Abstract
The chromosomal translocation t(11;14)(q13;q32) leading to cyclin-D1 overexpression plays an essential role in the development of mantle cell lymphoma (MCL), an aggressive tumor that remains incurable with current treatment strategies. Cyclin-D1 has been postulated as an effective therapeutic target, but the evaluation of this target has been hampered by our incomplete understanding of its oncogenic functions and by the lack of valid MCL murine models. To address these issues, we generated a cyclin-D1–driven mouse model in which cyclin-D1 expression can be regulated externally. These mice developed cyclin-D1–expressing lymphomas capable of recapitulating features of human MCL. We found that cyclin-D1 inactivation was not sufficient to induce lymphoma regression in vivo; however, using a combination of in vitro and in vivo assays, we identified a novel prosurvival cyclin-D1 function in MCL cells. Specifically, we found that cyclin-D1, besides increasing cell proliferation through deregulation of the cell cycle at the G1–S transition, sequestrates the proapoptotic protein BAX in the cytoplasm, thereby favoring BCL2’s antiapoptotic function. Accordingly, cyclin-D1 inhibition sensitized the lymphoma cells to apoptosis through BAX release. Thus, genetic or pharmacologic targeting of cyclin-D1 combined with a proapoptotic BH3 mimetic synergistically killed the cyclin-D1–expressing murine lymphomas, human MCL cell lines, and primary lymphoma cells. Our study identifies a role of cyclin-D1 in deregulating apoptosis in MCL cells, and highlights the potential benefit of simultaneously targeting cyclin-D1 and survival pathways in patients with MCL. This effective combination therapy also might be exploited in other cyclin-D1–expressing tumors.
Keywords: mouse model of MCL, ABT-737, cyclin-D1 inhibitor drugs, targeted therapy, oncogene addiction
Mantle cell lymphoma (MCL) is a distinct lymphoma entity that accounts for ∼6–8% of all cases of lymphoma (1, 2). MCL is thought to be derived from naïve pregerminal center B lymphocytes localized in primary follicles or in the mantle region of secondary follicles, and thus most tumors do not show somatic hypermutation of the Ig heavy-chain coding (IGH) genes. Cytologically, two major MCL subsets can be distinguished, the classical and blastoid/pleomorphic variants, which share a characteristic CD19+CD5+CD23− immunophenotype (2). Almost all MCL cases show the chromosomal translocation t(11;14)(q13;q32), which juxtaposes the CCND1 gene with IGH gene enhancers and causes overexpression of the cyclin-D1 protein. The best-known function ascribed to cyclin-D1 is in positive regulation of cell cycling. In MCL cells, constitutive cyclin-D1 activation maintains retinoblastoma (RB) protein in a phosphorylated state and promotes cell proliferation, thus likely initiating tumorigenesis (3). However, whether cyclin-D1 has additional oncogenic functions in the lymphoma cells has not been well addressed. In B lymphocytes, cyclin-D1 deregulation seems insufficient to induce neoplastic transformation, given that other genetic changes are required for the development of malignancy (3, 4). Numerous genes have been postulated as candidates that cooperate with cyclin-D1 to promote MCL development, including the cell-cycle regulators P16INK4a and CDK4; the DNA-damage sensor and repair genes ATM, TP53, and ARF; and components of the apoptotic machinery, such as BCL2L11 and BCL2 (3, 5–7). However, the functional mechanisms underlying the interplay of cyclin-D1 with these oncogenic proteins and their impact on MCL pathogenesis remain unexplored.
Clinically, there is no curative therapy for MCL; all treatment modalities, including combined immunochemotherapy and radiotherapy or intensive high-dose chemotherapy with stem cell transplantation, have failed to prevent disease recurrence and progression (8–10). In attempts to improve this poor outcome, attention has turned to novel therapies targeting specific regulatory pathways that are essential for the growth and maintenance of the transformed phenotype, some of which are currently undergoing clinical testing (8, 10, 11). However, a rigorous evaluation of the molecular targets that may be suitable for these compounds has not been conducted. Cyclin-D1, which plays a critical role in MCL development, has emerged as one of the most promising therapeutic targets, but its analysis has been hampered by the lack of useful MCL mouse models (10).
To investigate the function of cyclin-D1 in MCL development and its potential role as a therapeutic target, we generated a cyclin-D1–driven mouse model in which cyclin-D1 expression can be externally regulated. These mice developed lymphomas recapitulating some features of human MCL. Our study identifies a novel role for cyclin-D1 in deregulating apoptosis in MCL cells and highlights the potential benefits of simultaneously targeting cyclin-D1 and survival pathways in patients with MCL.
Results
Inhibition of Cyclin-D1 Has Moderate Effects on MCL Cell Growth but Enhances Sensitivity to Apoptosis.
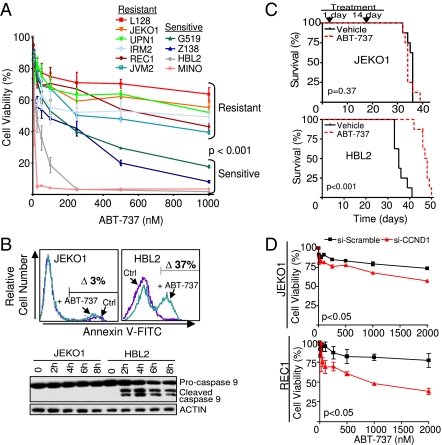
Cyclin-D1 has been postulated as a promising therapeutic target in MCL, based on its critical role in tumor development and its overexpression in virtually all cases. However, inhibition of cyclin-D1 in human MCL cell lines by siRNA resulted in a moderate effect on cell growth, leading to accumulation of cells in G1 phase of the cell cycle and to a minor increase in the apoptotic rates (Fig. S1A) (12). These data suggest that additional genetic pathways are contributing to the transformed phenotype in MCL cells. To identify the genes participating in lymphomagenesis and search for valid therapeutic targets, we developed a combined cellular-genomic-proteomics screen. In this approach, the cytotoxicity to compounds targeting cancer-related molecular pathways was tested in MCL cell lines and was correlated with their genomic, gene expression, and proteomic profiles (Fig. S1B). Although the MCL cell lines showed variable sensitivity to the drugs included in the screening (Fig. S1C), the BH3-only mimetic ABT-737, a small molecule that binds to BCL2, BCL-XL, and BCL-W (13), was selectively effective in some MCL cell lines, whereas other remained resistant (Fig. 1A and Fig. S1D). The reduced cell viability in the sensitive cells after exposure to the BH3 mimetic was associated with a marked increase in the apoptotic rates and with cleavage of caspase 9, indicating the involvement of the intrinsic apoptotic pathway (Fig. 1B and Fig. S1E). Similarly, ABT-737–sensitive cells were killed by this drug in vivo after i.v. inoculation into immunodeficient RAG2−/−γc−/− mice (lacking B, T, and dendritic cells) (14), whereas ABT-737–resistant MCL cells were not (Fig. 1C). Accordingly, longer survival of mice carrying the ABT-737–sensitive HBL2 cells was accompanied by a reduction in lymphoma volume and attenuation of tumor glycolytic activity (Fig. S1 F and G). We next checked whether cyclin-D1 silencing might influence ABT-737 sensitivity. Notably, simultaneous siRNA-mediated knockdown of cyclin-D1 and ABT-737 exposure were associated with a partial reversion of tumor resistance in ABT-737–resistant MCL cell lines (Fig. 1D and Fig. S1H). These data suggest that inhibition of cyclin-D1 may functionally interact with the apoptotic machinery to facilitate ABT-737–mediated apoptosis in MCL cells.
Fig. 1.
Inhibition of cyclin-D1 has moderate effects on MCL cell growth but enhances sensitivity to apoptosis. (A) ABT-737 was therapeutically effective in four MCL cell lines, whereas six lines remained resistant (P < 0.001). (B) Treatment with ABT-737 (250 nM) induced apoptosis in the sensitive cell lines, as revealed by annexin V staining and cleavage of caspase 9. Ctrl, control. (C) Kaplan–Meier overall survival (OS) curves for immunodeficient mice transplanted with MCL cells and treated with ABT-737 or vehicle. The ABT-737–treated mice transplanted with ABT-737–sensitive HBL2 cells had a longer OS than those treated with vehicle (median OS, 48 ± 2 d vs. 35 ± 3 d; P < 0.001). In contrast, mice carrying ABT-737–resistant JEKO1 cells had a similar outcome in the ABT-737–treated and control subgroups (median OS, 34 ± 2 d vs. 36 ± 1 d; P = 0.37). All experiments in mice were performed in duplicate with eight mice per group. (D) The combination of cyclin-D1 silencing and ABT-737 (250 nM) exposure resulted in a statistically significant decrease in cell viability, increase in apoptosis, and accumulation of cells in the G1 phase in two ABT-737–resistant MCL cell lines (Fig. S1H).
BCL2 Genomic Amplification and Protein Expression Determine Responses to ABT-737.
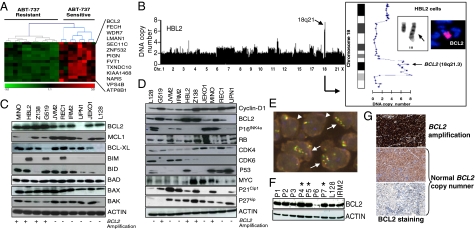
Analysis of the mechanisms underlying ABT-737 sensitivity revealed that the MCL-responsive cells were distinguished by a gene expression signature composed of 93 overexpressed genes, 13 of which (14%) mapped to chromosome bands 18q21-q22 (hypergeometric test, P = 6.85 × 10−15), including the BCL2 gene (Fig. 2A and Fig. S2A). Microarray-based comparative genomic hybridization (a-CGH) and fluorescence in situ hybridization (FISH) studies detected genomic amplification of chromosome 18q21 including the BCL2 gene locus in the four sensitive cell lines but not in any of the resistant tumors (Fig. 2B, Fig. S2B, and ref. 15). In addition, BCL2 protein was expressed in all but one of the MCL cell lines, at fivefold greater levels in those with 18q21 amplification (Fig. 2C and Fig. S2C). However, the expression of cyclin-D1 and of other proteins commonly altered in MCL and/or involved in apoptosis regulation was not correlated with ABT-737 sensitivity (Fig. 2 C and D). These data strongly indicate that BCL2 expression levels determine the response to ABT-737 in cyclin-D1–expressing MCL cells. To evaluate the clinical significance of our findings, we investigated the genomic and expression status of BCL2 in 183 primary cyclin-D1+ MCL specimens. Twenty-seven of the 183 specimens (15%) showed genomic gain or amplification of chromosome 18q21, including the BCL2 gene locus (Fig. 2E and Fig. S2D). BCL2 gain/amplification was correlated with higher levels of BCL2 protein expression, as determined by Western blot analysis (Fig. 2F). Immunohistochemistry (IHC) studies showed that almost all MCL biopsy specimens showed expression of BCL2, ranging from very low to high levels (Fig. 2G). Quantitative measurement of BCL2 expression assessed by IHC revealed that MCL cases with genomic gain/amplification of the BCL2 gene had a greater number of cells with BCL2 expression compared with nonamplified lymphomas (mean per tumor ± SEM, 13,000 ± 1,100 cells vs. 10,800 ± 540 cells; P = 0.05, Wilcoxon signed-rank test) (Fig. S2E and Table S1). Together, these data indicate that both cyclin-D1 and BCL2 are coexpressed in most patients with MCL, highlighting these molecules as potential targets for directed therapies.
Fig. 2.
ABT-737 sensitivity is associated with genomic amplification and overexpression of BCL2. (A) A gene expression signature based on the presence of overexpressed genes mapped to chromosome band 18q21-q22 subclassified ABT-737–sensitive and ABT-737–resistant lymphomas. (B) Genomic amplification of 18q21 was detected in the four ABT-sensitive MCL cell lines, but not in the resistant lymphomas (Fig. S2B). In HBL2 cells, a-CGH, cytogenetic, and FISH analyses (Inset) revealed high-level genomic amplification of chromosome 18q21.3 caused by a tandem duplication of BCL2 along the long arm of chromosome 18. (C) Western blot analysis of MCL cell lines measuring the expression of proteins involved in apoptosis regulation. (D) No association between ABT-737 sensitivity and alterations in proteins involved in cell-cycle control, DNA damage responses, or apoptosis was observed. (E) FISH study in a lymph node biopsy specimen from a patient with MCL (P4). Three cells had highly amplified BCL2 (green-red-yellow signals) with respect to centromeric chromosome 10 signals (blue spots) (cells marked with arrows). Two other cells had diploid karyotypes, as demonstrated by the number of green-yellow-red and blue signals (cells marked with arrowheads). (F) Western blot analysis of BCL2 protein in primary MCL cases and in the MCL cell lines L128 and IRM2. The cases with the highest BCL2 expression levels (P4, P5, and P7) corresponded to three cases with high-level amplification of the BCL2 gene represented in Fig. S2C. (G) IHC analysis revealed BCL2 expression of different intensities in almost all MCL cases; it was higher in lymphomas with genomic gain/amplification of BCL2.
Generation of Cyclin-D1–Expressing Lymphomas in Mice.
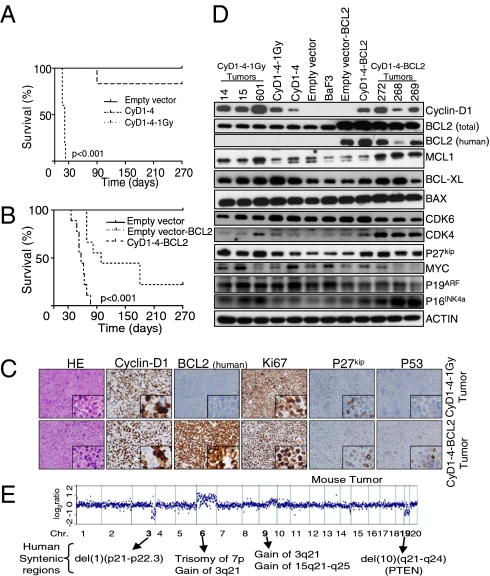
The foregoing results prompted us to explore the putative cyclin-D1/BCL2 interaction and their role as therapeutic targets in vivo. We generated a cyclin-D1–driven tumor model in mice in which cyclin-D1 expression could be regulated by doxycycline (Dox). For this, the human CCND1 gene was cloned into the Combit-TA vector (16) and stably transfected into mouse IL-3–dependent BaF3 pro-B lymphocytes, which were selected because of their similarity to the putative MCL cells of origin—naïve B lymphocytes with an active VDJ recombination program (Fig. S3 A and B). In two single-cell isolated cyclin-D1–expressing clones, CyD1-1 and CyD1-4, cyclin-D1 expression could be silenced within 48 h after exposure to Dox (Fig. S3C). In vitro cyclin-D1 overexpression did not give these cells the ability to grow independently of IL-3, nor did it substantially modify cell cycle or apoptotic rates, although it did increase cell proliferation. However, i.v. inoculation of CyD1-1 and CyD1-4 cells in RAG2−/−γc−/− mice did not induce tumor development (Fig. S3 D and E). We next tested whether additional genetic alterations induced by ionizing irradiation could promote transformation of cyclin-D1–expressing cells (17). Irradiated CyD1-1 and CyD1-4 cells were cultured without IL-3 and injected into immunodeficient mice. One of the CyD1-4 cell clones (obtained after irradiation with 1 Gy and hereinafter referred to as CyD1-4–1Gy) consistently developed tumors after 3–4 wk (median OS, 21 ± 4 d) (Fig. 3A). Genomic analysis of CyD1-4–1Gy cells with a-CGH revealed visible genomic alterations that were not present in the original CyD1-4 cells (see below), indicating that these acquired changes might have promoted cell transformation.
Fig. 3.
Characterization of cyclin-D1–expressing lymphomas in mice. (A) Injection of CyD1-4–1Gy cells in mice was associated with consistent development of tumors after 3–4 wk (nine mice per group). (B) CyD1-4–BCL2 cells did not grow independent of IL-3 in vitro, but induced tumor development in immunodeficient mice starting at week 6 (nine mice per group). (C) Histopathological and IHC studies of CyD1-4–1Gy and CyD1-4–BCL2 lymphomas revealed similar profiles to those of the pleomorphic variant of human MCL. HE, H&E staining. (D) Western blot analysis of mouse lymphomas identified common changes that are characteristic of human blastoid/pleomorphic MCL variants, such as overexpression of CDK4, P27kip, MYC, and MCL1 proteins. (E) Example of whole-genome a-CGH analysis of a mouse lymphoma, showing genomic alterations that overlap with those observed in the blastoid/pleomorphic variants of human MCL, such as the gains of human chromosomes 3q and 7p. In addition, other genomic changes that are characteristic of MCL cells, such as the loss of chromosome 1p21-p22, were identified.
To investigate whether BCL2 overexpression could similarly cooperate with cyclin-D1 to transform B lymphocytes, CyD1-4 cells were transfected with the human BCL2 gene cloned in the pcDNA3.1 vector (hereinafter, CyD1-4–BCL2 cells). Injection of 2.5 × 106 cells into immunodeficient mice was associated with tumor development starting at week 6 (median OS, 57 ± 11 d) (Fig. 3B). Isolated cell suspensions from cyclin-D1–driven lymphomas grew independently of IL-3 and could be transplanted into secondary RAG2−/−γc−/− recipients (Fig. S3F). Mouse lymphomas consistently involved bone marrow, peripheral blood, spleen and liver (Fig. S3G). Histopathologically, tumors were composed of an infiltrate of large and pleomorphic cells with a CD19+CD5−CD23−IgM− phenotype (confirmed by flow cytometry analysis of cell suspensions) and with a high proliferation rate, shown by Ki67 index of 100% and abundant mitoses, thus resembling the blastoid/pleomorphic variant of human MCL. The lymphomas showed coexpression of cyclin-D1 and BCL2 proteins. BCL2 expression was greather in CyD1-4–BCL2 lymphomas, whereas cyclin-D1 expression was fivefold greater in CyD1-4–1Gy1 lymphomas. P53 expression was detected by IHC in 5–30% of the tumor cells (Fig. 3C, Fig. S3 H and I, and Table S2). In addition, Western blot analysis identified changes typically found in blastoid MCL, such as overexpression of CDK4, P27kip, MYC, and MCL1 proteins (Fig. 3D) (18–21). Moreover, a-CGH studies of mouse lymphomas identified genomic alterations common to human MCL, such as gains of mouse chromosomes 6 and 9q (syntenic with gains of human chromosomes 3q21, 7p11-p22.3, and 15q21-q25) and deletions of chromosome 3q (syntenic with loss of 1p21-p22) and chromosome 19 (syntenic with the loss of human chromosome 10q21-q24, which harbors PTEN) (Fig. 3E and Fig. S3J) (7, 19). In summary, cyclin-D1–driven lymphomas recapitulated some of the cellular, histopathological, and genetic features of the blastoid/pleomorphic variants of human MCL, qualifying them as valid experimental models for testing directed therapies in vivo.
Combined Targeting of Cyclin-D1 and BCL2 Effectively Kills Lymphoma Cells.
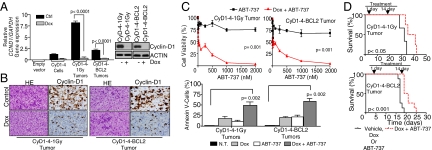
Administering Dox to the cyclin-D1–expressing lymphoma cells in culture or to the drinking water of mice led to down-regulation of cyclin-D1 levels by >95% within 48–72 h (Fig. 4 A and B and Fig. S4A). However, mouse lymphomas showed moderate differences in growth rate, cell cycle, and apoptotic indices after Dox-induced cyclin-D1 silencing or after exposure to ABT-737 in vitro (Fig. 4 B and C and Fig. S4B). Remarkably, simultaneous cyclin-D1 silencing and ABT-737 exposure induced prominent proliferative arrest and apoptosis more efficiently than cyclin-D1 inhibition or ABT-737 treatment alone, killing the lymphoma cells synergistically (Fig. 4C and Fig. S4 B and C). However, this synergy was not observed after cyclin-D1 silencing and exposure to the BH3 mimetic TW37 (22), bortezomib, or doxorubicin (Fig. S4D). In mice carrying cyclin-D1–induced lymphomas, the combination of ABT-737 with Dox-induced cyclin-D1 inhibition was associated with better responses, including a statistically significantly longer OS and clearance of lymphoma cells detected by imaging systems compared with control mice (Fig. 4D and Fig. S5). In vivo individual cyclin-D1 or BCL2 blocking did not modify cell morphology or proliferation rate, and did not induce signs of apoptosis. In contrast, cell proliferation and apoptotic changes were visible in the tumor biopsy specimens with the use of the combined therapy (Fig. S6). These data indicate that simultaneous inhibition of cyclin-D1 and BCL2 has synergistic antitumor activity on mouse lymphomas, recapitulating our previous results in human MCL.
Fig. 4.
Synergistic cooperation of cyclin-D1 and BCL2 to kill MCL in vivo. (A and B) Administration of Dox to mouse lymphoma cells in culture (4 μg/mL) and to the drinking water of mice led to cyclin-D1 silencing (>95%) within 48–72 h, as measured by qRT-PCR, Western blot analysis, and IHC analysis. (B) Liver biopsy specimens in mice treated and not treated with Dox are shown. HE, H&E staining. (C) Dox-induced cyclin-D1 inhibition or treatment with ABT-737 (250 nM) of mouse lymphomas was associated with moderate changes in growth and apoptotic rates. Notably, the simultaneous cyclin-D1 silencing and ABT-737 treatment had a synergistic therapeutic effect. NT, no treatment. (D) Mice engrafted with CyD1-4–1Gy or CyD1-4–BCL2 lymphomas received Dox, ABT-737, Dox plus ABT-737, or vehicle. Simultaneous therapeutic targeting of cyclin-D1 and BCL2 was associated with a statistically significantly longer OS in engrafted mice compared with the group of mice treated with vehicle, Dox, or ABT-737 (median OS, 38 ± 4 d vs. 32 ± 3 d for CyD1-4–1Gy lymphomas, P < 0.05; and 20 ± 2 d vs. 17 ± 1 d for CyD1-4–BCL2 lymphomas, P < 0.001). All experiments in mice were performed in duplicate, with eight mice per group.
Cyclin-D1 Sequestrates BAX and Inhibits ABT-737–Mediated Apoptosis.
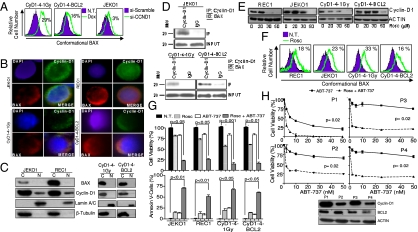
Based on the foregoing findings, we decided to investigate the mechanisms responsible for the enhanced therapeutic efficacy of ABT-737 in MCL cells after cyclin-D1 inhibition. Consistent with its role in cell-cycle regulation, a decrease in cyclin-D1 led to decreased phosphorylation of RB and increased P27kip expression in mouse lymphomas (Fig. S7A). However, no apparent changes were observed in the expression levels of the apoptotic modulators Bcl2, Mcl1, Bcl-xl, Bax, and Bak, among others (Fig. S7B). Notably, using flow cytometry, we found that the unbound fraction (active conformation) of BAX, a protein that functions as a final effector of the apoptotic cascades, was increased after cyclin-D1 silencing in the mouse lymphomas and in ABT-737–resistant MCL cell lines (Fig. 5A). Further analysis demonstrated that cyclin-D1 can complex with BAX in the cytoplasm of the lymphoma cells. Using immunofluorescence (IF) studies coupled with Western blot analysis of nuclear/cytoplasmic cellular protein fractions, we observed that most cyclin-D1 protein was present in the cytoplasm of both human and murine lymphomas, whereby it colocalized with BAX (Fig. 5 B and C). Accordingly, in the human MCL cell line JEKO1, the presence of BAX (but not of BAK) was detected by immunoblotting after cyclin-D1 immunoprecipitation. Likewise, in the cyclin-D1–expressing lymphomas, cyclin-D1 formed complexes with Bax, but not with Bak, Puma, Noxa, Bim, or Bad (Fig. 5D and Fig. S7C). As control for the experiment, the immunoprecipitation of a known partner of cyclin-D1, CDK4, was included (Fig. S7D). Silencing of Bax with siRNA in the lymphoma cells abrogated the therapeutic synergy between cyclin-D1 inhibition and ABT-737, indicating a prominent role of Bax in the resistance to the BH3 mimetic (Fig. S7E). These results reveal a mechanism in which the proapoptotic BAX protein is sequestrated by overexpressed cyclin-D1 in the cytoplasm of the lymphoma cells, thereby impeding apoptosis after ABT-737 exposure. After therapeutic depletion of cyclin-D1, BAX protein is released, exposing lymphoma cells to the ABT-737 action and facilitating apoptosis.
Fig. 5.
Inhibition of cyclin-D1 enhances apoptotic responses to ABT-737 therapy by modifying BAX conformational activation in MCL. (A) Flow cytometry revealed an increase in the unbound BAX protein fraction after cyclin-D1 silencing in CyD1-4–1Gy and CyD1-4–BCL2 lymphomas (31% ± 2% vs. 2% ± 2% and 19% ± 0.6% vs. 3% ± 0.5%; P < 0.001) and in the ABT-resistant JEKO1 cell line (4% ± 2% vs. 0.8% ± 0.2%; P < 0.05). Increments in the unbound fraction of BAX after cyclin-D1 silencing are shown. (B) IF analysis of cyclin-D1 (green) and BAX (red) in human MCL and murine lymphomas show that both proteins are detected in the cytoplasm, where they colocalize. Nuclei are contrasted with DAPI (blue). (C) Western blot analysis of cyclin-D1 and BAX in the cytoplasmic and nuclear cellular fractions of the human and murine lymphoma cells revealed that cyclin-D1 is preferentially present in the cytoplasm and only a small portion is observed in the nuclei, whereas BAX is a cytoplasmic protein. Lamin A/C and β-tubulin were used as controls of nuclear and cytoplasmic cellular fractions, respectively. (D) Immunoprecipitation of cyclin-D1 complexes shows the presence of BAX in JEKO1 cells and in CyD1-4–1Gy and CyD1-4–BCL2 lymphomas. (E) Roscovitine (Rosc) inhibited cyclin-D1 expression in human MCL cell lines and in mouse lymphomas in a dose-dependent manner. (F) Roscovitine (20–40 μM) increased the unbound BAX protein fraction in the MCL cell lines JEKO1 (24 ± 0.7% vs. 0.7 ± 0.09%; P < 0.001) and REC1 (23 ± 0.1% vs. 4.7 ± 0.01%; P < 0.01), and in CyD1-4–1Gy and CyD1-4–BCL2 mouse lymphomas (34 ± 2.9% vs. 1.22 ± 0.31% and 17 ± 0.01% vs. 0.8 ± 0.1%; P < 0.05). Increments in the unbound fraction of BAX after roscovitine exposure are shown. (G) Roscovitine (30 μM) had a synergistic therapeutic effect in combination with ABT-737 (250 nM) in the treatment of MCL-resistant cell lines and of CyD1-4–1Gy and CyD1-4–BCL2 mouse lymphomas. (H) Isolated mononuclear cells from four patients with MCL with leukemia disease were treated in vitro with roscovitine (10 μM) plus ABT-737 or ABT-737 alone. The global OS curve for the four patients after the different treatments is shown. (Inset) Western blot analysis for cyclin-D1 and BCL2 proteins.
Pharmacologic Inhibition of Cyclin-D1 and BCL2 Is an Effective Combination for Treating Human MCL.
Our experimental data suggest that therapy with ABT-737 could be selectively active in a fraction of MCL cases with increased BCL2 expression (∼15% of patients with MCL, according to Fig. 2 E–G and Fig. S2 D and E), but that with simultaneous cyclin-D1 silencing, the BH3 mimetic might be clinically effective in most patients with MCL irrespective of BCL2 expression. To begin to translate these findings to the clinical setting, we tested the therapeutic activity of the cyclin-D1/CDK inhibitor seliciclib (roscovitine), alone and in combination with ABT-737, in the human MCL cell lines and in the cyclin-D1–expressing mouse lymphomas (23). Roscovitine inhibited cyclin-D1 expression in all tested MCL cell lines and mouse lymphomas in a dose-dependent manner, increasing the unbound BAX protein fractions from 16% to 33% (Fig. 5 E and F). Thus, in the MCL cell lines as well as in the mouse lymphomas, the combination of roscovitine and ABT-737 resulted in decreased cell survival and massive apoptosis (Fig. 5G and Table S3). However, MCL cell lines represent advanced models of disease with therapeutic responses that might not be extrapolated to patient lymphomas. Therefore, fresh peripheral blood mononuclear cells were isolated from four unselected patients diagnosed with cyclin-D1+ MCL with leukemic disease and incubated with roscovitine and ABT-737. Responses to ABT-737 were correlated with BCL2 expression levels in three of the four cases. Nevertheless, the combination therapy of ABT-737 and roscovitine induced marked growth retardation and massive apoptosis in all cases irrespective of BCL2 expression, with responses comparable to those observed in the human MCL cell lines (Fig. 5H). Taken together, these data demonstrate that concomitant roscovitine and ABT-737 exposure is therapeutically effective in human MCL cell lines and in primary MCL cells. Our results highlight the potential benefit of simultaneously targeting cyclin-D1 and survival pathways for the effective treatment of most cases of human MCL, in agreement with the observations in the mouse lymphoma model. On the basis of these findings, we think that the synergistic combination of roscovitine and ABT-737 should be tested in a clinical trial with patients with MCL.
Discussion
Here we have defined a role of cyclin-D1 in deregulating apoptosis by interacting with BAX in the cytoplasm of MCL cells that may have therapeutic implications. A key element in our work is the murine lymphoma model generated by adding secondary changes to cyclin-D1–expressing B lymphocytes that were engrafted in immunocompromised mice. Silencing of cyclin-D1 in these mice showed that cyclin-D1 inhibition did not kill the lymphoma cells, but did sensitize them to apoptosis. Our data indicate the potential benefit of simultaneously targeting cyclin-D1 and survival pathways in patients with MCL.
The role of different oncogenes in initiating and maintaining cancer has previously been investigated in conditional transgenic mouse models. Remarkably, inactivation of some oncogenes was sufficient to revoke tumors, including BCR-ABL–induced leukemias, MYC-induced lymphomas and carcinomas, and lung carcinomas and melanomas after KRAS and HRAS inactivation (24). This concept has been termed “oncogene addiction.” However, in our mouse model, cyclin-D1 inhibition was not associated with lymphoma regression in vivo, indicating that this protein is not essential for MCL maintenance. This finding was not totally unexpected, given that the development of MCL differs from that of cancers driven by potent oncogenes. Thus, human MCL is thought to be generated by a multistep process initiated by CCND1/cyclin-D1 activation and followed by selected genetic changes that are necessary for tumor development (3). Indeed, our results are comparable with those from other mouse models that did not experience tumor regression after inactivation of the primary oncogene due to the presence of specific secondary genetic changes (16, 24). We speculate that in MCL cells, cyclin-D1 behaves as a weak oncogenic factor that is unable to induce malignancy directly, requiring other cooperating genetic hits to produce transformation. Likewise, single inactivation of cyclin-D1 is not sufficient to ensure tumor regression, but does unlock selected cooperating oncogenic pathways that may be used for therapeutic intervention. Accordingly, a potent therapeutic effect was observed after silencing cyclin-D1 and targeting the apoptotic machinery, which killed human and mouse lymphomas synergistically. These data suggest that cyclin-D1 may represent the “Achilles’ heel” in MCL cells that needs to be targeted to eventually cure the disease.
The mechanism underlying this effective therapeutic synergy may rely on the interaction of the overexpressed cyclin-D1 with the proapoptotic protein BAX, which was partially sequestrated in the cytoplasm of the lymphoma cells and functionally inactivated. Accordingly, therapeutic depletion of cyclin-D1 using siRNA or pharmacologic inhibitors released BAX protein, thereby facilitating ABT-737–mediated apoptosis. Our data indicate that along with deregulating the cell cycle, cyclin-D1 plays a role in modulating the apoptotic pathways in MCL cells. However, whether cyclin-D1 can also bind to BAX in other malignancies that commonly show cyclin-D1 expression, such as multiple myeloma or breast cancer, is currently not known. Nevertheless, the direct interaction of cyclin-D1 with BAX has previously been reported in human skin keratinocytes (25). In this line, cyclin-D1 has been shown to mediate apoptosis in B lymphocytes through modulation of molecular chaperones (26). Based on the putative interaction of cyclin-D1 with multiple proteins in the lymphoma cells and on its recently demonstrated role as a transcriptional regulator (27), we cannot discount the possibility that other factors participating in diverse molecular pathways are modified after cyclin-D1 inactivation. If so, then additional therapeutic synergies between anti–cyclin-D1 therapies and other compounds may exist, and these could be exploited to treat patients with MCL. In summary, our results provide a rational framework for the clinical evaluation of the combination of cyclin-D1/CDK inhibitors, such as roscovitine and ABT-737, in patients with MCL, a disease that urgently needs a curative therapy.
Materials and Methods
Human Cell Lines and Patient Samples.
Ten human-derived MCL cell lines and biopsy specimens from 183 patients diagnosed with MCL were studied. In addition, mononuclear cells isolated from fresh peripheral blood of four patients with leukemic MCL were used in the in vitro therapeutic experiments. All clinical samples were obtained before initiation of therapy. Human investigations were performed after approval by the Institutional Review Board on Scientific and Ethical Affairs.
Generation of Human Xenografts in Mice and of the Cyclin-D1–Driven Mouse Lymphoma Models
Mouse experiments were performed in the Center for Applied Medical Research's animal core facilities after approval by the local Animal Ethics Committee. Mouse xenografts were generated and monitored as reported previously (28). To generate the cyclin-D1–expressing lymphoma model, a 1.1-kb fragment of human CCND1 cDNA was amplified by PCR, digested with EcoRV, and ligated to the vector Combit-TA at the ScaI site (16).
Detailed descriptions of mouse generation and all experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Saul Rosenberg and Jane Hoff-Smith (Abbott Laboratories) for providing ABT-737, Drs. S. Wang and J. Chen (University of Michigan) for providing TW-37, Dr. I. Sanchez-Garcia for providing the Combit-TA vector, Drs. C. Panizo and F. Carbonell for providing MCL samples, Drs. M. Collantes and I. Peñuelas for performing the mouse imaging studies, Dr. E. Guruceaga for performing the bioinformatics analysis, Dr. E. Ciordia and E. Elizalde for support with animal care, Silvana B. De Lorenzo for technical assistance, and Philip Mason for manuscript editing. J.A.M.-C.’s laboratory was supported by grants from the Spanish Ministries of Science, Innovation and Health (PI081878, PS09/02437, and RTICC-ISCIII-RD06/0020/0088), the Navarra Government, the Ramon y Cajal career development program (to J.A.M.-C.), a Spanish Association Against Cancer (AECC) fellowship (to R.M.), the Cooperative Spanish-French Transpinenaic Research Grant on Innovative Therapies Against Leukemia/Lymphoma (CITTIL-FEDER) (to F.P. and J.A.M.-C.), and the Foundation for Applied Medical Research of the University of Navarra. M.E.F.-Z.’s laboratory was supported by the Mayo Clinic Cancer Center's Division of Oncology Research, Mayo/Iowa Lymphoma SPORE P50 CA097274, and the Leukemia and Lymphoma Society Translational Research Program. I.S.L.’s laboratory was supported by National Institutes of Health Grants CA109335 and CA122105 and the Dwoskin Family Foundation and Fidelity Foundation.
Footnotes
Conflict of interest statement: R.S. has received honoraria from Abbott.
Data deposition: Gene expression microarray data reported in this paper have been submitted to Gene Expression Omnibus (accession no. GSE25613).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018941108/-/DCSupplemental.
References
- 1.Harris NL, et al. The World Health Organization classification of hematological malignancies: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13:193–207. doi: 10.1038/modpathol.3880035. [DOI] [PubMed] [Google Scholar]
- 2.Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999;36:115–127. [PubMed] [Google Scholar]
- 3.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: Perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 4.Bodrug SE, et al. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagawa H, et al. Genome-wide array-based CGH for mantle cell lymphoma: Identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–1358. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- 6.Stilgenbauer S, et al. Molecular characterization of 11q deletions points to a pathogenic role of the ATM gene in mantle cell lymphoma. Blood. 1999;94:3262–3264. [PubMed] [Google Scholar]
- 7.Rubio-Moscardo F, et al. Mantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcome. Blood. 2005;105:4445–4454. doi: 10.1182/blood-2004-10-3907. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23:6409–6414. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 9.Dreyling M, Hiddemann W. Dose-intense treatment of mantle cell lymphoma: Can durable remission be achieved? Curr Opin Oncol. 2008;20:487–494. doi: 10.1097/CCO.0b013e32830b61c2. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor OA. Mantle cell lymphoma: Identifying novel molecular targets in growth and survival pathways. Hematology Am Soc Hematol Educ Program. 2007;2007:270–276. doi: 10.1182/asheducation-2007.1.270. [DOI] [PubMed] [Google Scholar]
- 11.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114:1469–1476. doi: 10.1182/blood-2009-02-179739. [DOI] [PubMed] [Google Scholar]
- 12.Klier M, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.Traggiai E, et al. Development of a human adaptive immune system in cord blood cell–transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 15.Mestre-Escorihuela C, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109:271–280. doi: 10.1182/blood-2006-06-026500. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Mancera PA, et al. Cancer development induced by graded expression of Snail in mice. Hum Mol Genet. 2005;14:3449–3461. doi: 10.1093/hmg/ddi373. [DOI] [PubMed] [Google Scholar]
- 17.Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci. 2006;97:575–581. doi: 10.1111/j.1349-7006.2006.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury JD, et al. Expression of Mcl-1 in mantle cell lymphoma is associated with high-grade morphology, a high proliferative state, and p53 overexpression. J Pathol. 2003;199:90–97. doi: 10.1002/path.1254. [DOI] [PubMed] [Google Scholar]
- 19.Beà S, et al. Increased number of chromosomal imbalances and high-level DNA amplifications in mantle cell lymphoma are associated with blastoid variants. Blood. 1999;93:4365–4374. [PubMed] [Google Scholar]
- 20.Hernández L, et al. CDK4 and MDM2 gene alterations mainly occur in highly proliferative and aggressive mantle cell lymphomas with wild-type INK4a/ARF locus. Cancer Res. 2005;65:2199–2206. doi: 10.1158/0008-5472.CAN-04-1526. [DOI] [PubMed] [Google Scholar]
- 21.Quintanilla-Martinez L, et al. Sequestration of p27Kip1 protein by cyclin D1 in typical and blastic variants of mantle cell lymphoma (MCL): Implications for pathogenesis. Blood. 2003;101:3181–3187. doi: 10.1182/blood-2002-01-0263. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 23.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 24.Felsher DW. Cancer revoked: Oncogenes as therapeutic targets. Nat Rev Cancer. 2003;3:375–380. doi: 10.1038/nrc1070. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed KM, Fan M, Nantajit D, Cao N, Li JJ. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene. 2008;27:6738–6748. doi: 10.1038/onc.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roué G, Pichereau V, Lincet H, Colomer D, Sola B. Cyclin D1 mediates resistance to apoptosis through up-regulation of molecular chaperones and consequent redistribution of cell death regulators. Oncogene. 2008;27:4909–4920. doi: 10.1038/onc.2008.126. [DOI] [PubMed] [Google Scholar]
- 27.Bienvenu F, et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature. 2010;463:374–378. doi: 10.1038/nature08684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter-Larrea JA, et al. Reversion of epigenetically mediated BIM silencing overcomes chemoresistance in Burkitt lymphoma. Blood. 2010;116:2531–2542. doi: 10.1182/blood-2010-02-268003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.