Abstract
ChIP coupled with next-generation sequencing (ChIP-seq) has revolutionized whole-genome mapping of DNA-binding protein sites. Although ChIP-seq rapidly gained support in eukaryotic systems, it remains underused in the mapping of bacterial transcriptional regulator-binding sites. Using the virulence-required iron-responsive ferric uptake regulator (Fur), we report a simple, broadly applicable ChIP-seq method in the pathogen Vibrio cholerae. Combining our ChIP-seq results with available microarray data, we clarify direct and indirect Fur regulation of known iron-responsive genes. We validate a subset of Fur-binding sites in vivo and show a common motif present in all Fur ChIP-seq peaks that has enhanced binding affinity for purified V. cholerae Fur. Further analysis shows that V. cholerae Fur directly regulates several additional genes associated with Fur-binding sites, expanding the role of this transcription factor into the regulation of ribosome formation, additional transport functions, and unique sRNAs.
Keywords: chromatin, immunoprecipitation, pathogenic, nickel
Transcriptional regulators play a critical role in cellular responses by altering gene-expression patterns that ultimately lead to proteomic and phenotypic changes. Expression profiles of bacteria lacking transcriptional regulators have been used successfully to broadly define genes impacted by the deleted regulator (1–4). Although providing informative data, transcriptional analysis alone cannot distinguish between direct and indirect regulatory effects or identify regulated genes that may be transcriptionally silent under the test conditions. The most direct means to locate genes controlled by a transcriptional regulator is through ChIP followed by sequence identification of the DNA fragments associated with the precipitated regulator (reviewed in ref. 5). Coupling ChIP with microarray technology (ChIP-ChIP) provided some of the first data on genome-wide transcriptional factor binding locations in both prokaryotic and eukaryotic systems (reviewed in refs. 6 and 7). Recent technological advances have allowed ChIP to be coupled with next-generation sequencing (ChIP-seq), which offers greater coverage, higher resolution and less noise and requires less sample than ChIP-ChIP (8).
After its introduction in 2007, ChIP-seq was embraced quickly for analysis of eukaryotic transcription factors (reviewed in ref. 8) but has yet to be used significantly to study bacterial transcriptional regulators. To our knowledge, only two investigations using ChIP-seq in bacteria have been published, reporting the mapping of the chromatin-remodeling proteins HN-S and Fis in Escherichia coli (9) and the DosR transcriptional regulator in Mycobacterium tuberculosis (10). This paucity of studies may reflect a lack of ChIP-quality antibodies for bacterial proteins (11) or suboptimal expression of the regulator of interest under laboratory conditions.
The bacterial ferric uptake regulator (Fur) protein regulates iron transport and homeostasis in many bacteria (reviewed in refs. 12–14). Iron is required for enzymatic functions; however, excess free iron can be detrimental by increasing oxidative damage (15). When bound to Fe2+, Fur binds to specific DNA sites called “Fur boxes” located near or in the promoter region of target genes, blocking access to RNA polymerase and repressing gene expression.
The description of the Fur box and its association with Fur is complex (refs. 16 and 17 and reviewed in refs. 13 and 18). A Fur box was described first in E. coli as a 19-bp palindromic sequence accommodating a Fur dimer (19); however, some Fur-binding sites in E. coli match only 11 of 19 predicted consensus bases. Fur boxes have been predicted in other bacteria, including Vibrio cholerae, based on motif alignments of 5′ UTRs of Fur-regulated genes (20). Several DNA-binding characteristics of Fur cannot be explained by a simple palindromic sequence. Foot-printing experiments show that Fur protects a region significantly larger than the Fur box, suggesting it interacts with DNA outside the predicted region. Furthermore, multiple Fur dimers can polymerize, wrapping around DNA into regions that do not have matching Fur box consensus sequences (21, 22). To help explain these and other phenomena, alternative Fur boxes have been proposed, including a sequence comprising three 6-bp repeats (23) and overlapping 13-bp 7-1-7 motifs (24).
Deletion of Fur attenuates several pathogenic bacteria including V. cholerae, the Gram-negative bacterium that causes the potentially lethal diarrheal disease cholera (1). Detailed microarray studies of V. cholerae Fur (vcFur)-dependent gene regulation have shown that vcFur is involved predominately in the negative regulation of iron acquisition or metabolism genes (1). Similar results have been observed in Fur studies in diverse groups of bacterial pathogens (1–4). In nearly all cases, expression studies have been performed during logarithmic growth and often in defined rich medium. However, vcFur protein levels have been shown to increase from 2,500 vcFur molecules per cell during logarithmic growth to 7,500 molecules per cell in stationary phase (25). The amount of Fur present may influence its binding to weak Fur boxes or competition with other DNA-binding factors. Different growth phases and conditions also may influence the amount of additional transcription factors or chromatin structural proteins that promote or hinder vcFur DNA binding. Although the general role of Fur in iron homeostasis is well documented, a better understanding of the vcFur regulon may help answer remaining questions about its complete role in gene regulation, its physical interaction with DNA, and its effect on host–pathogen interactions.
To define the vcFur regulon more completely, we demonstrate a simple, broadly applicable platform for mapping transcription factor-binding sites in V. cholerae using ChIP-seq. We compared available transcriptional responses of fur mutants with our genomic binding-location data to identify direct and indirect regulatory targets of vcFur as well as additional binding sites. Our results provide biochemical validation of previous direct vcFur regulatory predictions, define a vcFur box with enhanced binding to vcFur and implications for vcFur-DNA binding, and identify additional roles for vcFur in regulation of ribosome formation, transport functions, and sRNAs. Our results validate the use of this ChIP-seq method for bacterial transcriptional regulators, provide support for combining transcription regulatory binding-site information with available expression analysis, and significantly broaden the network of influence of this nearly ubiquitous bacterial transcriptional regulator.
Results
ChIP-Seq Identifies Direct and Indirect Targets Among vcFur-Regulated Genes.
Fur-dependent regulation of various genes has been shown to vary with growth phase and condition (26–28). vcFur protein levels triple upon entry into stationary phase (25), potentially altering the spectrum of its DNA-binding targets. We hypothesized that higher vcFur levels would better reveal the full spectrum of its genomic binding sites.
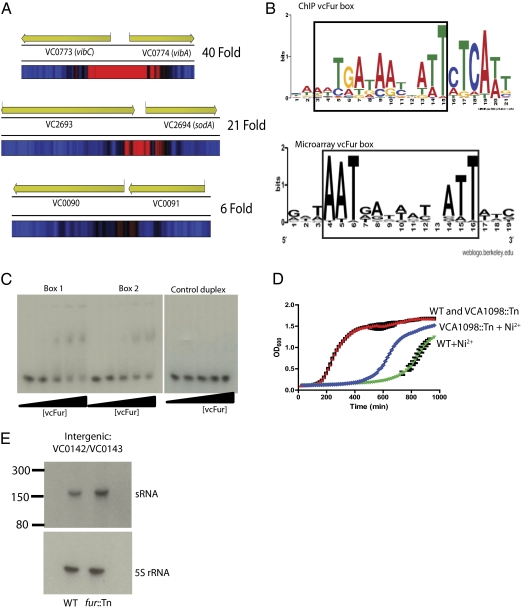
For our ChIP-seq experiments, we used inducible expression of an epitope-tagged vcFur in a fur::Tn V. cholerae background. This approach allowed us to control strong, rapid production of epitope-tagged vcFur. ChIP was performed using a high-affinity antibody against the 3× V5 epitope tag fused to the C terminus of vcFur. The V5 antibody does not show detectable cross-reaction with any proteins in V. cholerae or E. coli, and the 3× version of V5 greatly increases the efficiency of immunoprecipitation (29). Following induction and ChIP, we sequenced DNA bound to vcFur using HELICOS third generation sequencing (30). This platform sequences single DNA molecules directly, eliminating possible bias generated during library preparation and amplification required by other sequencing technologies (31, 32). Using the CLC genomics workbench, we analyzed three independent vcFur ChIP-seq runs using pre-ChIP input DNA as a comparison control. From each sequencing run, we aligned an average of 3,100,000, short (∼36 base) reads to the published N16961 V. cholerae genome (33). The alignments gave a total average coverage of 25-fold for chromosome 1 and 18-fold coverage of chromosome 2. This depth of coverage allowed us to use a low false-discovery rate (FDR) cutoff of 0.001% to call vcFur ChIP peaks. ChIP peaks are called when the sequence coverage of a given genomic region in the experimental sample exceeds the control sample at a rate specified by the FDR. ChIP peak enrichment ranged from five- to 300-fold over control. We compared the peak lists generated from all three samples and required that a peak be called in at least two of the three experiments to be considered a vcFur-binding site (Table S1) (34). The average ChIP peak length was ∼500 bp. We associated a vcFur peak with a gene if the peak overlapped the first codon (Fig. 1A and Table S2).
Fig. 1.
(A) Representative images of vcFur ChIP peaks called in our analysis. Blue represents background read coverage. Increasing red represents increased read coverage. The maximum fold increase for the peak is indicated beside each example. (B) Consensus vcFur box generated from complete set of vcFur ChIP peaks and vcFur box generated from alignments of 5′ UTRs of Fur-regulated genes from ref. 1. The region of sequence similarity is boxed. The height of the base indicates its proportional weight of prevalence in the motif. (C) Band-shift assay using purified vcFur. ChIP vcFur box (Box 1) and microarray vcFur box (Box 2) consensus sequence DNA duplexes were incubated with increasing concentrations of vcFur and separated by native electrophoresis. Box 1: gcCAAATGATAATTATTCTCATTgc; Box 2: cgGATAATGATAATTATTATCggcg. Binding of vcFur to a polyA/T duplex of equal length is shown as a control. Duplexes were used at a final concentration of 1 nM. vcFur was used at 0-, 3-, 6-, 12-, and 20-nM concentrations. vcFur bound Box 1 3.1 ± 1.1-fold more strongly than Box 2. (D) Growth of V. cholerae and a VCA1098::Tn mutant in LB ± 1 mM NiCl2. Strains grown with NiCl2 and curves for wild-type and VCA1098::Tn grown without nickel overlap are indicated. (E) Total RNA from exponentially growing V. cholerae and a fur::Tn mutant was separated and blotted with a probe for the predicted sRNA. A single-stranded RNA ladder was used to estimate the size of the sRNA.
A detailed transcriptome analysis comparing wild-type and fur-mutant V. cholerae previously described vcFur-dependent gene regulation during exponential growth in defined rich media (1). We mapped these data to our vcFur ChIP peaks to identify the gene set directly regulated by vcFur (Table 1). We identified 23 vcFur ChIP peaks associated with genes/small RNA (sRNA) previously shown to be regulated by vcFur. Two of these vcFur ChIP peaks overlapped the start codons of irgB (VC0474) and vibF (VC2209). Direct foot-printing assays have shown that both regions bind Fur (35, 36). The remaining 21 peaks provide direct evidence for vcFur binding to promoter regions of vcFur-regulated genes. These peaks include peaks associated with the major iron siderophore import genes as well as recognized vcFur-regulated genes, including the manganese-dependent superoxide dismutase sodA (1). Several of these genes are the first in apparent operons containing additional genes that also are derepressed in a fur mutant. For example, the ATP-binding cassette (ABC) transporter VCA0977 and hypothetical protein VCA0976 were implicated as an operon from expression data (1). Identification of a vcFur peak associated only with VCA0976 and the decreasing transcriptional induction toward VCA0977 supports the predicted operon structure. A single ChIP peak overlapped the start codons of VC0474/VC0475 and VC0773/VC0774. These pairs of known vcFur-regulated genes are transcribed divergently (33). Each of these peaks may contain two smaller peaks that were too close together to be resolved by our methods; however, we treat them as single peaks for the remainder of our analysis. Identification of vcFur ChIP peaks associated with many known vcFur-regulated genes strongly supports the effectiveness of our ChIP-seq procedure.
Table 1.
Known vcFur-regulated genes associated with vcFur ChIP-peaks
| Associated ORF (gene) | Function | fur/wild-type* expression ratio | Predicted Fur box sequence | Distance (bp) of Fur box to first codon |
| VC0091 | Putative O-methyl transferase | 4.8 | AAGATGATAATGAATCTCAAA | −8 |
| ryhB | Regulatory sRNA | ND | TAAATGAGAACTATTATTATT | 6 |
| VC0284 | Transport | 3.6 | CAAATGATAGTAATTTTCATT | 68 |
| VC0474 (irgB)† | IrgA regulator | 1.7 | TAAATGATAATTATTCTTAAT | 12 (VC0474) |
| VC0475 (irgA) | Enterobactin receptor | 32.5 | 111 (VC0745) | |
| VC0608 (fbpA) | Iron compound | 2.7 | GTAATGATAACCTTTATCAAT | −2 |
| VC0771 (vibB) | Vibriobactin synthesis | 11.7 | TAAATAATAACAATTATCATT | 145 |
| VC0773 (vibC)† | Vibriobactin synthesis | 11.0 | TTAATAAAAACTATTCTCATT | 71 (VC0773) |
| VC0774 (vibA) | Vibriobactin synthesis | 8.5 | 75 (VC0774) | |
| VC1264 (irpA) | Unknown | 5.4 | CAAATGATAATAATTTGCAAT | Overlap ATG |
| VC1548 | Unknown | 7.9 | ATAATGAGAGCGTTTCTCAAT | 258 |
| VC1572 | Unknown | 9.4 | AATGTGATAATAATTATCATT | 17 |
| VC1688 | Unknown | 2.6 | TTGTCGATAATAATTCTCATT | Overlap TTG |
| VC2209 (vibF) | Vibriobactin synthesis | 7.7 | TTAATAATAATCATTATCAAT | 58 |
| VC2210 (viuB) | Iron removal | 7.9 | CAAATGAGAATGTATATCATT | 118 |
| VC2211 (viuA) | Vibriobactin receptor | 5.4 | CAAATGTAAAGCATTCTCATT | 268 |
| VC2694 (sodA) | Superoxide dismutase | 7.5 | TTAATGATAATTAATATCATT | 15 |
| VCA0063 (ptrB) | Protease II | 4.9 | CAAATGATAATTGATCTTATT | 56 |
| VCA0232 (vctA) | Enterobactin receptor | 11.3 | CAAATGATAACGATTCGCATA | 29 |
| VCA0576 (hutA) | Heme transport | 20.4 | CAAATGATAGCAATTATCATT | 43 |
| VCA0625 (hasR) | Heme receptor | ND | CAATTGATAATTATTATCAAT | −51 |
| VCA0910 (tonB1) | Iron transport | 17.1 | TTAATAATAGCAATTATCAAT | 147 |
| VCA0976 | Unknown | ND | CATTTGAGAATAAATTGCATT | 33 |
*All expression data were taken from ref. 1.
†One peak was found overlapping the first codon of VC0474/VC0475 and VC0773/VC0774. The strongest vcFur box prediction from within each peak is shown.
ChIP-Seq Analysis Expands Number of vcFur-Binding Sites.
We identified an additional 34 vcFur ChIP peaks associated with genes/sRNAs (Table 2). We broadly categorized these genes into transport, transcriptional and translational regulation, motility, intermediary metabolism, and hypothetical ORFs. Four vcFur ChIP peaks overlapped the start codons of multiple closely neighboring genes. Lack of expression data indicating vcFur control precluded immediate association of the peaks with just one ORF.
Table 2.
New vcFur ChIP peak-associated ORFs and sRNAs
| Associated ORF (gene) | Known or predicted function | Category* | Predicted Fur box sequence | Distance (bp) of Fur box to first codon |
| VC0914 | Multidrug resistance | Transport | AATCTGTAAGCCTTTCTCAAT | 101 |
| VC1235 | Sodium/dicarboxylate symporter | TAAATGAGAACGATTTTCTTG | 24 | |
| VC2012 | Sodium-dependent transporter | TAACTAAGAATTAAATTCTAG | 46 | |
| VC2334 | CorA-like Mg2+/Co2+ transporter | AACTTGACCATCAATCTCTTT | 315 | |
| VCA0355 | Threonine efflux pump | TGTTTAAGAGTGATTCGCAAC | 122 | |
| VCA1098 | NikA/Ni2+ transporter | GACATGATTTTTATTCTCATG | 10 | |
| VC0878 | L31P, alternate ribosomal protein | Transcription/translation regulation | TAATTAATAATTATTCTCAAG | 40 |
| VC0534 | Sigma factor, RpoS | CCCTTGATAACGGATCTCAAA | 106 | |
| VC2080 | AraC/XylS family regulator | TTCTCTAACATTGTTCTAAAT | 150 | |
| Intergenic† VC0142/VC0143 | sRNA | CAATTGATAACCAATCTCAAC | ND† | |
| Intergenic† VCA0452/VCA0453 | sRNA | CCTTTGATAATGATTCTCAAT | ND† | |
| VC2190 (flgL) | Flagellar hook-associated protein | Motility | ATGTTGATCATTTTACGCAAG | 292 |
| VCA1091 (cheR-3) | chemotaxis | CGAATGGGAAGAATTCTAATC | 12 | |
| VC0019 | Valine-pyruvate aminotransferase | Intermediary metabolism | TGTTCAAGAACTATTCTAAAC | 61 |
| VC0034 (dsbA) | Disulfide interchange protein | CGTCAATGAGTTTTTCTCATT | 116 | |
| VC0238 | O-acetyltransferase | CAACTGAAAATGTTTCTCAAG | 640 | |
| VC2447 (eno) | Enolase | TGGGTGAGCAGGGTTCTCAAT | 195 | |
| VC0005 | unknown | Hypothetical | GCGCTAATAACCTTTCTCATC | 190 |
| VC0074 | unknown | CGTCTGAGAATAATTCGTATT | 9 | |
| VC0519 | unknown | CTCTTCTTTGAGATTCTCAAT | −6 | |
| VC0882 | unknown | AAACGGGTAAATTATCTCTTG | 227 | |
| VC0931 | unknown | CGAGCGAAAGTCATTCTCATC | −174 | |
| VC1009 | unknown | TAATTGATAATTATTATCATG | 31 | |
| VC2004 | unknown | TAACTGATAACGATTCTCATT | 2 | |
| VC2232 | unknown | ACCGCGAAAAAGATTCTCAAC | −133 | |
| VC2488 | unknown | ATGTTGAGAGCTTATCCCATT | 115 | |
| VC2498 | unknown | GCGTAGTTCATCATTATCATT | Overlap start | |
| VC2739 | unknown | AAATTGTACCGTATTCTCATC | 71 | |
| VCA0645 | unknown | GAGACCGAATCTATTCTCAAT | 53 | |
| VCA0948 | unknown | TACTCCTTCACTATTCTCAAT | 181 | |
| VC0052/VC0053/ VC0054§ | ATGACAGTCGGTATTATCATG | 1 (VC0052) | ||
| 14 (VC0053) | ||||
| 158 (VC0054) | ||||
| VC0559/VC0560§ | CTCACATTCACGAATATCATT | 102 (VC0559) | ||
| 80 (VC0560) | ||||
| VC0871/VC0872§ | CAAATGATAACTGCTCTCAAT | 221 (VC0871) | ||
| 11 (VC0872) | ||||
| VCA0894/VCA0895 | GTATCTACAGCTAATTTCATT | Overlap start (VCA0894) 166 (VCA0895) |
*General functional categorization only.
†vcFur ChIP peak was found in the intergenic region between the indicated genes.
†ND, start site not determined.
§vcFur peak overlapped the first codon if the indicated genes.
To validate binding of vcFur to these loci, we chose a subset of new peak locations and used quantitative PCR (qPCR) to determine the fold enrichment of these genomic loci in our vcFur ChIP samples compared with pre-ChIP control DNA (Table 3). For reference, we determined the enrichment of vcFur binding upstream of known regulator targets enterobactin receptor gene A (irgA) and tonB1 as well as two nontarget genomic loci [endonuclease IV (nfo) and isocitrate dehydrogenase (icd)] as negative controls. Quantification of fold enrichment of seven new vcFur peaks identified by ChIP-seq showed equivalent or greater enrichment than binding to irgA and tonB1 promoter regions. These results support our ChIP peaks as containing authentic vcFur-binding sites. Strikingly, six of these seven vcFur-binding sites showed greater enrichment by ChIP than the known vcFur-regulated tonB1 gene promoter. Furthermore, two of these vcFur-binding sites showed greater enrichment by ChIP than the irgA promoter region. Of specific note is a vcFur-binding site upstream of an alternative ribosomal protein, VC0878, that showed ∼300-fold enrichment.
Table 3.
Validation of vcFur binding to new genomic loci
| Associated ORF peak | Fold enrichment* |
| VC0475 (irgA) | 14.0 ± 2.6 |
| VCA0910 (tonB1) | 4.8 ± 0.8 |
| VC0519 | 7.6 ± 1.0 |
| VC0534 | 8.6 ± 1.2 |
| VC0560 | 10.8 ± 1.0 |
| VC0878 | 306.0 ± 111.4 |
| VC0914 | 6.2 ± 0.2 |
| VCA1098 | 4.8 ± 1.2 |
| Intergenic: VC0142/VC0143 | 39.2 ± 9.2 |
| VC2360 (nfo) | 1.0 ± 0.2 |
| VC1141 (icd) | 1.0 ± 0.2 |
*Mean ± SD of at least three independent samples.
ChIP-Seq Analysis Defines an Enhanced V. cholerae Fur Box.
A thorough microarray analysis of genes regulated by vcFur during exponential phase growth in defined rich medium used alignments of 5′ UTRs of vcFur-regulated genes to define the Fur box in V. cholerae (1). Our ChIP experiments identified several vcFur peaks in front of genes described in that study (Table 1) as well as several other vcFur binding locations (Table 2). We used the sequences of our complete list of vcFur peaks and de novo motif search software (37) to predict a vcFur box. We identified a consensus vcFur box that was present in every ChIP peak near the start site of the associated gene/sRNA (Fig. 1B and Tables 1 and 2). This consensus motif shares sequence similarity with the previously predicted vcFur box. In fact, the boxed regions share an identical sequence; however, the two predictions differ in their weighting of the significance of the bases. The vcFur ChIP box also has a 3′ weighted skew and a 21-bp rather than a 19-bp consensus sequence. We identified the locations of the microarray and ChIP-seq–defined vcFur boxes within their associated vcFur ChIP peak (Table S2). When ChIP-seq– and microarray-defined vcFur boxes were located in the same ChIP peak, both sites overlapped but were shifted three to five bases to the right or left. Interestingly, the distance from the predicted vcFur boxes to the first codon of the regulated gene varied considerably (Tables 1 and 2). Although we do not know the transcriptional start site for each gene, this result suggests that vcFur can exert a regulatory influence from a variable distance.
The vcFur ChIP Fur box had a more stringent requirement for residue position than the vcFur box predicted by microarray studies. We compared the binding of purified vcFur to the microarray- and ChIP-seq–defined vcFur boxes and a control polyA/T DNA duplex. We used vcFur reconstituted with Zn2+ because this ion is required for vcFur structure, does not oxidize quickly, and allows vcFur to bind known promoters as efficiently as vcFur containing Fe2+ (38). Purified vcFur bound both the microarray- and ChIP-seq–defined vcFur boxes but did not bind the control duplex (Fig. 1C). Interestingly, vcFur bound the ChIP-defined vcFur box 3.1 ± 1.1-fold more strongly than the previously predicted vcFur box (Fig. 1C). The identification of this consensus Fur box in all ChIP peaks further supports the validity of these genomic regions as true vcFur-binding sites.
ChIP-Seq–Identified Binding Sites Expand Set of vcFur-Regulated Genes and sRNAs.
We compared the transcript levels of four genes associated with new vcFur binding sites in wild-type and isogenic fur::Tn-mutant V. cholerae using qPCR. We measured expression levels from bacteria in exponential phase grown in rich media with excess iron. We measured the expression of the well-studied vcFur-regulated gene irgA as a reference (39). Importantly, we observed derepression of three of the four genes in the fur::Tn mutant (Table 4). The gene encoding sigma factor S (rpoS) was not differentially regulated under these conditions (Table 4). One gene, VCA1098, was particularly interesting because it shared strong homology with NikA, the nickel-binding periplasmic protein that is part of a nickel ABC transport system (40). A transposon insertion in VCA1098 increases the resistance of V. cholerae to elevated nickel levels, suggesting a role in nickel transport for this vcFur-regulated gene (Fig. 1D). The intergenic region between VC0142 and VC0143 has a strong, validated vcFur ChIP peak and was shown previously to express an sRNA (41). Northern blots using probes against this sRNA showed it also was derepressed in the fur::Tn mutant (Fig. 1E). We also identified an sRNA near the vcFur ChIP peak in the intergenic region between VCA0452 and VCA0453, but its expression was similar in both wild-type and fur::Tn-mutant samples.
Table 4.
vcFur-dependent regulation of genes associated with new vcFur ChIP peaks
| ORF (gene) | fur/wild-type expression* |
| VC0475 (irgA) | 216.2 ± 54.5 |
| VC0878 | 7.1 ± 1.4 |
| VCA1098 | 2.6 ± 1.1 |
| VC0914 | 5.0 ± 3.2 |
| VC0534 (rpoS) | 1.4 ± 0.3 |
*Mean ± SD of at least three independent samples.
Discussion
Next-generation sequencing offers increased sensitivity for genomic-directed experiments. ChIP-seq is unmatched for identifying regulator-binding sites in eukaryotic systems, and we show here its utility for mapping the genomic binding sites of a bacterial transcriptional regulator. Strong, specific antibody recognition of the target protein is a critical component of a successful ChIP experiment (11). The expression level of a target transcriptional regulator can vary widely depending on growth phase or condition, affecting its binding potential. To overcome these potential difficulties in genome-wide binding analysis, we propose using inducible plasmid-borne copies of the regulator that carry a specific, high-affinity, epitope tag. This epitope can be used with any regulator, providing a target for well-tested ChIP-quality antibodies. Furthermore, antibody recognition of this epitope is not sensitive to crosslinking. The benefit of overexpression is that the transcriptional regulator has a greater potential to bind low-affinity sites, outcompete interfering proteins, and bind sites that may not be optimal under laboratory conditions. This approach allows interrogation of all potential binding sites regardless of fluctuations in regulator abundance. Overexpression of a transcriptional regulator has the possibility of producing false-positive ChIP peaks by binding to nonspecific sites. Previous ChIP-ChIP studies identified bacterial regulators binding to degenerative consensus sequences or sites that could not be confirmed in vitro (reviewed in ref. 7). However, using vcFur as a test case with ChIP-seq, we have not found a false-positive ChIP peak and instead have identified several targets of vcFur regulation. The increased depth of sequence coverage of bacterial genomes with next-generation technologies permits the use of low FDRs when predicting bacterial regulator ChIP peaks, undoubtedly contributing to our success in identifying valid binding sites.
Computational methods have successfully predicted vcFur-binding sites in the V. cholerae genome (42, 43). However, no computational study was able to predict the spectrum of vcFur-binding sites identified in our ChIP-seq analysis. In fairness, bioinformatic algorithms must rely on currently available experimental data, which may be incomplete, to derive search motifs. It will be interesting to determine how the expanded set of vcFur-binding sites will affect computational predictions of Fur-binding sites.
Combining ChIP-seq analysis with available expression data, we can distinguish clearly between direct and indirect vcFur regulation and now suggest a much broader role for vcFur in direct regulation. Our analysis greatly expands the number of experimentally verified vcFur-binding sites and implicates several more genes as being under direct vcFur regulation. There are several possible reasons why the genes associated with our vcFur-binding sites were not identified in microarray studies. Targets such as sRNAs and small ORFs such as VC0878 were not included in the microarray and thus would not have been tested. In a fur::Tn mutant, our qPCR results showed up-regulation of irgA by more than 200-fold (Table 4). Using qPCR, we showed that three genes associated with new vcFur-binding sites were also up-regulated in a fur::Tn mutant, but to a much smaller extent than irgA (Table 4). The sensitivity of the microarray analysis may not have been high enough to detect these relatively small changes in gene expression, or these relatively small changes may not have been reproducible among experiments.
Our results show that a high degree of vcFur binding in a promoter region does not correlate directly with a high level of regulation of the associated gene (Tables 2 and 3). The context of the vcFur-binding site may be as important as, or more important than, the affinity of vcFur for the site. Other transcription factors, such as the aerobic respiratory control protein (ArcA), the fumarate and nitrate reduction regulatory protein (Fnr), and cyclic AMP receptor protein (CRP), may bind at sites near or overlapping the vcFur sites. Depending on the growth condition, these factors may increase or decrease vcFur binding significantly at a specific location.
We have shown direct regulation of three of the genes associated with new vcFur peaks using a fur::Tn mutant (Tables 2 and 4). VCA1098 shares strong homology with the ABC nickel transport subunit NikA (40), and our experiments show that disruption of VCA1098 decreases the sensitivity of V. cholerae to extracellular nickel (Fig. 1D). These results suggest that VCA1098 may play a role in nickel transport. Interestingly in Helicobacter mustelae, several genes annotated for iron acquisition have been shown recently to be used for nickel uptake (44). In addition NikA also has been shown to bind heme, suggesting a multifunctional use for VCA1098 (45). Our results also showed significant ChIP enrichment of vcFur binding to the promoter region of VC0878, the alternative L31 ribosomal protein. The VC0878 Bacillus subtilis homolog is regulated by the zinc uptake regulator Zur and helps buffer the bacterium against zinc starvation (46). Furthermore, zinc transport has been shown to be controlled by Fur in Pasteurella multocida (47). The direct association of genes potentially involved in Ni2+ and Zn2+ regulation suggests that vcFur may have additional roles beyond iron regulation.
We identified strong vcFur ChIP peaks in the intergenic regions between VC0142/VC0143 and VCA0452/VCA0453. These peaks are not located near the start site of any gene. Livny et al. (41) identified an sRNA in the intergenic region between VC0142/VC0143 during a computational screen for V. cholerae sRNAs. Our Northern blots showed increased expression of this sRNA in the fur::Tn mutant (Fig. 1E). Combined with the vcFur ChIP localization, we have shown that vcFur directly regulates this unstudied sRNA. We also identified an sRNA in the intergenic region between VCA0452/VCA0453 that did not show vcFur-dependent regulation; however, vcFur-dependent regulation may occur in different growth conditions.
Fur/RpoS cross-regulation has been observed in Vibrio vulnificus and E. coli (48, 49), but we did not observe up-regulation of rpoS in a fur::Tn mutant (Table 4). As with all other genes associated with vcFur-binding sites, strong vcFur-dependent regulation may require alternate growth conditions.
The identification of several additional vcFur-binding sites allowed us to predict an enhanced V. cholerae Fur box consensus sequence. This motif was found within the vcFur ChIP peaks near the translational start site of each associated ORF. In vitro assays showed that this motif bound vcFur slightly better than the previously predicted vcFur box that used only a subset of our binding region. Interestingly, both boxes share an identical span of bases. Our prediction has a more stringent requirement for the 3′ end, suggesting this region may provide increased binding to vcFur. The ability of our predicted motif to bind vcFur effectively and its presence in all identified ChIP peaks (vcFur-bound sites) help support the validity of the new vcFur-binding sites reported here.
Materials and Methods
Strains and plasmids are listed in Table S3. Sequencing was performed using the HeliScope Single Molecule Sequencer. Sequencing data analysis was performed using CLC genomic workbench software. 6XHis C-terminal tagged vcFur was purified by affinity chromatography. For detailed information, See SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. D. Ewen Cameron for support in using the V. cholerae transposon library. B.W.D. was supported by the Jane Coffin Childs Memorial Fund for Medical Research and by the National Research Council of Canada H. L. Holmes Award. This work was supported by National Institutes of Health Grant AI-018045 (to J.J.M.).
Footnotes
The authors declare no conflict of interest.
Data deposition: Sequences are available at the laboratory website, http://mekalanoslab.med.harvard.edu/resources.html.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107894108/-/DCSupplemental.
References
- 1.Mey AR, Wyckoff EE, Kanukurthy V, Fisher CR, Payne SM. Iron and Fur regulation in Vibrio cholerae and the role of Fur in virulence. Infect Immun. 2005;73:8167–8178. doi: 10.1128/IAI.73.12.8167-8178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo M, et al. Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun. 2010;78:4850–4859. doi: 10.1128/IAI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledala N, Sengupta M, Muthaiyan A, Wilkinson BJ, Jayaswal RK. Transcriptomic response of Listeria monocytogenes to iron limitation and Fur mutation. Appl Environ Microbiol. 2010;76:406–416. doi: 10.1128/AEM.01389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao H, et al. The iron-responsive Fur regulon in Yersinia pestis. J Bacteriol. 2008;190:3063–3075. doi: 10.1128/JB.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macquarrie KL, Fong AP, Morse RH, Tapscott SJ. Genome-wide transcription factor binding: beyond direct target regulation. Trends Genet. 2011;27:141–148. doi: 10.1016/j.tig.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson ME, Snyder M. High-throughput methods of regulatory element discovery. Biotechniques. 2006;41:673, 675, 677 passim. doi: 10.2144/000112322. [DOI] [PubMed] [Google Scholar]
- 7.Wade JT, Struhl K, Busby SJ, Grainger DC. Genomic analysis of protein-DNA interactions in bacteria: Insights into transcription and chromosome organization. Mol Microbiol. 2007;65:21–26. doi: 10.1111/j.1365-2958.2007.05781.x. [DOI] [PubMed] [Google Scholar]
- 8.Park PJ. ChIP-seq: Advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahramanoglou C, et al. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res. 2011;39:2073–2091. doi: 10.1093/nar/gkq934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lun DS, Sherrid A, Weiner B, Sherman DR, Galagan JE. A blind deconvolution approach to high-resolution mapping of transcription factor binding sites from ChIP-seq data. Genome Biol. 2009;10:1–12. doi: 10.1186/gb-2009-10-12-r142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goens G, Rusu D, Bultot L, Goval JJ, Magdalena J. Characterization and quality control of antibodies used in ChIP assays. Methods Mol Biol. 2009;567:27–43. doi: 10.1007/978-1-60327-414-2_2. [DOI] [PubMed] [Google Scholar]
- 12.Wyckoff EE, Mey AR, Payne SM. Iron acquisition in Vibrio cholerae. Biometals. 2007;20:405–416. doi: 10.1007/s10534-006-9073-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 14.Hantke K. Iron and metal regulation in bacteria. Curr Opin Microbiol. 2001;4:172–177. doi: 10.1016/s1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 15.Sutton HC, Winterbourn CC. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6:53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- 16.Calderwood SB, Mekalanos JJ. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lorenzo V, Wee S, Herrero M, Neilands JB. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (Fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escolar L, Pérez-Martín J, de Lorenzo V. Opening the iron box: Transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ptashne M. A Genetic Switch. Cambridge, United Kingdom: Cell Press and Blackwell Scientific; 1992. [Google Scholar]
- 20.Goldberg MB, Boyko SA, Calderwood SB. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli Fur system. J Bacteriol. 1990;172:6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavrrar JL, Christoffersen CA, McIntosh MA. Fur–DNA interactions at the bidirectional fepDGC-entS promoter region in Escherichia coli. J Mol Biol. 2002;322:983–995. doi: 10.1016/s0022-2836(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 22.Le Cam E, Frechon D, Barray M, Fourcade A, Delain E. Observation of binding and polymerization of Fur repressor onto operator-containing DNA with electron and atomic force microscopes. Proc Natl Acad Sci USA. 1994;91:11816–11820. doi: 10.1073/pnas.91.25.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escolar L, Pérez-Martín J, de Lorenzo V. Binding of the fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J Mol Biol. 1998;283:537–547. doi: 10.1006/jmbi.1998.2119. [DOI] [PubMed] [Google Scholar]
- 24.Baichoo N, Helmann JD. Recognition of DNA by Fur: A reinterpretation of the Fur box consensus sequence. J Bacteriol. 2002;184:5826–5832. doi: 10.1128/JB.184.21.5826-5832.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watnick PI, Eto T, Takahashi H, Calderwood SB. Purification of Vibrio cholerae Fur and estimation of its intracellular abundance by antibody sandwich enzyme-linked immunosorbent assay. J Bacteriol. 1997;179:243–247. doi: 10.1128/jb.179.1.243-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YW, Park SA, Lee HW, Lee NG. Alteration of growth-phase-dependent protein regulation by a fur mutation in Helicobacter pylori. FEMS Microbiol Lett. 2009;294:102–110. doi: 10.1111/j.1574-6968.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- 27.Fiorini F, Stefanini S, Valenti P, Chiancone E, De Biase D. Transcription of the Listeria monocytogenes fri gene is growth-phase dependent and is repressed directly by Fur, the ferric uptake regulator. Gene. 2008;410:113–121. doi: 10.1016/j.gene.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Merrell DS, et al. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71:6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moqtaderi Z, Struhl K. Expanding the repertoire of plasmids for PCR-mediated epitope tagging in yeast. Yeast. 2008;25:287–292. doi: 10.1002/yea.1581. [DOI] [PubMed] [Google Scholar]
- 30.Milos P. Helicos BioSciences. Pharmacogenomics. 2008;9:477–480. doi: 10.2217/14622416.9.4.477. [DOI] [PubMed] [Google Scholar]
- 31.Kozarewa I, et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat Methods. 2009;6:291–295. doi: 10.1038/nmeth.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aird D, et al. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 2011;12:1–14. doi: 10.1186/gb-2011-12-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Håndstad T, Rye MB, Drabløs F, Sætrom P. A ChIP-seq benchmark shows that sequence conservation mainly improves detection of strong transcription factor binding sites. PLoS ONE. 2011;6:e18430. doi: 10.1371/journal.pone.0018430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterton JR, Choi MH, Watnick PI, Carroll PA, Calderwood SB. Vibrio cholerae VibF is required for vibriobactin synthesis and is a member of the family of nonribosomal peptide synthetases. J Bacteriol. 2000;182:1731–1738. doi: 10.1128/jb.182.6.1731-1738.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watnick PI, Butterton JR, Calderwood SB. The interaction of the Vibrio cholerae transcription factors, Fur and IrgB, with the overlapping promoters of two virulence genes, irgA and irgB. Gene. 1998;209:65–70. doi: 10.1016/s0378-1119(98)00018-3. [DOI] [PubMed] [Google Scholar]
- 37.Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 38.Sheikh MA, Taylor GL. Crystal structure of the Vibrio cholerae ferric uptake regulator (Fur) reveals insights into metal co-ordination. Mol Microbiol. 2009;72:1208–1220. doi: 10.1111/j.1365-2958.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg MB, DiRita VJ, Calderwood SB. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;58:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro C, Wu LF, Mandrand-Berthelot MA. The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport system for nickel. Mol Microbiol. 1993;9:1181–1191. doi: 10.1111/j.1365-2958.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 41.Livny J, Fogel MA, Davis BM, Waldor MK. sRNAPredict: An integrative computational approach to identify sRNAs in bacterial genomes. Nucleic Acids Res. 2005;33:4096–4105. doi: 10.1093/nar/gki715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad R, Hjerde E, Hansen GA, Haugen P, Willassen NP. Prediction and experimental testing of ferric uptake regulator regulons in vibrios. J Mol Microbiol Biotechnol. 2009;16:159–168. doi: 10.1159/000128322. [DOI] [PubMed] [Google Scholar]
- 43.Panina EM, Mironov AA, Gelfand MS. Comparative analysis of FUR regulons in gamma-proteobacteria. Nucleic Acids Res. 2001;29:5195–5206. doi: 10.1093/nar/29.24.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoof J, Kuipers EJ, Klaver G, van Vliet AH. An ABC transporter and a TonB ortholog contribute to Helicobacter mustelae nickel and cobalt acquisition. Infect Immun. 2010;78:4261–4267. doi: 10.1128/IAI.00365-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd M, Heath MD, Poole RK. NikA binds heme: A new role for an Escherichia coli periplasmic nickel-binding protein. Biochemistry. 2007;46:5030–5037. doi: 10.1021/bi700183u. [DOI] [PubMed] [Google Scholar]
- 46.Gabriel SE, Helmann JD. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol. 2009;191:6116–6122. doi: 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garrido ME, et al. The high-affinity zinc-uptake system znuACB is under control of the iron-uptake regulator (Fur) gene in the animal pathogen Pasteurella multocida. FEMS Microbiol Lett. 2003;221:31–37. doi: 10.1016/S0378-1097(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 48.Guillemet ML, Moreau PL. Fur-dependent detoxification of organic acids by rpoS mutants during prolonged incubation under aerobic, phosphate starvation conditions. J Bacteriol. 2008;190:5567–5575. doi: 10.1128/JB.00577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HJ, et al. Regulation of fur expression by RpoS and fur in Vibrio vulnificus. J Bacteriol. 2003;185:5891–5896. doi: 10.1128/JB.185.19.5891-5896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.