Abstract
IgG-mediated anaphylaxis occurs in mice and may contribute to human reactions to infused drugs. To distinguish IgE- from putative IgG-mediated human anaphylaxis, we developed blood markers for murine anaphylaxis and evaluated their human relevance. Both IgG- and IgE-mediated anaphylaxis were characterized by decreased basophil and monocyte percentages and an increased neutrophil percentage in mouse blood. IgE- but not IgG-mediated murine anaphylaxis was accompanied by large increases in IL-4 secretion, plasma soluble IL-4 receptor-α (IL-4Rα) concentration, and T-cell membrane IL-4Rα expression. T-cell IL-4Rα expression also increased when mice that express human Fcε receptor Iα were sensitized with IgG-depleted serum from a peanut-allergic individual and challenged with peanut extract. Increased T-cell IL-4Rα expression is likely to also be a marker for human IgE-mediated anaphylaxis, because IgE-activated human basophils secrete IL-4, and IL-4 increases human T-cell IL-4Rα expression in vitro. Murine IgG- but not IgE-mediated anaphylaxis was characterized by decreased neutrophil Fcγ receptor III (FcγRIII) expression that was observed even when the antigen dose was insufficient to induce shock. Human neutrophils cultured with IgG immune complexes also lost FcγRIII. These observations suggest that decreased blood neutrophil FcγRIII expression without increased IL-4Rα expression can be used to determine whether and when IgG-mediated anaphylaxis occurs in man.
Keywords: rodent, histamine, platelet activating factor, mast cell
Anaphylaxis (immune-mediated shock) results from rapid release of large quantities of vasoactive mediators that increase vascular permeability, cause smooth muscle contraction, and decrease cardiac output (1, 2). In mice, two pathways that lead to anaphylaxis have been defined: the classic pathway, which is mediated by IgE, Fcε receptor I (FcεRI), mast cells, and histamine > platelet activating factor (PAF); and the alternative pathway, which is mediated by IgG, Fcγ receptor III (FcγRIII), and macrophage and basophil secretion of PAF (3–5). Although the kinetics and clinical features of these two types of anaphylaxis are generally similar, considerably more Ab and antigen are required to induce IgG- than IgE-mediated anaphylaxis (3). This most likely reflects the much higher affinity of IgE binding by FcεRI than IgG binding by FcγRIII (6).
In humans, the IgE pathway of anaphylaxis has been well characterized, whereas the existence of an IgG pathway is controversial (1, 7–9). Although increased serum levels of tryptase, which is released by degranulating mast cells, suggests IgE-mediated anaphylaxis (10–12), many cases of human anaphylaxis are not accompanied by elevated serum tryptase or detectable antigen-specific IgE (2, 7). Such cases could reflect an IgG-dependent (or IgM- or complement-dependent) anaphylaxis mechanism but might also be explained by the short half-life of tryptase in blood, the secretion of relatively small amounts of tryptase in mild anaphylaxis, and the association of antigen-specific IgE with high-affinity mast cell and basophil receptors (Rs) in the absence of detectable levels in blood (13, 14). Additionally, it remains possible that human anaphylaxis accompanied by elevated serum tryptase can result from IgG-mediated mast cell activation.
By analogy with murine IgG-mediated anaphylaxis, human IgG-mediated anaphylaxis would be expected to occur when individuals with relatively high concentrations of antigen-specific IgG are inoculated with relatively large quantities of the antigens bound by those Abs (14). Cases of anaphylaxis that develop after repeated infusion of large amounts of dextran (15), aprotinin (16), von Willebrand's factor (to individuals deficient in this clotting factor) (17), or therapeutic IgG mAbs (18, 19), in which anaphylaxis developed in the presence of detectable IgG but not IgE Abs to the infused compound and in the absence of a detectable increase in serum tryptase (7), may be the most likely candidates for human IgG-mediated anaphylaxis. The rapid increase in the use of therapeutic IgG mAbs (20, 21), the frequent development of IgG Abs to these mAbs in treated patients (8), and the fairly high frequency of significant infusion reactions in patients treated with these mAbs (19, 22) make it important to determine whether at least some of these reactions represent IgG-mediated anaphylaxis.
However, although the existence of IgG-mediated anaphylaxis in the mouse has been demonstrable in studies that induce disease passively through sensitization with IgG Abs and that use mice deficient in FcεRI, FcγRIII, IgE, or mast cells (3, 4, 23), these methods cannot be ethically used to determine whether IgG-mediated anaphylaxis exists in man. Instead, it would be desirable to develop blood markers that could distinguish IgE- from IgG-mediated anaphylaxis. With this in mind, we have evaluated whether changes in serum and cellular markers in blood could distinguish murine IgE- from IgG-mediated anaphylaxis and, where positive, have evaluated whether the same markers might be useful in humans. Our observations demonstrate changes in blood parameters that are specific for IgE- or IgG-mediated anaphylaxis. Use of these markers should facilitate determination of whether IgG immune complexes (IC) can cause human anaphylaxis.
Results
IgE- and IgG-Mediated Anaphylaxis Induce Similar Changes in Populations of Nucleated Blood Cells.
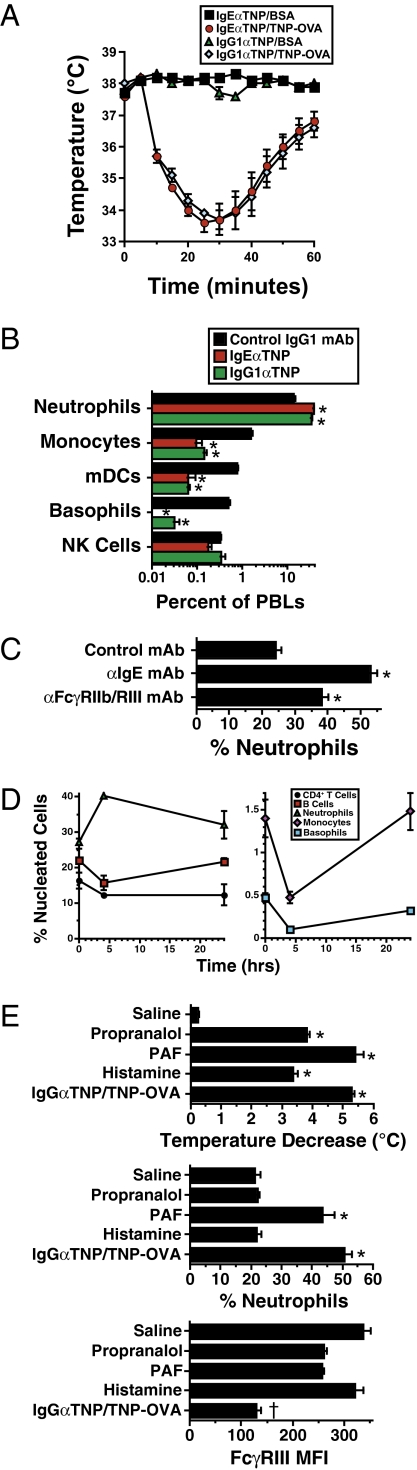
Because murine basophils and mast cells, but neither macrophages nor neutrophils, express IgERs, whereas all of these cells express IgGRs (6, 24), we hypothesized that IgG-mediated anaphylaxis would induce changes in blood neutrophil and macrophage populations that were not induced by IgE-mediated anaphylaxis. Contrary to our expectations, induction of IgE- and IgG-mediated passive anaphylaxis of similar severity (Fig. 1A) caused considerable decreases in the percentages of basophils, myeloid dendritic cells, and monocytes and increases in the percentage of neutrophils in blood (Fig. 1B and Fig. S1A). Similar results were observed when anaphylaxis was induced by injecting mice with anti-IgE or anti-FcγRIIb/RIII mAb (Fig. 1C). Kinetic studies of anaphylaxis induced by anti-IgE mAb (Fig. 1D, Left) or anti-FcγRII/RIII mAb (Fig. 1D, Right) demonstrated that these changes were marked 4 h after disease induction but much less apparent by 24 h. No increase in the percentage of blood neutrophils was observed when shock was induced by injection of histamine or propranalol, but shock induced by PAF injection was accompanied by an increased percentage of neutrophils in blood (Fig. 1E). Thus, these changes in populations of blood leukocytes may be general markers for severe shock and cannot be used to distinguish IgE- from IgG-mediated anaphylaxis.
Fig. 1.
Percentage of neutrophils in peripheral blood increases in both IgE- and IgG-mediated anaphylaxis. (A) BALB/c mice (four per group in all experiments unless otherwise stated) were passively immunized i.v. with 10 μg of IgEαTNP mAb, 100 μg of IgG1αTNP mAb, or 100 μg of a control IgG1 mAb and challenged the next day with 40 μg of TNP-OVA or BSA. Rectal temperatures were determined every 5 min for the next 1 h. Means and SEs are shown in all figures. (B) BALB/c mice were passively immunized i.v. with 10 μg of IgEαTNP mAb or 100 μg of IgG1αTNP mAb and challenged the next day with 40 μg of TNP-OVA. Percentages of neutrophils, monocytes, myeloid dendritic cells (mDCs), basophils, and natural killer (NK) cells in peripheral blood drawn 4 h later were determined by flow cytometry in this and subsequent figures. (C) BALB/c mice were challenged i.v. with 100 μg of αIgE or 500 μg of αFcγRIIb/RIII mAb and bled 2 h later. (D) BALB/c mice were challenged with 100 μg of αIgE mAb (Left) or 500 μg of αFcγRII/RIII mAb (Right) and bled immediately or 4 or 24 h later. (E) Mice were injected i.v. with 0.26 mg of propranolol, 450 ng of PAF, or 4.3 mg of histamine, or sensitized with 100 μg of IgG1αTNP, and challenged 24 h later with 50 μg of TNP-OVA. Rectal temperatures were followed for 60 min after challenge. Mice were bled 4 h after challenge, and the percentage of neutrophils in blood and neutrophil FcγRIII expression were determined. *Statistically significant difference between the marked group and the control group; †statistically significant difference between the marked group and all other groups in the same set. The same symbols have the same meaning in all subsequent figures. ND, not detectable.
IgE- but Not IgG-Mediated Anaphylaxis Induces Large Increases in Secretion of IL-4 and Expression of IL-4Rα.
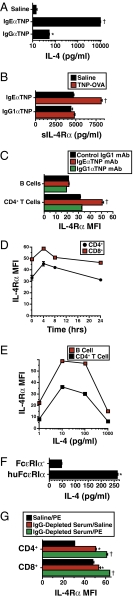
Cross-linking of basophil FcεRI but not FcγRIII induces considerable secretion of IL-4 (25), and IL-4 can increase the concentration of soluble (s) IL-4Rα in serum (26) and expression of IL-4Rα on T lymphocytes (27). Consequently, we hypothesized that these changes would occur during IgE- but not IgG-mediated anaphylaxis. Indeed, IL-4 production, as detected by the in vivo cytokine capture assay (IVCCA), increased ≈1,000-fold in IgE-mediated anaphylaxis, whereas IgG-mediated anaphylaxis had no effect at antigen doses up to 200 μg and only a slight effect at an antigen dose of 1 mg (Fig. 2A and Figs. S1B and S2). Similarly, serum levels of sIL-4Rα increased by 60–70% and T-cell membrane IL-4Rα increased by 40–70% during IgE-mediated anaphylaxis in BALB/c mice, whereas IgG-mediated anaphylaxis had little of no effect on these parameters (Figs. 2 B and C and Figs. S1 C and D and S2). The increase in cell membrane IL-4Rα was statistically significant and at least 25% in each of five independent experiments (average 38.3% ± 6.2%). The percentage increase in cell membrane IL-4Rα expression was greater on CD4+ T cells than on CD8+ T cells or B cells and peaked ≈4 h after disease initiation (Fig. 2D). An IgE-dependent, FcγRIII-independent increase in IL-4 secretion and sIL-4Rα and CD4+ T-cell IL-4Rα expression was also observed in mice immunized actively with goat anti-mouse IgD antibody (4) and challenged i.v. with the relevant antigen (goat IgG) (Fig. S3).
Fig. 2.
IL-4 secretion and T-cell membrane and secreted IL-4Rα increase after IgE- but not IgG-mediated anaphylaxis. (A) BALB/c mice were primed with 10 μg of IgEαTNP or 100 μg of IgG1αTNP mAb and challenged i.v. 24 h later with 40 μg of TNP-OVA. Mice were bled 4 h later. IL-4 secretion was evaluated by IVCCA, with biotin-αIL-4 mAb injected at the time of antigen challenge. (B) Soluble IL-4Rα was measured by ELISA in sera obtained 4 h after TNP-OVA challenge. (C) Cell membrane IL-4Rα expression on B and CD4+ T cells obtained 4 h after TNP-OVA challenge was evaluated by flow cytometry. (D) BALB/c mice were primed with IgEαTNP mAb, challenged with TNP-OVA 24 h later, and bled at the time of challenge or 4, 8, or 24 h later. Flow cytometry was used to determine IL-4Rα expression on peripheral blood CD4+ and CD8+ T cells. (E) Human PBNCs were cultured at 37 °C for 24 h in DMEM with 10% FBS plus 0–1,000 pg/mL of IL-4. After incubation, B-cell and CD4+ T-cell IL-4Rα expression was evaluated by flow cytometry. (F) FcεR1α-deficient mice and mice that express human but not mouse FcεRIα were primed with 250 μg of IgG-depleted serum from a peanut-allergic individual. Mice were challenged i.v. 24 h later with 100 μg of peanut extract. IL-4 secretion was evaluated by IVCCA. (G) IL-4Rα expression by T cells from the same mice was evaluated by flow cytometry.
Two experiments were performed to determine whether increased T-cell IL-4Rα might be a relevant marker for human IgE-mediated anaphylaxis. First, an in vitro study confirmed previous evidence that IL-4 increases human CD4+ T-cell IL-4Rα expression (28) by demonstrating that this response was strongly induced by 10 pg/mL of IL-4 (Fig. 2E). In contrast to our in vivo mouse studies, however, IL-4 increased membrane IL-4Rα expression by B cells as well as T cells. Second, in vivo studies performed with mice that express human FcεRIα in place of mouse FcεRIα (29) and, consequently, bind human rather than mouse IgE, demonstrated that priming with IgG-depleted serum from a peanut-allergic individual, followed by challenge with peanut extract, induced significant increases in IL-4 secretion and CD4+ and CD8+ T-cell IL-4Rα expression (Fig. 2 F and G and Fig. S4); the increase in CD4+ T-cell IL-4Rα expression averaged 22% ± 2% in three experiments performed with IgG-depleted sera from three different peanut-allergic individuals and was significant in each experiment. Taken together, these results reveal a unique marker that is specific for IgE-mediated anaphylaxis in the mouse and support the belief that the same marker may apply to humans.
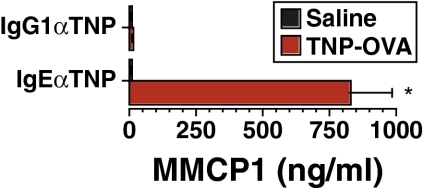
Increased Serum Mouse Mast Cell Protease 1 (MMCP1) Levels Are Specific for IgE-Mediated Anaphylaxis.
Currently, increases in serum tryptase levels are the preferred assay for detection of mast cell-mediated anaphylaxis in humans (1, 10). To determine whether a similar increase in a mast cell-specific protease could differentiate IgE- from IgG-mediated anaphylaxis in mice, we determined serum MMCP1 levels before and after IgE- and IgG-mediated anaphylaxis in this species. Results indeed demonstrated a considerable rise in serum MMCP1 levels after IgE- but not IgG-mediated anaphylaxis (Fig. 3).
Fig. 3.
Increased serum MMCP1 concentration is a specific marker for IgE-mediated anaphylaxis. BALB/c mice were primed with 10 μg of IgEαTNP or with 100 μg of IgG1αTNP and challenged i.v. 12 h later with 50 μg of TNP-OVA. MMCP1 concentration in blood obtained 4 h after challenge was measured by ELISA.
Decreased Neutrophil FcγRIII Expression Differentiates IgG- from IgE-Mediated Anaphylaxis.
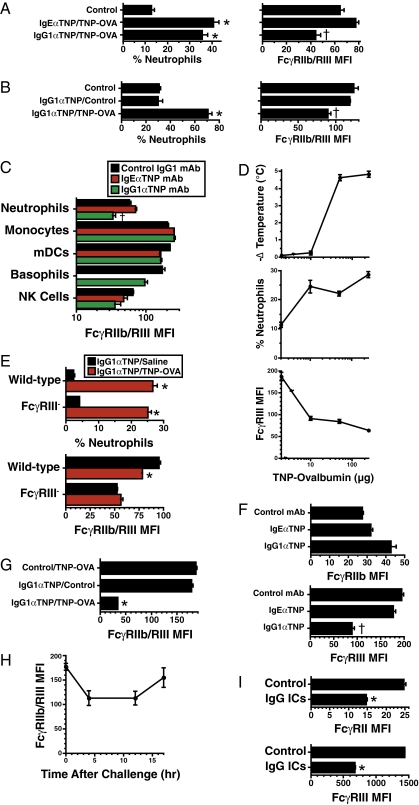
Serum IgG levels are generally much higher than IgE levels. Consequently, although IgE IC can interact with mouse FcγRIIb and FcγRIII, at least in vitro at 4 °C (30), the quantity of IgG IC is likely to be considerably higher than the quantity of IgE IC in the same animal. With this in mind, we hypothesized that IgG- but not IgE-mediated anaphylaxis might be accompanied by a change in FcγRIII expression by nucleated blood cells. Experiments in which cells were stained with the mAb 2.4G2, which binds to both FcγRIIb and FcγRIII (6, 31) (Fig. S5, Left), demonstrated that IgG- but not IgE-mediated anaphylaxis decreases neutrophil FcγRIIb/III expression (Fig. 4A and Fig. S2). This decrease reflected decreased neutrophil plasma membrane FcγR expression rather than blocking of FcγRs by IgG IC (Fig. S6) and required both priming with an antigen-specific IgG mAb and challenge with the specific antigen (Fig. 4B). This effect was prominent on neutrophils and basophils but was not observed for monocytes or dendritic cells (Fig. 4C). Neutrophil loss of FcγRIII [detected with an FcγRIII-specific mAb (Fig. S5, Right)] was a sensitive indicator of in vivo generation of antigen/IgG IC; it was observed even when mice were challenged with an antigen dose too small to induce shock (Fig. 4D). The in vivo generation of IgG IC was not accompanied by decreased neutrophil FcγRIIb expression in mice that lacked the stimulatory FcγR, FcγRIII (Fig. 4E), and IgG-mediated anaphylaxis was accompanied by a decrease in FcγRIII but not FcγRIIb expression on neutrophils in wild-type mice (Fig. 4F) in experiments that used mAbs specific for FcγRIII or FcγRIIb, respectively (Figs. S5 and S7). Consistent with this, there was a large percentage decrease in neutrophil FcγR expression in FcγRIIb-deficient mice stained with anti-FcγRII/RIII mAb (Fig. 4G), and the average IgG anaphylaxis-associated decrease in FcγR expression in six independent experiments in which neutrophils were stained with an FcγRIII-specific mAb (60.1% ± 5.9%) was considerably larger than that observed when neutrophils were stained with anti-FcγRII/RIII mAb (average 28.4% ± 3.9% in four independent experiments). The decrease in neutrophil FcγR expression was detectable by 4 h after the induction of IgG-mediated anaphylaxis and lasted for at least 12 h (Fig. 4H). Decreases in neutrophil FcγRIII expression sometimes occurred during PAF and propranalol-induced shock but have been much smaller than those that accompany IgG-mediated anaphylaxis (Fig. 1E).
Fig. 4.
Decreased neutrophil FcγRIII expression is a specific marker for IgG-mediated anaphylaxis. (A) BALB/c mice primed with IgEαTNP or IgG1αTNP were challenged i.v. 18 h later with TNP-OVA and bled 2 h after that. The percentage of neutrophils in blood and MFI of neutrophil staining with anti-FcγRIIb/RIII mAb were determined by flow cytometry. (B) The percentage of neutrophils in blood and neutrophil FcγRIIb/RIII expression were determined 2 h after OVA (control) or TNP-OVA challenge of IgGαTNP mAb-primed mice. (C) Mice primed with IgEαTNP or IgG1αTNP were challenged i.v. 16 h later with TNP-OVA and bled 2 h after that. FcγRIIb/RIII median fluorescence intensity (MFI) for neutrophils, myeloid dendritic cells (mDCs), natural killer (NK) cells, monocytes, and basophils was evaluated by flow cytometry. (D) BALB/c mice primed with IgG1αTNP were challenged i.v. 12 h later with 0–250 μg of TNP-OVA. Rectal temperatures were followed for 1 h after challenge, and maximum temperature drops were determined (Top). The percentage of neutrophils and neutrophil FcγRIII expression were evaluated (Middle and Bottom). (E) C57BL/6 wild-type and FcγRIII-deficient mice were primed with IgG1αTNP, challenged i.v. 12 h later with TNP-OVA, and bled 4 h after that. The percentage of neutrophils and neutrophil FcγRIII expression were determined. (F) BALB/c mice primed with IgEαTNP or IgG1αTNP were challenged 12 h later with TNP-OVA and bled 4 h after challenge. Neutrophil FcγRIIb and FcγRIII expression was evaluated. (G) FcγRIIb-deficient mice primed with IgG1αTNP or isotype control mAb were challenged 12 h later with TNP-OVA or saline and bled 2 h after that. Neutrophils were stained with fluorochrome-labeled αFcγRIIb/RIII mAb and evaluated by flow cytometry for surface fluorescence. (H) BALB/c mice primed with IgG1αTNP were challenged 12 h later with TNP-OVA and bled 0–17 h after challenge. Neutrophil FcγRIIb/RIII expression was evaluated. (I) Human PBNCs were cultured for 4 h at 37 °C in RPMI plus 15% human plasma with or without 0.25 mg/mL of anti-human IgG/human IgG ICs; then evaluated for neutrophil FcγRIII and FcγRIIa/b expression.
To determine whether IgG ICs could cause a similar decrease in human neutrophil FcγRIII, human neutrophils were cultured for 4 h with IgG ICs. Results (Fig. 4I) demonstrate that IgG ICs induce a decrease of nearly 50% in FcγRII (FcγRIIa and/or FcγRIIb, both of which are bound by the mAb used) and >60% in FcγRIII expression by these cells (Fig. 4I). Taken together, these results indicate that decreased neutrophil FcγRIII expression is a robust marker that differentiates IgG- from IgE-mediated anaphylaxis in mice and that is likely to also reflect an acute increase in the concentration of IgG ICs in humans.
Discussion
The possibility that IgG-mediated anaphylaxis is responsible for infusion reactions in individuals who are repeatedly administered chimeric and humanized human mAbs (19, 22) led us to attempt to identify blood markers that might differentiate IgE-mediated anaphylaxis from putative IgG-mediated anaphylaxis in humans. Because it is not feasible to directly demonstrate such markers in man, we used the strategy of establishing these markers in mice, in which IgE- and IgG-mediated anaphylaxis can be rigorously defined, then performing preliminary studies to evaluate whether these markers may be applicable in humans. Our results demonstrate that murine IgE-mediated anaphylaxis is characterized by (i) an increased percentage of neutrophils and decreased percentages of basophils and monocytes in blood; (ii) increased levels of MMCP1, sIL-4Rα, and CD4+ T-cell IL-4Rα expression; and (iii) little or no change in neutrophil levels of FcγRIII. In contrast, murine IgG-mediated anaphylaxis, although accompanied by changes in blood basophil, monocyte, and neutrophil levels that are similar to those observed in IgE-mediated anaphylaxis, is characterized by a substantial decrease in neutrophil FcγRIII expression but little change in serum levels of MMCP1 or sIL-4Rα or T-cell IL-4Rα expression. Neutrophil loss of FcγRIII is a sensitive indicator of formation of the IgG ICs that cause IgG-mediated anaphylaxis; this loss is observed after challenge with an antigen dose too small to induce shock. Thus, murine IgG-mediated anaphylaxis can be ruled out by the lack of a decrease in neutrophil FcγRIII expression.
Although we cannot prove that changes similar to those observed in mice can distinguish human IgE-mediated anaphylaxis from putative IgG-mediated anaphylaxis, previous observations and our preliminary studies are consistent with this possibility: (i) human basophils, like mouse basophils, are induced to secrete IL-4 by FcεRI cross-linking (32); (ii) IL-4 induces human T cells to express increased IL-4Rα (28); (iii) cross-linking of humanized FcεRI in chimeric mice by allergen binding to human IgE increases T-cell IL-4Rα expression; and (iv) human neutrophils lose FcγRIII when cultured with IgG ICs.
This view is reinforced by the fact that the differences between markers for IgE- and IgG-mediated anaphylaxis make sense: although basophils express both FcεRI and FcγRIII and can be activated through both Rs, only activation through FcεRI induces a strong IL-4 response (25, 33), which, in turn, induces increases in both soluble and T-cell membrane IL-4Rα expression. Similarly, although mast cells can be induced under special conditions to release granules that contain MMCP1 in response to FcγRIII cross-linking (34), FcεRI cross-linking is normally a much more potent trigger for mast cell degranulation. It also seems logical that IgG but not IgE ICs cause neutrophils to decrease their expression of FcγRIII. This may not, however, result simply from FcγR binding or cross-linking by IgG ICs, because such complexes fail to induce monocytes or dendritic cells to decrease FcγR expression or to cause a consistent loss of FcγRIIb by neutrophils in FcγRIII-deficient mice. Most likely, loss of FcγRIII during IgG-mediated anaphylaxis requires signaling through FcγRIII that results in receptor internalization or shedding; signaling that occurs in neutrophils but not in some other cell types. In contrast, some of the effects of anaphylaxis on blood do not seem to be anaphylaxis-specific. The decreased percentage of monocytes and increased percentage of neutrophils in blood, for example, was also induced by infusion of PAF and may well be a response to severe shock, regardless of the mechanism responsible for shock induction.
Taken together, our results suggest an approach that can be used to detect whether IgG-mediated anaphylaxis occurs in humans, and, if so, a set of assays that could help to diagnose human IgG-mediated anaphylaxis. As noted in the Introduction, considerable evidence already supports the existence of human IgG-mediated anaphylaxis—anaphylaxis that occurs after exposure to a high dose of an antigen that is recognized by serum IgG Abs in the absence of detectable IgE Abs or an increase in serum tryptase. It would be reasonable to initially look for evidence of human IgG-mediated anaphylaxis, using our mouse-validated criteria, in individuals who are being infused repeatedly with a foreign antigen, such as a chimeric mAb, at fixed intervals. This would require using cells and plasma obtained from the same individual before development of anaphylaxis or >24 h after recovery from anaphylaxis, because there may well be considerable variation in basal plasma sIL-4Rα, T-cell membrane IL-4Rα, and neutrophil FcγRIII levels among different individuals. It would make less sense to look for evidence of human IgG-mediated anaphylaxis in situations in which anaphylaxis is mediated only by IgE in the mouse; for example, allergen ingestion (35–37) or in situations in which anaphylaxis is induced by inoculation with low quantities of allergen, such as venom inoculation by stinging insects.
Finally, it should be noted that there is a practical reason to distinguish IgE- from IgG-mediated anaphylaxis. Because development of IgG- but not IgE-mediated anaphylaxis requires relatively large amounts of antigen, IgG Abs can protect against IgE-mediated anaphylaxis by neutralizing antigen before its binding by FcεRI-associated IgE on mast cells and basophils (3). Immunization with the involved antigen can be therapeutic in such circumstances by raising the concentration of blocking IgG Abs. In contrast, increasing specific IgG Ab levels would likely be detrimental when anaphylaxis is caused by IgG ICs. In addition, the different cell types, Rs, and mediators involved in IgE- vs. IgG-mediated anaphylaxis suggest that knowledge of the specific type of anaphylaxis involved will be required to optimize prophylactic and therapeutic approaches.
Materials and Methods
Mice.
BALB/c mice were purchased from the National Cancer Institute. C57BL/6 FcγRIIb-deficient (38) mice were a gift of Jeffrey Ravetch (Rockefeller University, New York, NY). BALB/c FcγRIII-deficient mice (39) were purchased from Jackson Laboratories. FVB/N IgE-deficient mice (5) were a gift of Hans Oettgen (Children's Hospital Boston, Boston, MA). Mice that express human rather than murine FcεRIα (27) were a gift of Jean-Pierre Kinet (Harvard University, Cambridge, MA). All experimental procedures were performed with approval from the Institutional Animal Care and Use Committees of the Cincinnati Children's Hospital Research Foundation and the Cincinnati Veterans Affairs Medical Center.
Reagents.
Details about reagents used are provided in SI Materials and Methods.
Immunofluorescence Staining.
Single-cell leukocyte suspensions were generated from mouse spleen and from peripheral blood collected by tail vein bleeding into EDTA-coated tubes (BD Bioscience), followed by erythrocyte lysis with ACK lysis buffer (BioWhittaker). Human blood was collected into EDTA-coated tubes, and peripheral blood nucleated cells (PBNCs) were prepared by gradient centrifugation with Ficoll-Paque Plus (GE Healthcare). Cells were stained for 30 min on ice with 1 μg each of appropriately labeled Abs. All samples were analyzed on a FACSCalibur (BD Bioscience). Data analysis was performed with CellQuest software (BD Bioscience). Light scatter gates were set to exclude most nonlymphoid cells and cells that had died before fixation except in the cases in which ToPro3 exclusion was used to gate out dead cells. Specific cell types were identified by the following characteristics: mouse neutrophils, Ly6GhighCD11b+CD4− and relatively high forward and side light scatter; mouse myeloid dendritic cells, CD11b+CD11c+F4/80−; mouse NK cells, CD49b+IgE−; mouse monocytes, F4/80+CD11b+CD11c−; mouse basophils, IgE+CD49b+CD4−; human neutrophils, CD15+CD16+CD32lowCD163− and relatively high forward and side light scatter.
Measurement of IL-4, Soluble IL-4Rα, and MMCP1.
In vivo IL-4 secretion was measured by IVCCA (40, 41). Mice were injected with biotinylated anti–IL-4 mAb (BVD4-1D11) (42) at the time of trinitrophenyl ovalbumin (TNP-OVA) challenge. Serum was collected 2 h later, unless other indicated. For measurement of soluble IL-4Rα by ELISA, plates were coated with anti-IL-4Rα mAb (clone M1) (43) and blocked with SuperBlock (Pierce Biotechnology). Serial dilutions of serum or plasma were added to wells, followed sequentially by biotinylated affinity-purified goat anti–IL-4Rα polyclonal Ab (26), HRP-streptavidin, and SuperSignal ELISA substrate (Pierce Biotechnology). Serum levels of MMCP1 were measured in blood drawn 2 or 4 h after antigen challenge, unless otherwise indicated, with an ELISA kit purchased from Moredun Scientific.
Anaphylaxis.
Mice were primed i.v. with 10 μg of IgE anti-TNP (IgE-mediated anaphylaxis) or 100 μg of IgG anti-TNP (IgG-mediated anaphylaxis) mAb unless otherwise indicated, then challenged i.v. 24 h later with TNP-OVA. The severity of the anaphylactic shock was assessed by change in rectal temperature (4, 44). Histamine and PAF antagonists were used in some experiments to suppress anaphylaxis (4).
Studies with Human Leukocytes.
Human PBNCs were incubated in RPMI-1640 supplemented with 15% human plasma that contained 13.2 mg/mL of human IgG before dilution with or without IgG ICs (0.25 mg/mL) for 4 h at 37 °C, washed twice with cold HBSS supplemented with 10% newborn bovine serum and 0.2% NaN3, then stained and analyzed by flow cytometry. Experiments with human cells were approved by the institutional review board at Cincinnati Children's Hospital Medical Center and obtained with informed consent from the donor.
Statistics.
Differences in temperature, concentrations of MMCP1, IL-4, (s)IL-4R, and fluorescence intensity between groups of mice or groups of samples were compared using the Mann–Whitney test (GraphPad Prism 4.0; GraphPad software). A P value of <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Hans Oettgen, Jeffrey Ravitch, and Jean-Pierre Kinet for their generous gifts of transgenic mice. This work was supported by a Department of Veterans Affairs Merit Award (to F.D.F.) and National Institutes of Health Grant R21 AI079947 (to F.D.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105695108/-/DCSupplemental.
References
- 1.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S161–S181. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 2.Golden DB. Patterns of anaphylaxis: Acute and late phase features of allergic reactions. Novartis Found Symp. 2004;257:101–110; discussion 110–115, 157–160, 276–285. [PubMed] [Google Scholar]
- 3.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc γ RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 5.Oettgen HC, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 6.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 7.Cheifetz A, et al. The incidence and management of infusion reactions to infliximab: A large center experience. Am J Gastroenterol. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CL, et al. Consequences of immunogenicity to the therapeutic monoclonal antibodies ReoPro and Remicade. Dev Biol (Basel) 2003;112:37–53. [PubMed] [Google Scholar]
- 9.Kolho KL, Ruuska T, Savilahti E. Severe adverse reactions to Infliximab therapy are common in young children with inflammatory bowel disease. Acta Paediatr. 2007;96:128–130. doi: 10.1111/j.1651-2227.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Simons FE, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120(1 Suppl):S2–S24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551–1555. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz LB. Effector cells of anaphylaxis: Mast cells and basophils. Novartis Found Symp. 2004;257:65–74; discussion 74–79, 98–100, 276–285. [PubMed] [Google Scholar]
- 13.Lin RY, et al. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J Allergy Clin Immunol. 2000;106:65–71. doi: 10.1067/mai.2000.107600. [DOI] [PubMed] [Google Scholar]
- 14.Simons FE, et al. Practical allergy (PRACTALL) report: Risk assessment in anaphylaxis. Allergy. 2008;63:35–37. doi: 10.1111/j.1398-9995.2007.01605.x. [DOI] [PubMed] [Google Scholar]
- 15.Hedin H, et al. Incidence, pathomechanism and prevention of dextran-induced anaphylactoid /anaphylactic reactions in man. Dev Biol Stand. 1980;48:179–189. [PubMed] [Google Scholar]
- 16.Umeda Y, et al. Anaphylactic shock related to aprotinin induced by anti-aprotinin immunoglobulin G antibody alone; report of a case. Kyobu Geka. 2007;60:69–71. in Japanese. [PubMed] [Google Scholar]
- 17.Bergamaschini L, et al. Posttransfusion anaphylactic reactions in a patient with severe von Willebrand disease: Role of complement and alloantibodies to von Willebrand factor. J Lab Clin Med. 1995;125:348–355. [PubMed] [Google Scholar]
- 18.Klastersky J. Adverse effects of the humanized antibodies used as cancer therapeutics. Curr Opin Oncol. 2006;18:316–320. doi: 10.1097/01.cco.0000228734.32261.62. [DOI] [PubMed] [Google Scholar]
- 19.Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med. 2005;72:250–256. [PubMed] [Google Scholar]
- 20.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 21.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campi P, Benucci M, Manfredi M, Demoly P. Hypersensitivity reactions to biological agents with special emphasis on tumor necrosis factor-α antagonists. Curr Opin Allergy Clin Immunol. 2007;7:393–403. doi: 10.1097/ACI.0b013e3282ef96df. [DOI] [PubMed] [Google Scholar]
- 23.Khodoun M, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinet JP. The high-affinity IgE receptor (Fc ε RI): From physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 25.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khodoun M, et al. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J Immunol. 2007;179:6429–6438. doi: 10.4049/jimmunol.179.10.6429. [DOI] [PubMed] [Google Scholar]
- 27.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci USA. 1988;85:8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armitage RJ, Beckmann MP, Idzerda RL, Alpert A, Fanslow WC. Regulation of interleukin 4 receptors on human T cells. Int Immunol. 1990;2:1039–1045. doi: 10.1093/intimm/2.11.1039. [DOI] [PubMed] [Google Scholar]
- 29.Dombrowicz D, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 30.Takizawa F, Adamczewski M, Kinet JP. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as Fc γ RII and Fc γ RIII. J Exp Med. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacGlashan D, Jr, et al. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- 33.Tsujimura Y, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Dombrowicz D, et al. Absence of Fc epsilonRI α chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR β and γ chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandt EB, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–214. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 37.Leung DY, et al. Avon Longitudinal Study of Parents and Children Study Team Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 38.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(γ)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 39.Hazenbos WL, et al. Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16) J Immunol. 1998;161:3026–3032. [PubMed] [Google Scholar]
- 40.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 41.Finkelman FD, Morris SC, Orekhova T, Sehy D. The in vivo cytokine capture assay (IVCCA) for measurement of in vivo cytokine production in the mouse. Curr Protoc Immunol. 2003 doi: 10.1002/0471142735.im0628s54. Chapter 6:Unit 6.28. [DOI] [PubMed] [Google Scholar]
- 42.Abrams JS, et al. Strategies of anti-cytokine monoclonal antibody development: Immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 43.Beckmann MP, et al. Monoclonal antibodies block murine IL-4 receptor function. J Immunol. 1990;144:4212–4217. [PubMed] [Google Scholar]
- 44.Miyajima I, et al. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.