Abstract
Mutations in the gene encoding the transcription factor autoimmune regulator (AIRE) are responsible for autoimmune polyendocrinopathy candidiasis ectodermal dystrophy syndrome. AIRE directs expression of tissue-restricted antigens in the thymic medulla and in lymph node stromal cells and thereby substantially contributes to induction of immunological tolerance to self-antigens. Data from experimental mouse models showed that AIRE deficiency leads to impaired deletion of autospecific T-cell precursors. However, a potential role for AIRE in the function of regulatory T-cell populations, which are known to play a central role in prevention of immunopathology, has remained elusive. Regulatory T cells of CD8+CD28low phenotype efficiently control immune responses in experimental autoimmune and colitis models in mice. Here we show that CD8+CD28low regulatory T lymphocytes from AIRE-deficient mice are transcriptionally and phenotypically normal and exert efficient suppression of in vitro immune responses, but completely fail to prevent experimental colitis in vivo. Our data therefore demonstrate that AIRE plays an important role in the in vivo function of a naturally occurring regulatory T-cell population.
Keywords: inflammatory bowel disease, suppressor T cells, repertoire
Immunological homeostasis of the organism is maintained by a large variety of mechanisms. One of these mechanisms involves induction of tolerance to self-antigens, which is at least in part acquired during T-cell development in the thymus, where autospecific T-cell precursors undergo negative selection and either die by apoptosis or are rendered functionally anergic (1). Despite the substantial quantitative impact of these mechanisms (2), some autospecific T cells leave the thymus (3). Such cells are kept under control by peripheral tolerance mechanisms that include induction of apoptosis and anergy (4) and the activity of CD4+ or CD8+ regulatory T-lymphocyte populations (Treg) (5, 6). Treg not only control immune responses to self- but also to non-self-antigens, for example during infection and pregnancy as well as in the gut (7–9). Their therapeutic potential has been demonstrated in experimental autoimmune and transplantation models (10–12).
Whereas the best-studied Treg is of CD4+Foxp3+ phenotype, other Treg have also been described and may play very important roles in physiology. One of these populations expresses CD8 and low levels of the costimulatory molecule CD28. These cells were originally described in cultures of human lymphocytes that, after repeated in vitro stimulation, lost proliferative capacity (13). Later, they were identified in unmanipulated mice and shown to suppress in vitro T-cell responses and to prevent experimental autoimmune encephalomyelitis (14). We showed that experimental colitis induced by injection of naïve T cells into immunodeficient animals could efficiently be prevented by coinjection of CD8+CD28low Treg (15).
Mutations in the gene encoding the transcription factor autoimmune regulator (AIRE) are responsible for the immune disorder autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) (16, 17). This disease is mainly characterized by chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenocortical insufficiency. Also, AIRE-deficient (AIRE°) mice develop an autoimmune pathology similar to APECED (18–22). Interestingly, it was recently shown that also candidiasis is probably due to an autoimmune disorder: APECED patients had neutralizing antibodies to Th17-associated cytokines known to be involved in immunity to fungi (23, 24). These observations indicate that AIRE is required for the control of immune responses to self-antigens. AIRE promotes promiscuous expression of tissue-restricted antigens in the thymic medulla and by lymph node stromal cells, thereby inducing immunological tolerance (25–27). Evidence from T-cell receptor (TCR)-transgenic mouse models shows that expression of neo-self-antigens leads to deletion of specific thymocytes, and that in AIRE° mice, deletion of TCR-transgenic thymocytes was severely impaired (28–30). AIRE also drives expression of chemokines by mTEC (31), for example XCL1, which is involved in the medullary accumulation of thymic dendritic cells (32). In AIRE° mice, marginally reduced numbers of CD4+Foxp3+ Treg may develop. However, molecular analysis of the TCR repertoire of WT and AIRE° CD4+Foxp3+ Treg failed to detect any difference, and these cells have unaltered in vitro and in vivo suppressive activity (20, 21, 30, 32, 33). In APECED patients, decreased expression of Foxp3 and impaired suppressive function of Treg were observed (34).
Because diarrhea and other gastrointestinal disorders are among the multiple symptoms of, and sometimes even dominate, the clinical picture of APECED syndrome (35), it is important to understand the role of AIRE in development of T-cell populations thought to play a key role in the maintenance of immunological homeostasis in the gut. Human CD8+ T cells from healthy colon biopsies have in vitro suppressive activity. In contrast, CD8+ T cells from colon biopsies affected with inflammatory bowel disease (IBD) lack such in vitro suppressive activity (36). In mice, CD8+ cells play a crucial role in the induction of intestinal tolerance to orally administered antigens (37). We previously demonstrated that CD8+CD28low T cells prevent experimental colitis in mice (15). Also, intestinal CD8αα+ T cells have been shown to control experimental colitis (38). Combined, these data strongly suggest an important role for CD8+ Treg in control of immune homeostasis in the intestines. We therefore assessed the capacity of AIRE° CD8+CD28low Treg to prevent experimental IBD.
Results
Quantitatively Unaltered Development of Transcriptionally and Phenotypically Normal CD8+CD28low Treg in AIRE° Mice.
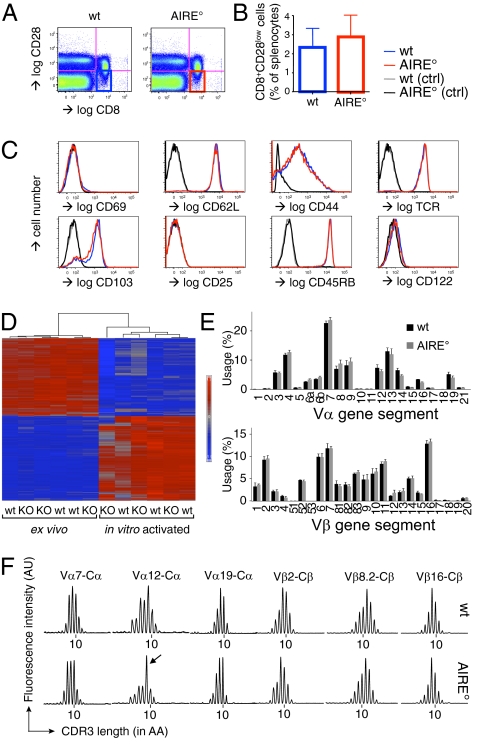
Despite elegant attempts (30), no direct experimental data exist showing that AIRE deficiency impinges on Treg function. We embarked on a study of the influence of AIRE on the in vitro and in vivo suppressor activity of CD8+CD28low Treg. Mice carrying a 13-bp mutation in the AIRE gene that disrupts the PHD1 domain in exon 8 were previously discussed (21). It was demonstrated that these mice developed mild symptoms of autoimmune pathology, similar to other AIRE-deficient mice on the C57BL/6 background. Given that CD28 is down-modulated on chronically activated T lymphocytes (39), we first compared the numbers and phenotypes of CD8+CD28low splenic T cells from AIRE° C57BL/6 mice and their WT (AIRE+/+) littermates (Fig. 1). CD28low cells were defined as those expressing levels not exceeding the level found with an isotype-matched control antibody (Fig. 1A) (15). AIRE° and WT mice had similar percentages and absolute numbers of CD8+CD28low cells in the spleen (Fig. 1B). As shown in Fig. 1C, these cells expressed neither the early activation marker CD69 nor the IL-2Rα chain CD25, also up-regulated upon T-cell activation. Moreover, they expressed low levels of the activation marker CD44 and high levels of naïve T-cell marker CD45RB. The lymph node homing receptor l-selectin (CD62L) was expressed at high levels on both WT and AIRE° CD8+CD28low splenic T cells. WT and AIRE° CD8+CD28low cells uniformly expressed high levels of TCR. Combined, these data show that WT as well as AIRE° CD8+CD28low T cells had a naïve phenotype. The integrin αE chain CD103 is expressed on the majority of CD8+CD28low T cells and, again, no difference between WT and AIRE° cells was observed. The IL-2Rβ chain CD122 is expressed at hardly detectable levels on WT and AIRE° CD8+CD28low cells (Fig. 1C), indicating that they constitute a population distinct from CD122highCD8+ regulatory cells (40) that have a CD28+ phenotype (Fig. S1A). In contrast to the uniform naïve phenotype of CD8+CD28low splenic cells, the CD8+CD28+ population, which at the population level lacks in vitro and in vivo regulatory capacity (15), contains activated (CD44high) cells. However, again, no phenotypic difference between WT and AIRE° CD8+CD28+ T cells was observed (Fig. S1A).
Fig. 1.
AIRE° and WT mice have normal numbers of transcriptionally and phenotypically unaltered CD8+CD28low Treg with similar but apparently distinct TCR repertoires. (A) Definition of WT (AIRE+/+) and AIRE° (AIRE°/°) C57BL/6 CD8+CD28low cells from spleen. Flow cytometry was performed using the indicated antibodies. (B) WT and AIRE° littermates had similar percentages of splenic CD8+CD28low Treg (gated as in A). Indicated are mean values ± SD (n = 3). (C) Phenotype of CD8+CD28low cells, electronically gated as shown in A. Expression profiles of markers indicated in the figure. Control (ctrl) stainings were performed using isotype-matched antibodies. Shown are typical results of three independently performed experiments. (D) Heatmap of the differentially expressed genes (according to fold change) in resting compared with activated, WT versus AIRE° CD8+CD28low Treg. Genes with statistical differential average expression (adjusted P < 0.05) with a fold change >2 when comparing activated and native cells are represented in this heatmap. Red indicates increased expression; blue indicates decreased expression. The dendrogram shows that WT and AIRE° (KO) samples are not clustering within the activated and native states. (E) Vα or Vβ segment use of WT versus AIRE° CD8+CD28low splenic Treg, as measured by semiquantitative RT-PCR. Indicated are mean values ± SD (n = 3 samples consisting of pooled cells from three mice). (F) CDR3 length distribution of indicated variable domains. Shown are typical results for indicated Vα and Vβ CDR3s. “10” indicates a CDR3 length of 10 amino acids; other peaks are separated by 3 nucleotides = 1 amino acid. Immunoscope results for all Vα and Vβ CDR3s for all analyzed mice are shown in Fig. S2 D and E. The arrow indicates the relatively increased signal for the 9-amino acid-long Vα12 CDR3 in AIRE° Treg.
We next analyzed the gene expression profiles of WT versus AIRE° CD8+CD28low Treg (Fig. 1D and Fig. S1 B and C). RNA was extracted from freshly isolated and from in vitro activated cells, and genome-wide gene expression profiling was performed. Microarray data showed distinct grouping of the freshly isolated versus activated samples (Fig. 1D and Fig. S1B), but no subgrouping was observed between the WT and AIRE° samples within these activation states. ANOVA revealed 2,190 genes with differential average expression between resting and activated cells (adjusted P < 0.05 and absolute fold change ≥ 2) (Fig. 1D) but did not reveal any genes with significant differential average expression between AIRE° and WT cells, with adjusted P values approaching 1 for both the activated and freshly isolated AIRE° versus WT contrasts (Fig. S1C). Together, these data show that unaltered numbers of transcriptionally and phenotypically normal CD8+CD28low Treg develop in AIRE° mice.
TCR Repertoires of WT and AIRE° CD8+CD28low Treg Are Very Similar but Not Identical.
AIRE drives ectopic expression of tissue antigens by mTEC, including that of the XCL1 chemokine involved in accumulation of thymic dendritic cells in the medulla (32, 41). Because the thymic medulla and dendritic cells play a central role in shaping the T-cell repertoire, the repertoires of WT versus AIRE° CD8+CD28low Treg may be different. To assess this possibility, we analyzed the TCR repertoire of peripheral CD8+CD28low Treg. We found that TCR Vα and Vβ use was very diverse, but it was indistinguishable between WT and AIRE° Treg (Fig. 1E). Moreover, immunoscope analysis of all Vα and Vβ domains revealed similar near-normal distributions of CDR3 lengths in WT versus AIRE° CD8+CD28low Treg (Fig. 1F and Fig. S1 D and E). However, a reproducible increase in the signal of 9-amino acid-long Vα12 CDR3 was observed in AIRE° compared with WT CD8+CD28low Treg (Fig. 1F and Fig. S1D). Together, these data show that the CD8+CD28low TCR repertoire is very diverse and similar between WT and AIRE° mice, but also clearly indicate that they are not identical.
CD8+CD28low Treg from AIRE-Deficient Mice Exert Normal in Vitro Suppressive Activity.
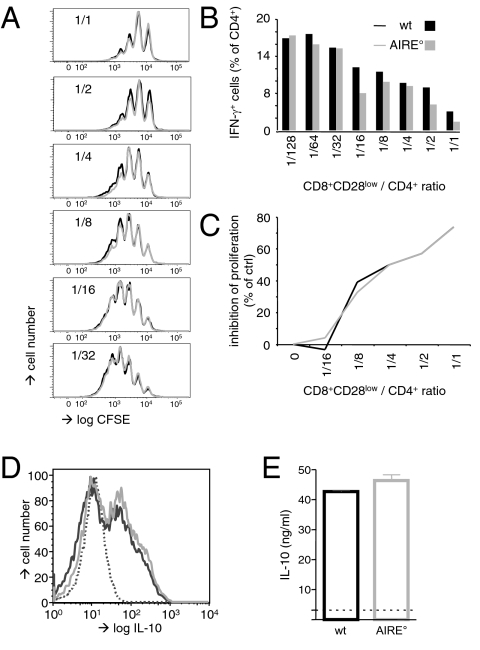
We next compared the in vitro suppressive capacities of WT versus AIRE° CD8+CD28low Treg. It was previously shown that freshly isolated CD8+CD28low cells exert in vitro and in vivo suppressive activity (14, 15). Titrated numbers of magnetic bead-sorted Treg were therefore put in culture with 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled responder CD4+ T cells. T-cell stimulation was achieved using antigen-presenting cells (APC) and anti-CD3 antibody. Proliferation of and IFN-γ production by CD4+ responder T cells were as efficiently suppressed by AIRE° as by WT CD8+CD28low Treg (Fig. 2 A and B). Also, when stimulated with allogeneic APC, proliferation was as efficiently inhibited by AIRE° as by WT Treg (Fig. 2C). Prestimulation of Treg substantially increases their in vitro suppressive activity. We therefore assessed whether WT and AIRE° CD8+CD28low Treg increased their in vitro suppressive activity to the same extent. Magnetic bead-sorted splenic Treg were expanded in vitro with allogeneic APC for 1 wk and then used in our in vitro suppression assays (Fig. S2). Upon in vitro stimulation with APC and anti-CD3 antibody, thus activated WT and AIRE° CD8+CD28low Treg equally efficiently inhibited proliferation and IFN-γ production by conventional CD4+ T cells (Fig. S2 A and B). Also, in mixed lymphocyte cultures, preactivated WT and AIRE° Treg inhibited proliferation with equal efficiency (Fig. S2C). In all three cases, prestimulation substantially increased suppressive activity (cf. Fig. 2 A–C and Fig. S2 A–C, respectively).
Fig. 2.
AIRE° and WT CD8+CD28low cells have identical activity in in vitro suppression assays and produce similar levels of IL-10. (A) CD8+CD28low Treg isolated from WT (AIRE+/+) and AIRE° (AIRE°/°) C57BL/6 (B6) mice were cultured with CFSE-labeled responder B6 CD4+ T cells and APC in the presence of anti-CD3ε antibody. Proliferation of CD4+ cells was assessed by FACS analysis of CFSE dilution. (B) As in A, but responder CD4+ T cells were analyzed by flow cytometry for IFN-γ production. (C) B6 CD4+ T cells were cultured with DBA/2 APC in the presence of B6 AIRE° or WT CD8+CD28low Treg (as indicated) at indicated Treg:CD4 ratios. Proliferation in these mixed lymphocyte reactions was assessed by measuring incorporation of [3H]thymidine. (D) CD8+CD28low Treg were activated in vitro with anti-CD3ε antibody and APC over 5 d and then intracellularly stained with antibody specific for IL-10 or with isotype-matched control antibody (dotted line). (E) As in D, but supernatants of cultures were analyzed by ELISA for IL-10. The broken line indicates background value, determined in the absence of anti-CD3ε antibody in the in vitro culture. Results are representative of those obtained in at least three independent experiments.
IL10-deficient CD8+CD28low Treg less efficiently suppress in vitro T-cell activation than WT cells (15). Together with our observation that WT and AIRE° CD8+CD28low Treg have identical in vitro suppressive capacity, this suggests that IL-10 production by WT and AIRE° Treg is probably similar. To verify this hypothesis, we stimulated freshly isolated WT and AIRE° Treg with anti-CD3 antibody and APC. Five days later, the frequency of IL-10-producing cells was assessed by flow cytometry. We observed that a similar proportion of WT and AIRE° Treg produced this central anti-inflammatory cytokine (Fig. 2D). Secretion of IL-10 by in vitro activated WT and AIRE° Treg was assessed by ELISA and, again, was found to be similar (Fig. 2E). Combined, these data show that AIRE deficiency does not perturb in vitro suppressor function of and IL-10 production by CD8+CD28low Treg.
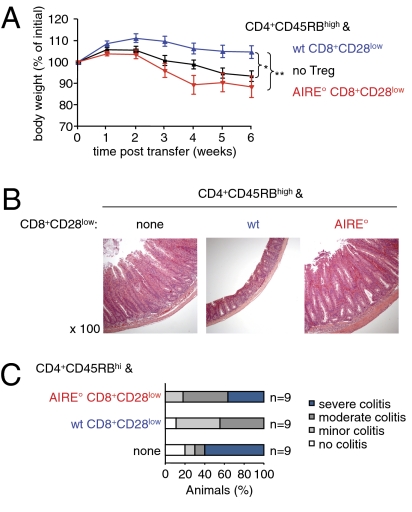
AIRE-Deficient CD8+CD28low Treg Are Incapable of Preventing Experimental Colitis.
We previously published that CD8+CD28low Treg prevented experimental IBD in mice (15). To study the in vivo function of AIRE° Treg, we therefore assessed whether they were capable of preventing this pathology. Immunocompromised RAG-2-deficient (RAG°) mice were injected with syngeneic flow-sorted naïve CD4+CD45RBhigh cells. These mice developed weight loss and histological signs of colitis such as substantial mucosal thickening, disappearance of goblet cells, elongation of crypts, and infiltration by mononuclear cells (Fig. 3 A and B). When the colitogenic population was coinjected with syngeneic WT CD8+CD28low cells, weight loss and histological signs of colitis were prevented, confirming our previously published data (Fig. 3 A–C). In contrast, coadministration of AIRE° CD8+CD28low Treg did not prevent intestinal pathology (Fig. 3 A and B). The clinical scores of colons from mice injected with colitogenic cells and AIRE° CD8+CD28low Treg were as severe as those from mice injected with colitogenic T cells alone (Fig. 3C). Injection of AIRE° CD8+CD28low cells alone did not induce any signs of colitis, excluding the hypothetical possibility that this population contains colitogenic cells that cannot be suppressed by CD8+CD28low Treg (Fig. S3).
Fig. 3.
AIRE° CD8+CD28low T cells fail to prevent colitis. RAG-2° hosts were i.v. injected with syngeneic CD4+CD45RBhigh colitogenic T cells with or without the indicated CD8+CD28low Treg (“wt”, AIRE+/+; “AIRE°”, AIRE°/°). (A) Evolution of weight of animals. Shown is the mean weight ± SD as a percentage of weight at the start of the experiment (*P < 0.05, **P < 0.01, Mann–Whitney test; n = 9 per group; three independent experiments). (B) Mice were euthanized 6 wk after injection of T cells. Microscopic sections of distal colon were stained with hematoxylin and eosin and examined for histological signs of colitis. Shown results are representative of those obtained in three independent experiments. (C) Colons of mice were examined as in B and clinical scores of colitis were attributed (n = 9 from three independent experiments).
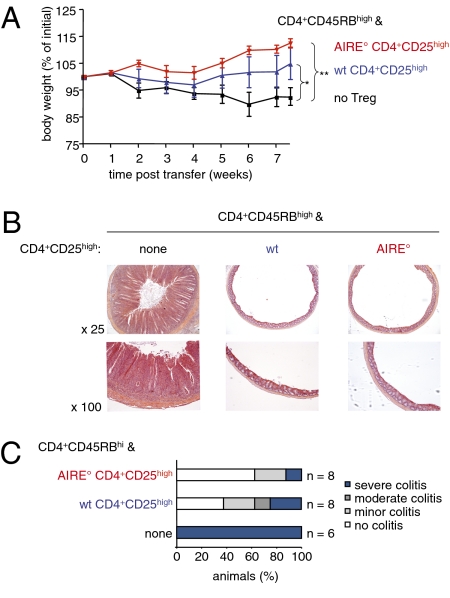
It was previously reported that AIRE° CD4+CD25high Treg prevented experimental colitis (30), and our results were therefore rather surprising. However, the AIRE-deficient mice we used did not carry the same mutation as the mice used by Anderson and colleagues. Moreover, the experimental setup was not strictly identical to ours. We therefore assessed whether CD4+CD25high Treg from our AIRE° animals could prevent colitis induced by injection of CD4+CD45RBhigh cells. Mice injected with colitogenic T cells developed weight loss and histological signs of colitis such as substantial mucosal thickening, disappearance of goblet cells, elongation of crypts, and infiltration by mononuclear cells (Fig. 4 A and B). In contrast, mice coinjected with CD4+CD25high Treg from WT but also from AIRE° animals showed substantially reduced signs of pathology (Fig. 4 A and B). Clinical scores attributed to the histological analysis of all experimental mice confirmed that both WT and mutant CD4+CD25high Treg efficiently prevented development of intestinal pathology (Fig. 4C). The incapacity of AIRE° Treg to prevent experimental colitis described here therefore appears to be a specific property of the CD8+CD28low population.
Fig. 4.
AIRE° CD4+CD25high Treg do not show any defect in prevention of colitis. Colitis was induced as in Fig. 3. (A) Evolution of weight of animals. Shown is the mean weight ± SD as a percentage of weight at the start of the experiment (*P < 0.05, **P < 0.01, Mann–Whitney test; n = 6 without Treg, n = 8 with WT Treg, n = 8 with AIRE° Treg; two independent experiments). (B) Mice were euthanized 7 wk after injection of T cells. Microscopic sections of distal colon were stained with hematoxylin and eosin and examined for histological signs of colitis. Shown results are representative of those obtained in two independent experiments. (C) Colons of mice were examined as in B and clinical scores of colitis were attributed (n values as indicated, from two independent experiments).
Discussion
In this report, we show that AIRE deficiency leads to a defect of the in vivo function of a Treg population that appears to play an important role in maintenance of immunological homeostasis in the intestines. Because the in vitro suppressor activity and IL-10 production of WT versus AIRE° CD8+CD28low Treg were strictly identical and no difference could be detected in phenotype nor in transcriptional pattern, we feel that the identified defect is most likely due to very subtly altered shaping of the CD8+CD28low Treg TCR repertoire by intrathymic or peripheral mechanisms. This hypothesis is supported by careful analysis of Vα and Vβ CDR3s that showed that the TCR repertoires of WT versus AIRE° CD8+CD28low Treg are diverse and very similar, but also revealed that they are not identical.
To date, no other evidence has been published showing that AIRE deficiency leads to impaired in vivo Treg function. In nude mice transplanted with a WT and an AIRE° thymus, an autoimmune attack of several organs occurred. This result showed that the autoimmune syndrome developing in AIRE° mice was not due to defective dominant (i.e., Treg-mediated) tolerance. Moreover, CD4+CD25high Treg from these mice were as effective as WT Treg in preventing experimental colitis (30). On the other hand, transgenic expression of model antigens expressed under control of the AIRE promoter enhanced development of CD4+Foxp3+ Treg expressing specific transgenic TCR (42). Whereas the latter observation strongly suggested that proteins expressed under control of AIRE can modulate Treg repertoire selection, it failed to show that AIRE deficiency leads to altered in vivo Treg function. In contrast, here we identified an in vivo functional defect of the CD8+CD28low Treg from AIRE° mice.
Expression of AIRE in T lymphocytes has never been found, and it appears therefore unlikely that this transcription factor intrinsically affects CD8+CD28low Treg function. This postulate is supported by the indistinguishable transcriptional patterns we found in WT versus AIRE° CD8+CD28low Treg. In in vitro experiments, we found that freshly isolated WT and AIRE° Treg equally well inhibited proliferation and IFN-γ production by conventional T cells. IL-10-deficient CD8+CD28low Treg were incapable of preventing experimental colitis, which showed that this cytokine plays a nonredundant role in vivo (15). We therefore also assessed production of this cytokine and found that, upon a 1-wk activation period in vitro, CD8+CD28low Treg from WT and AIRE° mice similarly produced and secreted IL-10. Moreover, the substantially increased in vitro suppressor activity of WT Treg upon in vitro prestimulation was also found (and to the same extent) for AIRE° CD8+CD28low cells. Increased suppressor activity of Treg upon in vitro activation probably reflects differentiation of naïve Treg to effector cells, a process known to take several days for conventional T cells (43). Combined, these data establish that the intrinsic suppressive capacity of the AIRE° CD8+CD28low Treg is unaltered. This was an important point to make because it was previously shown that the CD4+CD25+ Treg of autoimmune diabetes-prone nonobese diabetic (NOD) mice is contaminated with a substantial number of cells not expressing Foxp3. This observation probably accounts for the observed reduced in vitro and in vivo regulatory capacity of NOD CD4+CD25+ T cells (44).
It appears therefore more likely that AIRE somehow affects shaping of the TCR repertoire of CD8+CD28low Treg. Extensive analysis of TCR Vα and Vβ use and CDR3 length distribution showed that the TCR repertoires of WT and AIRE° CD8+CD28low Treg are very diverse and very similar. This result parallels the finding of apparently similar TCR repertoires of WT versus AIRE° CD4+Foxp3+ Treg (33). However, using immunoscope analysis a very reproducible difference was observed, showing that the TCR repertoires of CD8+CD28low cells from WT versus AIRE° mice are not identical. Substantially more work will need to be done to precisely characterize the difference between the TCR repertoires of CD8+CD28low Treg from WT and AIRE° mice and to identify the specificities involved in prevention of colitis apparently lacking in AIRE° mice.
AIRE is expressed in the thymic medulla, where it controls expression of tissue-restricted antigens (25, 26). Also, lymph node stromal cells have been reported to express AIRE, to transcribe a distinct subset of genes encoding tissue-restricted antigens, and to induce peripheral tolerance (45–49). We have found mature CD8+CD28low T cells in the thymus, suggesting that they may develop in this primary lymphoid organ (Fig. S4). However, the CD28low phenotype of these Treg suggests a chronically activated state, and they may therefore also differentiate in the periphery (39). Hence, identification of the site of CD8+CD28low Treg development merits further investigation. We propose that within this site, AIRE allows stromal cells to express tissue-restricted antigens that play an important role in selection of the CD8+CD28low Treg repertoire. In the absence of AIRE, a Treg repertoire would develop that is no longer capable of preventing experimental colitis.
Whereas AIRE° CD8+CD28low Treg failed to control experimental colitis, CD4+CD25high Treg from the same donors fully prevented the pathology, confirming a previous report (30). This difference suggests that the repertoire of CD8+CD28low Treg involved in prevention of colitis is very limited. The observation that AIRE° CD8+CD28low Treg fail to control colitis also suggests that these cells recognize self-antigens. Our experimental model may therefore provide an ideal tool to evaluate the intriguing question of the antigen specificity of colitis-preventing Treg.
The observation that CD8+CD28low Treg from AIRE° mice fail to control experimental colitis may provide an explanation for the gastrointestinal disorders observed in patients affected with APECED syndrome (35). In contrast, AIRE° mice do not develop colitis. A tentative explanation for this observation is provided by the unaltered capacity of mouse AIRE° CD4+CD25+ Treg to prevent intestinal inflammation. Human CD8+ T cells from healthy colon biopsies have in vitro suppressive activity. In contrast, CD8+ T cells from colon biopsies affected with IBD lack such in vitro suppressive activity (36). These results therefore suggest an important role for defective CD8+ Treg function in human IBD and may explain the difference between the effects of human and mouse AIRE deficiency on chronic intestinal inflammation.
In conclusion, AIRE-deficient CD8+CD28low Treg fail to prevent experimental colitis. It will now be important to further characterize the mechanisms by which AIRE deficiency perturbs the studied in vivo function of this Treg population and to identify the antigen(s) recognized.
Materials and Methods
Mice.
All mice were used at 6–10 wk of age. DBA/2 and C57BL/6 mice were from Janvier. RAG° C57BL/6 mice were bred in our specific pathogen free animal facility. AIRE-deficient C57BL/6 mice (21) were maintained in a heterozygous breeding colony; AIRE+/+ littermates were used as controls. The health status of the mice in the animal facility was periodically monitored according to Federation for Laboratory Animal Science Associations (FELASA) guidelines and found free of monitored pathogens. The regional animal experimentation ethics committee approved performed experiments (reference no. MP/01/39/10/06).
Flow Cytometry Analysis.
The following reagents were used: FITC-labeled anti-CD8α, IFN-γ, CD103, and CD45RB; anti-CD4-PE; anti-CD4-APC; anti-IL10-APC; anti-CD28-biotin; streptavidin-PE; all from eBioscience; anti-CD69-FITC; CD122-FITC; APC-conjugated anti-CD62L, -CD25, and -TCRβ; anti-CD44-PeCy5; anti-CD8α-AF700; all from BD Pharmingen.
For FACS analysis, cells were incubated with antibodies in staining buffer (PBS and 2.5% FCS) for 20 min and then washed. Labeled cells were analyzed on a FACS LSRII (Becton Dickinson) using DIVA (Becton Dickinson) and FlowJo software (Tree Star).
Isolation of T-Cell Subsets.
CD8+CD28low Treg and CD4+CD45RBhigh colitogenic T cells were isolated as previously described (15). For isolation of CD4+CD25high cells, erythrocyte-depleted splenocytes were incubated with anti-FcγRII/III (2.4G2), anti-CD8 (53.6.7), anti-MHC class II (M5), and anti-B220 (RA3-6B2) and thus labeled cells eliminated using Dynabeads coated with sheep anti-rat IgG (Dynal Biotech). The resulting population was labeled with anti-CD25-PE and CD4+CD25high cells were enriched with anti-PE microbeads (Miltenyi). CD4+CD25high T cells were routinely >95% pure. Responder CD4+ T cells used in in vitro assays were enriched from erythrocyte-depleted splenocytes by Dynabead-mediated depletion of FcγRIII+, MHC class II+, CD8+, and B220+ cells.
Microarray Analysis of Treg Transcriptomes.
Total RNA was extracted using TRIzol (Invitrogen) for lysis of cells and phase separation according to the manufacturer's instructions. RNA was then purified from the aqueous phase using the RNeasy Mini Kit (QIAGEN). All RNA had a quality score (RNA integrity number) of at least 8.5, assessed using a Bioanalyzer 2100 (Agilent Technologies). Genome-wide gene expression profiling was performed using the Affymetrix GeneChip Mouse Gene 1.0 ST array. Labeling and array hybridization, washing, staining, and scanning of the arrays were performed according to the manufacturer's instructions.
Statistical Analysis.
All analysis was performed using the Partek Genomics Suite, version 6.4. Raw expression data were processed with the robust multichip average (MA) function and quantile normalization. These data were analyzed using ANOVA. A principal components analysis plot of the expression data were constructed (Fig. S1B). A heatmap of the up-regulated and down-regulated genes (by fold change) comparing native and activated cells was generated using unsupervised clustering. An MA plot of the average expression of activated AIRE° versus activated WT samples was generated.
TCR Repertoire Analysis.
RNA was extracted using the RNeasy Micro Kit (QIAGEN) and reverse-transcribed using oligo(dT) and SuperScript II (Invitrogen). Vα and Vβ expression pattern was assessed by real-time PCR using V-specific primers and probes (50, 51). Amplified products were used as template for a runoff reaction with fluorescent-tagged oligonucleotides. We have used the nomenclature from Arden et al. for the TCRβ chain (52) and the IMGT server referred to by Lefranc for the TCRα chain (53).
In Vitro Suppression Assays.
CD4+ responder (105) and titrated numbers of CD8+CD28low Treg were cultured in the presence of allogenic APC (5 × 105) for 96 h and 1 μCi of [3H]thymidine was added to the cultures for the last 16 h. Alternatively, CD4+ effector cells were stained in vitro with CFSE (Sigma-Aldrich). CFSE-labeled responders (105) were cultured with titrated numbers of CD8+CD28low Treg in the presence of APC (5 × 105) and 10 μg/mL anti-CD3ε mAb 2C11. After 3 d of culture, cells were stained with anti-CD4-APC and proliferation of CD4+ responder cells was assessed by FACS.
Detection of Cytokine Production.
Cells were restimulated with phorbol 12-myrestate 13-acetate (PMA) (50 ng/mL) and ionomycin (1 μg/mL) (Sigma) for 4 h at 37 °C. For intracellular detection, Brefeldin A (10 μg/mL; eBioscience) was added for the last 2 h, cells were stained for the indicated surface markers, fixed with 2% paraformaldehyde for 30 min at 4 °C, permeabilized in 0.5% saponin, 1% BSA, 1 μg/mL rat IgG in PBS for 30 min at RT, and then incubated for 30 min at RT with FITC-conjugated anti-IFN-γ or anti-IL-10 in permeabilization buffer. IL-10 detection by ELISA was performed using JES-2AS and SXC-1 antibodies.
Induction and Clinical and Histological Assessment of Colitis.
C57BL/6 RAG-2° mice were i.v. injected with 4 × 105 syngeneic WT CD4+CD45RBhigh T cells with or without 2 × 105 syngeneic WT or AIRE° CD8+CD28low or CD4+CD25high cells. Mice were weighed weekly and euthanized after 6–8 wk. Histological analysis of distal colon and determination of clinical scores were performed as previously described (15).
Supplementary Material
Acknowledgments
We thank Drs. Lucette Pelletier, Jean-Charles Guéry, and Christophe Viret for critical reading of the manuscript and the staffs of the animal, flow cytometry, and histopathology facilities for excellent technical support. This work was financially supported by grants from the Association François Aupetit (2007 and 2008); the Association pour la Recherche sur le Cancer (to C.P.); Fundação para a Ciência e a Tecnologia Fellowship SFRH/BPD/72483/2010 (to R.V.); EuroThymaide Consortium Contract LSHB-CT-2003-503410; National Health and Medical Research Council of Australia Grants 171601, 461204, 257501, 264573, and 406700; Swedish Society of Medicine Grant SLS-96661; the Swedish Endocrine Society; and Swedish Research Council Grant 524-2010-6723 (to B.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE30129).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107136108/-/DCSupplemental.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.van Meerwijk JPM, et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 5.Pomié C, Ménager-Marcq I, van Meerwijk JP. Murine CD8+ regulatory T lymphocytes: The new era. Hum Immunol. 2008;69:708–714. doi: 10.1016/j.humimm.2008.08.288. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: A common quest for host homeostasis. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 8.Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev. 2006;212:330–343. doi: 10.1111/j.0105-2896.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 10.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 11.Joffre O, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang JY, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol. 2008;69:681–686. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 14.Najafian N, et al. Regulatory functions of CD8+CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ménager-Marcq I, Pomié C, Romagnoli P, van Meerwijk JPM. CD8+CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–1785. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 17.Finnish-German APECED Consortium An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 19.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 21.Hubert FX, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182:3902–3918. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 22.Pöntynen N, et al. Aire deficient mice do not develop the same profile of tissue-specific autoantibodies as APECED patients. J Autoimmun. 2006;27:96–104. doi: 10.1016/j.jaut.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Kisand K, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puel A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 26.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 27.Gardner JM, Fletcher AL, Anderson MS, Turley SJ. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21:582–589. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 29.Liston A, et al. Gene dosage—Limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Laan M, et al. Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J Immunol. 2009;183:7682–7691. doi: 10.4049/jimmunol.0804133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 2010;184:6865–6873. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kekäläinen E, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 35.Husebye ES, Perheentupa J, Rautemaa R, Kämpe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265:514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 36.Brimnes J, et al. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 37.Grdic D, Hörnquist E, Kjerrulf M, Lycke NY. Lack of local suppression in orally tolerant CD8-deficient mice reveals a critical regulatory role of CD8+ T cells in the normal gut mucosa. J Immunol. 1998;160:754–762. [PubMed] [Google Scholar]
- 38.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallejo AN. CD28 extinction in human T cells: Altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+CD122+ cells regulatory T cells or memory T cells? Hum Immunol. 2008;69:751–754. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- 41.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 42.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 43.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: Intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 44.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner JM, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 47.Nichols LA, et al. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 48.Cohen JN, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabaniols JP, Fazilleau N, Casrouge A, Kourilsky P, Kanellopoulos JM. Most α/β T cell receptor diversity is due to terminal deoxynucleotidyl transferase. J Exp Med. 2001;194:1385–1390. doi: 10.1084/jem.194.9.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fazilleau N, et al. T cell repertoire diversity is required for relapses in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2007;178:4865–4875. doi: 10.4049/jimmunol.178.8.4865. [DOI] [PubMed] [Google Scholar]
- 52.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 53.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2003;31:307–310. doi: 10.1093/nar/gkg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.