P = 0.02, Kruskal–Wallis one-way ANOVA; Tpit+/+, n = 3; Tpit+/−, n = 5; Tpit−/−, n = 5; mean ± SEM). (H) Cell volume assessed by FACS for adult LH-cerulean cells of the same Tpit genotypes as before show a statistically significant decrease of approximately 40% cell volume in Tpit−/− pituitaries (P = 0.03, Kruskal–Wallis one-way ANOVA).
Discussion
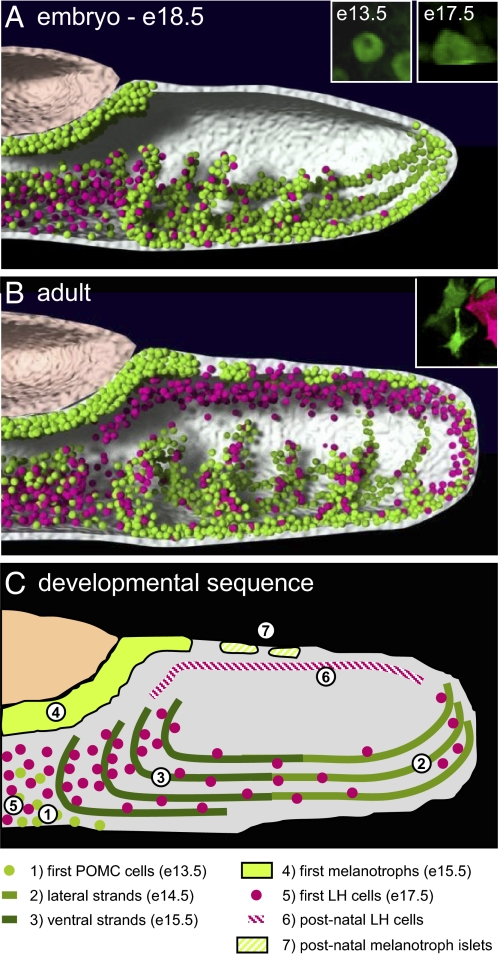
By using pituitary-scale 3D imaging during development (Fig. 5A) and in adults (Fig. 5B), the present work revealed the unique 3D topological organization of cell networks for two low-abundance pituitary lineages (each representing ≤10% of adult AL cells), the corticotropes and gonadotropes, that derive from a putative common precursor. These networks occupy different, yet interdigitated, positions within the adult gland. In particular, the position of the two networks relative to the microvasculature is different: whereas gonadotropes are usually in close contact with capillaries, the corticotropes compensate their distance from blood vessels by a different cytoarchitecture that includes cytoplasmic projections or cytonemes. Cell shape and network topology are thus adapted to provide equal access of cells to vasculature within the context of the other and more abundant lineages that occupy the bulk of the gland. Whereas cell networks may serve an important role in endocrine function by the rapid exchange of signals, as shown for the GH cell network (6, 7, 14), their presence may also reflect the developmental program of organogenesis (Fig. 5C). These two functions are not mutually exclusive and most likely reflect concerted developmental purposes.
Fig. 5.
Schematic representation of POMC (green) and LH (magenta) cell networks in developing E18.5 (A) and adult pituitary (B). Insets: Photographs of POMC (green) and LH (red) cells at indicated times of development to illustrate changes in cell shape. (C) Developmental sequence of appearance/changes in POMC and LH cell networks. 1, The first POMC cells appear on ventral AL at approximately E13.5. 2, By E14.5, strands of POMC cells extend from lateral tips of the AL along the ventral surface. 3, The ventral strands of POMC cells penetrate the AL. 4, The first IL melanotropes appear at E15.5. 5, The first LH cells appear on ventral AL surface at E17.5 and populate medial AL. 6, Postnatal appearance of LH cell network on dorsal side of AL. These postnatal LH cells have few contacts with POMC cells. 7, Postnatal appearance of POMC- and Pax7-positive melanotrope islets on dorsal surface of AL.
Homotypic Cell Networks and Pituitary Cell Function.
Contrary to previous belief and patchwork appearance on tissue sections, anterior pituitary cells are organized in homotypic networks that are readily visible on 3D imaging. Cell interactions within networks are likely critical for concerted rapid responses (6, 14) and may contribute to adaptation to new endocrine conditions in adult (7).
Homotypic networks and cell shape are also adapted to provide equivalent access to the vasculature for quick cell responses to circulating signals. Indeed, some cells have a cellular architecture that allows them to be, broadly speaking, further away from capillaries but nonetheless in contact through cytonemes that directly reach the perivascular space. Interestingly, it is the corticotropes, which are the first cells to differentiate during organogenesis, that adopt this cellular architecture and thus adapt their cell shape and homotypic network organization to the appearance of other lineages.
Close associations between cells and microvasculature ensure (i) integration of hypothalamic inputs, which are gradually distributed within the parenchyma; (ii) appropriate supplies of oxygen (and nutrients), which are finely adjusted depending on cell consumption; and (iii) delivery of hormonal products toward the perivascular space, from which they will pass the endothelial cell barrier into the bloodstream (14). Thus, although corticotropes possess somas that are distant from the capillaries, the generation of ACTH secretion burst is not slower compared with other hormones, as primed ACTH vesicles are oriented along the processes that contact vessels (15, 16) and small secreted products such as ACTH circulate rapidly (∼10 μm/sec) within the extracellular space (14).
Close associations between homotypic cell networks may be the basis for paracrine interactions. In the present work, the deficit of corticotropes in Tpit−/− AL results in a significant decrease of gonadotrope cell volume in parallel with increased gonadotrope number. The later is likely a result of the alternate cell fate adopted by their common precursors (10). However, the 30% to 40% decrease in gonadotrope cell volume of Tpit−/− pituitaries (Fig. 4H) is most likely caused by the absence of signals provided by corticotropes, as Tpit is not expressed in gonadotropes (10, 17). These signals could be secreted but are more likely to be membrane-associated given the close contacts between cells of these two lineages. Paracrine interactions have been suggested previously to modulate hormone responsiveness but never to involve control of cell volume (18). The network organization of pituitary lineages and their heterotypic interactions may thus serve an important maintenance function in the adult gland by coordinating the balance between lineages, and thus contribute to adjust cell number, volume, and function. Conversely, disruption of these networks may be implicated in subtle forms of hormone imbalance/disorders (19).
Ontogeny of Corticotrope and Gonadotrope Networks.
Our results provide support for a pituitary-scale model of temporally precise control of positional determination of gonadotropes and corticotropes, two non–Pit-1–dependent cell lineages (Fig. 5C). It is noteworthy that the first terminally differentiated cells of both lineages appear as single cells on the ventral surface of the developing gland (Fig. 5C, 1 and 5, respectively). For corticotropes, available markers, such as Tpit and POMC, appear within 12 h of each other, such that it is not possible to know whether the cells were determined earlier. For gonadotropes, however, hormone or LH-cerulean are detected from E16, but the earlier expression of SF1 at E13.5 suggests that cells destined to become gonadotropes may have been determined earlier (20), possibly concomitant with corticotropes, in agreement with their origin from a common progenitor (10). It should be noted, however, that gonadotropes may be heterogeneous and that the present work documented the appearance and organization of LH-expressing cells. As FSH-expressing gonadotropes do not completely overlap with LH expression, in particular during development (21), their organization may differ.
The early-born corticotropes represent likely candidates for establishment of a 3D scaffold that serves to organize networks of other lineages, as development of the GH cell network does not commence until the E15.5/E16.5 transition (6) and the first wave of gonadotropes appears in close proximity to corticotropes at approximately E17.5. Indeed, soon after their appearance, corticotropes regroup on the caudal–ventral side of the gland and at the tips of the lateral wings of the forming gland (Fig. 5C, 2) to form strands of cells that then penetrate within the body of the AL (Fig. 5C, 3). It is unclear whether clustering of corticotropes arises from de novo differentiation or whether differentiated corticotropes migrate to associate in this fashion. The E15.5 to E18.5 period of pituitary organogenesis is also characterized by a change in corticotrope shape and the formation of extended cell processes (Fig. 5 A and B, Insets). This occurs at the same time as the lateral expansion of cells from Pit-1–dependent lineages within the medial AL (22). In particular, appearance of GH cells within the body of the AL may contribute to the “stretching” of POMC cells that are already organized in strands. POMC cell cytonemes may thus form rather passively as a result of this action and of strong homotypic interactions; alternatively, an active process of cytoplasmic outgrowth may lead to intercalation of POMC cells within the extensive GH cell network (6).
It is along the preformed corticotrope network that gonadotropes differentiate and cluster. In the midventral AL, gonadotropes rapidly establish homotypic contacts but also heterotypic contact with corticotropes (Fig. 5 B and C). In contrast, at the AL lateral ends, isolated LH-cerulean–positive gonadotropes are more often observed in isolation from each other but in close contact with the corticotrope network. Again, the apparent replacement of corticotropes by gonadotropes in Tpit−/− pituitaries, particularly at the lateral ends of the AL, supports the model that they share a common precursor (10). The close association of LH cells with the corticotrope cell network as they differentiate during organogenesis further suggests that cells of these lineages share an ability for cell–cell interactions that precedes terminal differentiation. This idea is supported by the recent characterization of primitive pituitary adenomas that share markers of both lineages (23). Nonetheless, LH-cerulean cells do not adopt an organization similar to that of corticotropes in the Tpit−/− AL, suggesting that the strand organization of corticotropes is an intrinsic property. Further, POMC cells exhibit the most important change in cell shape during organogenesis, starting from small round cells to become polygonal with long cytonemes (Fig. 5 A and B, Insets). Thus, corticotropes may have a cell-autonomous program to form a network that scaffolds other cell networks.
After birth, further positional determination takes place with the appearance of gonadotropes along the Rathke cleft and the dorsal side of the AL (Fig. 5C, 6). This second wave of gonadotrope differentiation yields sheets of homotypically connected cells that rarely contact AL corticotropes or POMC/Pax7–positive cells (of presumed IL origin), which form sparsely distributed clusters of polygonal cells on the dorsal AL surface (Fig. 5C, 7). This arrangement of dorsal gonadotropes persists into adulthood and may be related to recently proposed subgroups of functionally distinct gonadotropes (21).
Perspective: Revisiting Pituitary Biology?
The current view of pituitary organogenesis was largely developed from spatiotemporal expression patterns of transcription factors and signaling molecules using mostly midsagittal pituitary sections (24). The bulk of previous studies did not analyze postnatal pituitary cell position and did not account for the fact that the medial portion of the pituitary gland only represents approximately 20% of adult pituitary cells (6). The results of the present study thus demand a revised assessment of pituitary organogenesis because (i) corticotropes and gonadotropes form large-scale cell networks that may function in a similar manner to the somatotrope network to integrate and propagate cell responses; (ii) both Pit1-dependent (6) and Pit1-independent (i.e., in the present study) lineages undergo developmental networking of terminally differentiated endocrine cells, suggesting a generalized paradigm of cell networking; (iii) a positional determination of 3D cell networks is present and may involve morphogen gradients and/or transcriptional factors from the lateral wings toward the medial parts of the gland; and (iv) cell networks interact during development, as evidenced by the dependence of gonadotropes on the presence of a fully differentiated corticotrope scaffold. The interdigitated cell network revealed in the present study in mice is consistent with the more clustered pituitary cell organization observed in other species such as fish (25) in which cell–cell coupling was shown (26). Homotypic cell networks may thus represent a different evolutionary solution to a dependence on cell coupling for efficient hormone response. Further, interdigitated cell networks may favor heterotypic interactions and effective coordination of different endocrine axes.
In summary, this pituitary-scale 3D imaging study of the positional determination of corticotropes and gonadotropes across a lifespan has highlighted the importance of pituitary cell networking in mouse pituitary development. The developmental processes that guide formation of endocrine cell networks clearly have implications for the proper functioning of the adult pituitary gland.
Materials and Methods
Tpit mutant (10) and POMC–EGFP transgenic mice (8) were described previously. Production of LH-cerulean transgenic mice is described in SI Materials and Methods, and their characterization is described in Fig. S1. For imaging, tissues were fixed in situ by using solution PBS with 4% paraformaldehyde (PFA) and stored in PBS solution until image acquisition. Immunostaining was performed as previously described (9). Animal experimentation was approved by the animal ethics review committee of Institut de Recherches Cliniques de Montréal.
Multiphoton and confocal images of pituitaries were acquired as previously reported (6) and are detailed in SI Materials and Methods. Pituitary LH-cerulean–positive cells were analyzed by FACS using a BD Biosciences cell sorter and Summit software (version 4.3; Beckman-Coulter).
Supplementary Material
Acknowledgments
We are indebted to many colleagues for discussions and suggestions on this work. We are grateful to Noriko Uetani for her artistic contribution to illustrations, to Éric Massicotte for expert assistance and FACS analyses, and to Lise Laroche for secretarial support. This work was supported by Canadian Institutes of Health Research grants (to J.D.); Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université Montpellier 1 and 2, Agence Nationale de la Recherche (Pit-Net Project), Institut Fédératif de Recherches 3, and Région Languedoc Roussillon Grants (to P.M.); the Irish National Biophotonics and Imaging Platform; the Irish Government's Programme for Research in Third-Level Institutions, Cycle 4; and Ireland's European Union Structural Funds Programmes 2007–2013.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105929108/-/DCSupplemental.
References
- 1.Melmed S, Drouin J. Pituitary development. In: Melmed S, editor. The Pituitary. Oxford: Academic Press; 2010. pp. 3–19. [Google Scholar]
- 2.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris GW. The function of the pituitary stalk. Bull Johns Hopkins Hosp. 1955;97:358–375. [PubMed] [Google Scholar]
- 4.Schally AV. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978;202:18–28. doi: 10.1126/science.99816. [DOI] [PubMed] [Google Scholar]
- 5.Guillemin R. Peptides in the brain: The new endocrinology of the neuron. Science. 1978;202:390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefont X, et al. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci USA. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Cardenas C, et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA. 2010;107:21878–21883. doi: 10.1073/pnas.1010849107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie PL, Budry L, Balsalobre A, Drouin J. Developmental dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Mol Endocrinol. 2008;22:1647–1657. doi: 10.1210/me.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29:1895–1908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulichino AM, et al. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17:738–747. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 12.Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol. 2001;15:734–746. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- 13.Bilodeau S, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20:2871–2886. doi: 10.1101/gad.1444606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafont C, et al. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA. 2010;107:4465–4470. doi: 10.1073/pnas.0902599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriarty GC. Adenohypophysis: Ultrastructural cytochemistry. A review. J Histochem Cytochem. 1973;21:855–894. doi: 10.1177/21.10.855. [DOI] [PubMed] [Google Scholar]
- 16.Moriarty GC, Halmi NS, Moriarty M. The effect of stress on the cytology and immunocytochemistry of pars intermedia cells in the rat pituitary. Endocrinology. 1975;96:1426–1436. doi: 10.1210/endo-96-6-1426. [DOI] [PubMed] [Google Scholar]
- 17.Lamolet B, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 18.Denef C. Paracrinicity: The story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waite E, et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology. 2010;151:234–243. doi: 10.1210/en.2009-0539. [DOI] [PubMed] [Google Scholar]
- 20.Ingraham HA, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 21.Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107:16372–16377. doi: 10.1073/pnas.1000423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, et al. ACTH and alpha-subunit are co-expressed in rare human pituitary corticotroph cell adenomas proposed to originate from ACTH-committed early pituitary progenitor cells. Endocr Pathol. 2008;19:17–26. doi: 10.1007/s12022-008-9014-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
- 25.Liu NA, et al. In vivo time-lapse imaging delineates the zebrafish pituitary proopiomelanocortin lineage boundary regulated by FGF3 signal. Dev Biol. 2008;319:192–200. doi: 10.1016/j.ydbio.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levavi-Sivan B, Bloch CL, Gutnick MJ, Fleidervish IA. Electrotonic coupling in the anterior pituitary of a teleost fish. Endocrinology. 2005;146:1048–1052. doi: 10.1210/en.2004-1415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.