Abstract
Predicted effects of climate change include high extinction risk for many species, but confidence in these predictions is undermined by a perceived lack of empirical support. Many studies have now documented ecological responses to recent climate change, providing the opportunity to test whether the magnitude and nature of recent responses match predictions. Here, we perform a global and multitaxon metaanalysis to show that empirical evidence for the realized effects of climate change supports predictions of future extinction risk. We use International Union for Conservation of Nature (IUCN) Red List criteria as a common scale to estimate extinction risks from a wide range of climate impacts, ecological responses, and methods of analysis, and we compare predictions with observations. Mean extinction probability across studies making predictions of the future effects of climate change was 7% by 2100 compared with 15% based on observed responses. After taking account of possible bias in the type of climate change impact analyzed and the parts of the world and taxa studied, there was less discrepancy between the two approaches: predictions suggested a mean extinction probability of 10% across taxa and regions, whereas empirical evidence gave a mean probability of 14%. As well as mean overall extinction probability, observations also supported predictions in terms of variability in extinction risk and the relative risk associated with broad taxonomic groups and geographic regions. These results suggest that predictions are robust to methodological assumptions and provide strong empirical support for the assertion that anthropogenic climate change is now a major threat to global biodiversity.
Keywords: anthropogenic warming, elevated temperature, extinction crisis, climate warming
Many scientists argue that we are entering the sixth great mass extinction and that anthropogenic climate change is one of the major threats to global biodiversity (1–3). Comprehensive, multitaxon reviews suggest that 10–70% of plant and animal species assessed so far could be at increased risk of extinction from climate change (4) or that by 2050, climate-induced changes in habitat will commit 15–37% of species to extinction (1). Both these estimates are based on approaches that can be sensitive to ecological and methodological assumptions (5–8), and the latter study considers only geographical range shifts resulting from changes in temperature and rainfall (1). Many species are also expected to be adversely affected by changes in sea-level and ocean chemistry (9), and the impacts of climate change may include breakdowns in biological interactions as species respond individualistically to climate change (10), loss of habitat because of sea-level rise (11), and higher mortality because of increased ocean acidity (12). The spectrum of approaches used to predict ecological responses to climate change has also broadened in recent years, enabling more robust estimates of future changes to be made (13). Here, we use International Union for Conservation of Nature (IUCN) Red List Criteria (14) to derive estimates of extinction risk from a wide range of climate impacts, ecological responses, and methods of analysis. Importantly, the broad evidence base that now exists for realized ecological responses to recent climate change allows us to validate future predictions by comparison with responses that have already been observed.
We identified 130 observed and 188 predicted ecological responses to climate change using a robust review of 10 leading scientific journals from 2005 to 2009 (Methods). The responses included documented changes to extinction risk, population size, and geographic range size for 305 taxa from all major groups of organisms, covering a high proportion of the global terrestrial and marine surface (Table S1). All 318 climate change responses were expressed in terms of extinction risk using IUCN Red List criteria, which is possible, because the threshold values used to assign IUCN categories on the basis of population decline are linearly related to the logit transform of threshold extinction risk values (Fig. S1). Estimates of the mean extinction risk for taxa and the proportion subject to varying degrees of extinction risk were derived with an intercept-only generalized linear model with an inflated β-error distribution and logit link function (Methods).
A range of factors relating to the selection of study systems (climate impact type, taxon, and region) and the publication of results could influence whether the sampled climate change responses gave unbiased estimates of mean extinction risk. To determine whether there was publication bias, we investigated whether extinction risk was related to the journal in which the study was published and also, created a funnel plot of extinction risk against sample size (Fig. S2). The presence of asymmetry in a funnel plot signifies bias to the publication of significant results (15). To account for biases in the type of impact studied, we incorporated impact type as a factor into models and compared the results of averaging across impact types with those results obtained by averaging across studies. To account for possible phylogenetic nonindependence of extinction risk, we constructed a phylogenetic tree and added the residual of each tip relative to its branch to the mean across all tips in instances where branches were significant. Research carried out in regions where taxa are disproportionately threatened by climate change could also bias overall estimates of threat, and therefore, we controlled for spatial patterns in extinction risk by spatially averaging our results (Methods).
To examine whether there were consistent ecoregional and taxonomic patterns across studies making predictions and studies reporting empirical data, we subdivided our data into three major taxonomic groups (plants, invertebrates, and vertebrates) and four major ecoregions: (i) polar and boreal, (ii) temperate, (iii) tropical and subtropical, and (iv) marine; we compared observations with predictions.
Results and Discussion
Across all studies, the mean extinction risk over 90 y (i.e., to 2100) was 11.2%. Separating projections of extinction risk based on predicted and observed responses yielded a mean extinction risk of 6.7% based on predictions but 14.7% based on observations (Table 1).
Table 1.
Projected extinction risk by 2100 based on observations and predictions
| Expected extinction risk |
>50% probability of extinction |
Threatened with extinction |
|||||||
| Method | All | Observed | Predicted | All | Observed | Predicted | All | Observed | Predicted |
| Estimate derived from values given in each study | 0.112 | 0.147 | 0.067 | 0.069 | 0.120 | 0.019 | 0.291 | 0.318 | 0.076 |
| Estimate obtained by averaging across impact types | 0.116 | 0.158 | 0.061 | 0.073 | 0.132 | 0.035 | 0.298 | 0.333 | 0.204 |
| Estimate obtained by averaging across taxa | 0.104 | 0.140 | 0.061 | 0.051 | 0.104 | 0.049 | 0.296 | 0.329 | 0.375 |
| Estimate obtained by spatially averaging across the globe | 0.118 | 0.139 | 0.103 | 0.000 | 0.002 | 0.000 | 0.620 | 0.648 | 0.600 |
Expected extinction risk is based on the β-distribution of observed or predicted extinction risks (npredicted = 188; nobserved = 130). Taxa categorized as threatened were those taxa exceeding a modeled extinction risk by 2100 of 0.09. IUCN categories: CR, critically endangered; EN, endangered; VU, vulnerable.
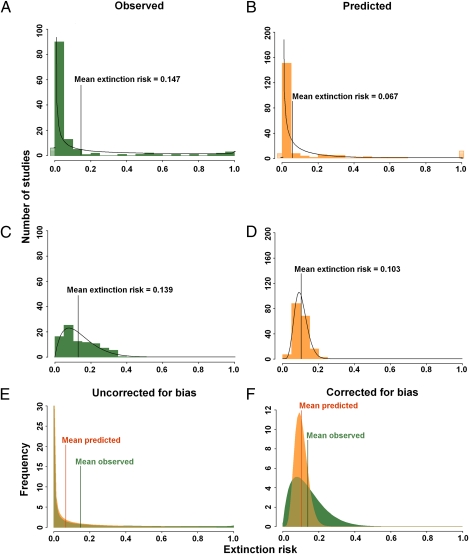
The proportion of taxa qualifying as threatened by 2100 using IUCN criteria would be 7.6% based on predictions and 31.7% based on observations. The proportion of taxa more likely to go extinct than not was 1.9% based on predictions but 12.0% based on observations (Table 1). The degree of variability in extinction risk across observations and predictions is similar (Fig. 1), with the majority of taxa (>80%) at low risk (<5%) of extinction; this finding suggests that predictions are not invalidated by methodological assumptions. The symmetry of the funnel plots of extinction risk against sample size (Fig. S2) suggests very little evidence of publication bias. However, extinction risk for both observations and predictions was affected by climate impact type. More studies reported threats from changes in temperature and rainfall, but the few studies on the effects of reductions in sea ice and changes in ocean circulation patterns showed higher predicted extinction risk (Table S2). More studies on effects such as changes in oceanic circulation patterns and acidity on marine organisms would improve estimates of extinction risk. Nevertheless, models that controlled for climate impact type did not lead to marked changes in mean extinction risk, either for empirical observations or predictions (Fig. 1 and Table 1).
Fig. 1.
Proportion of taxa subject to varying degrees of extinction risk by 2100. Actual proportion derived from studies (histogram bars) together with a fitted β-probability function (black curve). The horizontal hatched bars (actual) and horizontal black lines (modeled) represent the number of studies with an extinction risk of zero or one. (A and B) Uncorrected estimates derived from observed (A) and predicted (B) data. (C and D) Estimates accounting for biases (Methods) derived from observed (C) and predicted (D) data. (E and F) Modeled probability density functions (green, observed; orange, predicted) overlaid to show that, when uncorrected (E), the variance in extinction risk derived from observed and predicted data is similar, and when corrected (F), the means are similar.
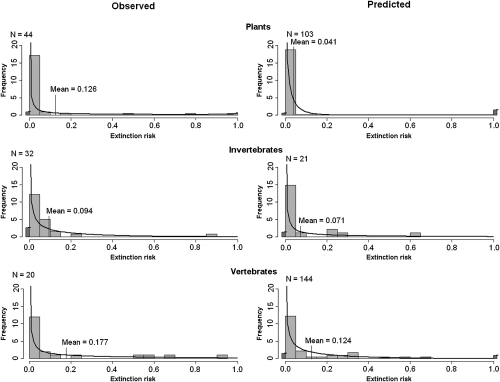
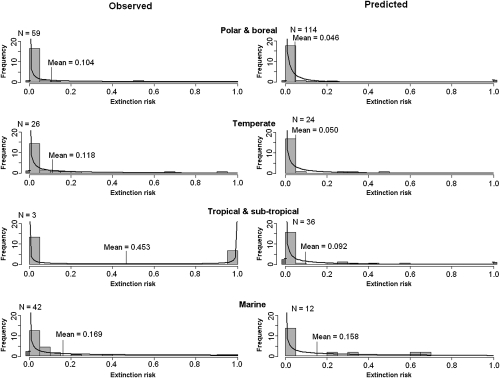
There was evidence of phylogenetic nonindependence of extinction risk, with both observations and predictions suggesting high levels of threat to vertebrates and lower levels of threat to plants and invertebrates. This finding was also supported by comparisons of extinction risk within each of these three major taxonomic groups (Fig. 2). There was a high degree of consistency between observations and predictions, with most taxa observed to be at high risk also predicted to be highly threatened in the future. After accounting for phylogeny, estimated extinction risks from observations decreased to 14%, whereas those risks from predictions did not change (Table 1). Thus, observed responses may be slightly exaggerated by work on more threatened taxa. Spatial averaging of results did not alter estimates of observed extinction risk (13.9%), whereas predicted risk increased (10.3%), implying that models of future effects of climate change may, to some extent, have neglected regions subject to higher levels of threat (Table 1). Comparisons of extinction risk in broad ecoregions suggest that marine taxa are particularly threatened and that taxa in the terrestrial tropics and subtropics are less threatened than those taxa in terrestrial temperate and high latitude areas, and the relative threat predicted for different regions was quite well-supported by empirical observations (Fig. 3). However, for both observations and predictions, there were few studies from the terrestrial tropics: the larger number of predictions from the subtropical and tropical category is mostly of tree responses in Mexico (16). In the marine environment, there is a bias to research on corals, which may be disproportionately affected by climate change. More research from tropical areas, where most species occur and are expected to have climates for which no current analog exists (17) and on a greater variety of marine taxa, would improve estimates of the realized threat to biodiversity from climate change.
Fig. 2.
Frequency distribution of extinction risk by 2100 in (Left) observed and (Right) predicted studies of taxa: (Top Left and Top Right) plants, (Middle Left and Middle Right) invertebrates, and (Bottom Left and Bottom Right) vertebrates. Actual proportion derived from studies (histogram bars) together with a fitted β-probability function (black curve). The dark bars (actual) and horizontal black lines (modeled) represent the frequency of studies with an extinction risk of zero or one. Data are scaled such that the total area of histogram bars and under the modeled extinction risk line is equal to one. N is the number of samples in each category.
Fig. 3.
Frequency distribution of extinction risk by 2100 in (Left) observed and (Right) predicted studies of ecoregions: (row 1) polar and boreal, (row 2) temperate, (row 3) tropical and subtropical, and (row 4) marine. Actual proportion derived from studies (histogram bars) together with a fitted β-probability function (black curve). The dark bars (actual) and horizontal black lines (modeled) represent the frequency of studies with an extinction risk of zero or one. Data are scaled such that the total area of histogram bars and under the modeled extinction risk line is equal to one. N is the number of samples in each category.
Our approach, using IUCN criteria to translate between population or range changes and extinction risk, has allowed us to include more examples than simply population viability studies, which estimate extinction risk directly. We, thus, reduce possible bias in threat levels that could result, because particular methodologies might focus on endangered species. However, we make the assumption that the threshold values for criteria relating to decline and extinction risk are comparable (SI Methods). Although the rules used to assign taxa to IUCN categories represent internationally accepted decision tools in conservation biology (14, 18) and broad consistency between criteria was sought during their development (19), variation among species makes it difficult to validate the equivalence of thresholds for different criteria (20). Moreover, phylogenetic nonindependence of extinction risk could also reflect difficulties in assessments for poorly known taxa, with generally fewer experts and less data available (14). For example, the apparent low threat to invertebrates may partly reflect the lack of detailed understanding of climate threats facing most insects, with the majority of studies being on Lepidoptera (21). Direct predictions of extinction, as determined, for example, by population viability analyses, yielded higher estimates of extinction risk than estimates provided by changes in population and range size (Fig. S3). However, we suspect that this finding is primarily caused by these studies focusing on particularly endangered species. Our results were not unduly sensitive to the assumed relationship between range change, population change, and extinction risk (Table S3), suggesting that our overall estimates of extinction risk are relatively robust to any lack of equivalency among IUCN criteria. Furthermore, interactions between taxonomic group and method did not significantly affect estimates of extinction risk, suggesting that variation in the degree of difficulty in estimating extinction risk across categories is unlikely to invalidate our cross-taxonomic findings.
Estimated extinction risk was not related to the journal of publication or the time period over which observations were carried out or predictions were made. The sample size in each study could still influence the reliability of results, but we did not use formal metaregression techniques for two reasons. First, it was our intention to report means and variances in extinction risk across taxa rather than provide a single measure. Each estimate is derived from different taxa and could stem from any one of a number of different responses. Consequently, the effect being measured is not common across studies, and there is no reason to attribute a higher weighting to studies with larger sample sizes. Second, many studies do not report complete or comparable measures of uncertainty. Therefore, the use of metaregression to calculate the uncertainty in extinction risk across all studies would not be valid. Consequently, we attached the same weighting to all studies, irrespective of sample size. This weighting is unlikely to cause major bias in estimates of extinction risk, because there was no evidence of a consistent relationship of reported extinction risks with either sample size or the number of species studied (SI Methods and Fig. S3).
There are many unknowns when projecting declines in biodiversity, and the values here should be interpreted with caution. Nevertheless, our results were robust to publication, taxonomic, geographical, and impact-type biases, and assumed theoretical relationships between extinction risk, population decline, and range change. Furthermore, the degree of variance is also similar across observations and predictions, suggesting that predictions are not invalidated by methodological assumptions. Given that climate change is expected to accelerate and hence, exacerbate impacts, empirical evidence suggests that many predictions of extinction risk may be somewhat conservative. However, in terms of consistent phylogenetic and ecoregional patterns, the results suggest that realized ecological responses to climate change support predictions of future change. Our estimates of extinction risk are lower than previous estimates of the proportion of species committed to extinction by 2050 (1), but they are within the same order of magnitude. Moreover, commitment to extinction is not the same as extinction risk, because decades may elapse between habitat loss or climate change and the resultant species-level extinctions (22). Consequently, one would expect estimated extinction risk over a specified period to be lower.
Our results lend support to the contention, based on entirely different data and methods (1), that anthropogenic climate warming at least ranks alongside other recognized threats to global biodiversity. Based on published results, we endeavor to distinguish between responses to climate and other drivers of change, although in many cases, the mechanisms behind species responses to climate change are not known. Several studies suggest that changes to biotic interactions have led to increased extinction risk for at least one interacting species (10, 23). Habitat degradation (24), invasive species (25), and overexploitation (26) play additional roles, and interactions among these threats and climate change will increasingly threaten populations of species. In addition, rapid climate change has the potential to overwhelm the capacity for adaptation in many populations, reducing the ability to resist and recover from other environmental stressors (27). Our metaanalysis showing high predicted levels of extinction, backed up by consistent data for changes that have already occurred, shows the need to give climate change high priority in conservation planning and to communicate its potentially wide-ranging consequences to policy makers and the wider public.
Methods
Details of the studies and methods are provided in SI Methods and Table S1.
Selection of Studies.
We reviewed 1,120 papers published from 2005 on in 10 leading journals focused on general science, ecology, or conservation. We searched all papers with climate change in the title, abstract, or keywords in Biological Conservation, Conservation Biology, Ecological Applications, Ecology Letters, Journal of Applied Ecology, Nature, Proceedings of the Royal Society of London Series B Biological Sciences, and Science, and all papers with climate change and biodiversity in Global Change Biology and PNAS. Those papers in which extinction probabilities, IUCN Red List categories, or a change in population size or range were reported were short-listed for metaanalysis. We extracted data only from those articles in which changes could primarily be attributed to climate change or where climate was distinguished from other effects. From each paper, we recorded the taxon and number of species, the start and end of the study period, the type of climate change impact (changes in temperature and/or rainfall, ocean circulation patterns, ocean acidity, or sea ice, or responses to habitat change such as loss of habitat because of sea-level rise), and the number of spatial and temporal replicates. We also specified whether the response was observed or predicted. In all, we extracted data from 74 studies (32 observations and 42 predictions), providing 318 (130 observations and 188 predictions) taxon-specific climate change response estimates (SI Methods and Table S1).
Estimating Extinction Risk.
We used IUCN Red List criteria to derive estimates of extinction risk from changes in population or range size, with a change in range size measured as the change in the area occupied. We assumed that a change in range is directly equivalent to a change in population size, an approach that is likely to give conservative estimates of population decline (28). Extinction risks can be standardized over any given period using multiple event probability theories (Eq. 1):
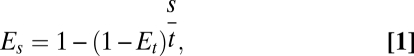 |
where Es is the extinction probability of the desired time period s and Et is the extinction probability over time period t. When extinction probabilities associated with each of the three IUCN Red List categories are standardized to 55.628 y and logit transforms are applied to ensure a continuous range of values, there is a perfectly linear relationship with the equivalent population size reductions over 10 y in each of the categories (Fig. S1). It is, thus, possible to infer extinction risk for any given change in population size, including increases. We also included studies where species had been assigned to IUCN Red List categories by assuming conservatively that their extinction risk corresponded to the threshold value for the category in which they had been placed. We assessed extinction risk over a 90-y period to give estimates for 2100. Full details of the method used to infer extinction risk from each study are given in Table S1. Because extinction estimates are constrained to values between zero and one and were zero- and one-inflated, estimates of the mean extinction risk for taxa and the proportion subject to varying degrees of extinction risk were derived by fitting a zero- and one-inflated β-error distribution to the data with a logit link function using the R (29) package GAMLSS (30).
To test for publication bias to studies that reported a high extinction risk, we examined the relationship between extinction risk and sample size. To test for researcher bias to species particularly threatened by climate change, we applied the same method using the number of species studied instead of sample size. There was no evidence of either researcher or publication bias (SI Methods and Fig. S2).
The potential effects of mean time of study, journal, and impact type on extinction estimates were assessed using a generalized linear model in R (29). All combinations of variables, including the null model, were tried, and the final model was selected using Akaike's Information Criterion (31). For both observed and predicted data, models in which impact type was included yielded the lowest Akaike's Information Criterion. We, thus, averaged across impact types to give a revised estimate.
To examine whether there were consistent taxonomic patterns across studies making predictions and empirical data, we subdivided our data into three major taxonomic groups: plants, invertebrates, and vertebrates. Bacteria, fungi, and taxa such as algae that were resolved to insufficient taxonomic detail were excluded from these analyses. To examine whether there were consistent ecoregional patterns across studies, we subdivided our data into four major ecoregions: (i) polar and boreal (ice sheets, tundra, and taiga), including studies of high altitude taxa at mid-latitudes, (ii) temperate (forest and steppe), (iii) tropical and subtropical, including xeric and Mediterranean habitats, and (iv) marine (all latitudes). Freshwater wetland taxa were assigned to the ecoregion in which the wetland was located. Studies spanning more than one ecoregion were excluded from these analyses.
Phylogenetic Relationships.
A composite phylogeny of all study taxa was constructed using information contained in ref. 32, with branch lengths scaled to be approximately equal to time since divergence. Often, extinction estimates were for groups of species only, and in such instances, a dummy species was created that branched from the node encompassing all species within the group. Using the standardized normal residuals from the Generalized Linear Model (GLM) modeling, the mean residual value across all descendant terminal taxa was then calculated for each branch using the analysis of traits function in Phylocom 4.1 (33). The significances of branch values relative to the mean value across all terminal taxa were calculated by randomizing values for each taxon across all tips. To control for the extent to which particular taxa differed in terms of their extinction risk when calculating global estimates, the residual of the tip relative to the branch was added to the mean across all tips in instances where branches were significant. To test the robustness of our results to uncertainties associated with divergence time estimation, we also ran our analyses on the same tree but with branch lengths set to one. This change did not affect observed estimates of extinction risk, but predicted estimates increased from 6.1% to 6.5%.
Spatial Relationships.
The geographical boundaries of all study sites from which extinction estimates were derived were mapped as polygons in ArcGIS 9.2 using a Cylindrical Equal Area projection (ESRI). The centroids of all study areas were then calculated, and spatial kriging with a spherical semivariogram model was performed using the Spatial Analyst tool in ArcGIS. Because it is not possible to define a projection that preserves true distances between all points on the globe, a North Pole Azimuthal Equidistant projection was used to perform spatial kriging in the northern hemisphere, and a South Pole Azimuthal Equidistant projection was used to perform spatial kriging in the southern hemisphere. The two hemispheres were then joined and converted back to a cylindrical equal area projection with a 1-km2 resolution. The individual pixel values were then exported as an ASCII file, and a zero- and one-inflated β-error distribution with a logit link function was fitted to these data to estimate the mean extinction risk and the proportion of taxa subject to varying degrees of extinction risk.
Supplementary Material
Acknowledgments
We thank J. Bennie, M. Evans, C. Parmesan, T. Tregenza, and two anonymous reviewers for their feedback. This research was partly funded by the European Social Fund Project 09099NCO5.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017352108/-/DCSupplemental.
References
- 1.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 2.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 3.Wake DB, Vredenburg VT. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 5.Araujo MB, Pearson RG, Thuiller W, Erhard M. Validation of species-climate impact models under climate change. Glob Change Biol. 2005;11:1504–1513. [Google Scholar]
- 6.Thuiller W, et al. Biodiversity conservation: Uncertainty in predictions of extinction risk. Nature. 2004;430:1–33. doi: 10.1038/nature02716. [DOI] [PubMed] [Google Scholar]
- 7.Pearson RG, et al. Model-based uncertainty in species range prediction. J Biogeogr. 2006;33:1704–1711. [Google Scholar]
- 8.Thuiller W. Patterns and uncertainties of species' range shifts under climate change. Glob Change Biol. 2004;10:2020–2027. doi: 10.1111/gcb.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson RA, et al. Travelling through a warming world: Climate change and migratory species. Endanger Species Res. 2009;7:87–99. [Google Scholar]
- 10.Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc R Soc Lond B Biol Sci. 1998;265:1867–1870. [Google Scholar]
- 11.Fish MR, et al. Predicting the impact of sea-level rise on Caribbean sea turtle nesting habitat. Conserv Biol. 2005;19:482–491. [Google Scholar]
- 12.Orr JC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 13.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Mace GM, et al. Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv Biol. 2008;22:1424–1442. doi: 10.1111/j.1523-1739.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Mendoza L, Arriaga L. Modeling the effect of climate change on the distribution of oak and pine species of Mexico. Conserv Biol. 2007;21:1545–1555. doi: 10.1111/j.1523-1739.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322:258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 18.Akcakaya HR, et al. Making consistent IUCN classifications under uncertainty. Conserv Biol. 2000;14:1001–1013. [Google Scholar]
- 19.IUCN Standards and Petitions Working Group. Guidelines for Using the IUCN Red List Categories and Criteria. Gland, Switzerland: World Conservation Union; 2010. [Google Scholar]
- 20.Akcakaya HR, Butchart SHM, Mace GM, Stuart SN, Hilton-Taylor C. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Glob Change Biol. 2006;12:2037–2043. [Google Scholar]
- 21.Wilson RJ, Maclean IMD. Recent evidence for the climate change threat to Lepidoptera and other insects. J Insect Conserv. 2011;15:259–268. [Google Scholar]
- 22.Krauss J, et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett. 2010;13:597–605. doi: 10.1111/j.1461-0248.2010.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 24.Travis JMJ. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc Biol Sci. 2003;270:467–473. doi: 10.1098/rspb.2002.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dukes JS, Mooney HA. Does global change increase the success of biological invaders? Trends Ecol Evol. 1999;14:135–139. doi: 10.1016/s0169-5347(98)01554-7. [DOI] [PubMed] [Google Scholar]
- 26.Schindler DW. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can J Fish Aquat Sci. 2001;58:18–29. [Google Scholar]
- 27.Jump AS, Peñuelas J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol Lett. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 28.Cowley MJR, et al. Flight areas of British butterflies: Assessing species status and decline. Proc R Soc Lond B Biol Sci. 1999;266:1587–1592. [Google Scholar]
- 29.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 30.Stasinopoulos DM, Rigby RA. Generalized additive models for location scale and shape (GAMLSS) in R. J Stat Softw. 2007;23:1–46. [Google Scholar]
- 31.Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 32.Maddison DR. The Tree of Life Web Project. 2007. Available at http://tolweb.org/. Accessed on October 15, 2010.
- 33.Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.