Abstract
In the postnatal period, cerebellar granule cells express a set of the maturation gene battery in an activity-dependent manner and establish synaptic function in the cerebellar circuitry. Using primary cultures combined with specific inhibition of signaling cascades, the present investigation revealed that the expression of the maturation genes, including the NMDA glutamate receptor NR2C and GABAA receptor GABAARα6 genes, is controlled by strikingly unified signaling mechanisms that operate sequentially through stimulation of AMPA and NMDA receptors, Na+ channels [voltage-gated Na channel type II (Nav1.2)], and voltage-dependent Ca2+ channels. This signaling then induces the Ets variant gene 1 (Etv1/Er81) transcription factor of the ETS family in an activity-dependent manner. Consistent with the culture study, the ChIP assay indicated that Etv1 up-regulates the maturation genes in a developmentally regulated manner. This activation, as revealed by the luciferase assay, occurrs by interacting with the Etv1-interacting motifs present in the promoter region. Importantly, in vivo knockdown of Etv1 by DNA electroporation in the developing cerebellum prevents the up-regulation of the maturation genes but has no effects on preceding developmental processes occurring in the granule cells. Etv1 thus orchestrates the activity-dependent gene regulation in the terminal maturation program and specifies the identity of cerebellar granule cells.
Keywords: cell culture, membrane potential, neuronal cell excitation, synaptic maturation, transcriptional regulation
A large body of evidence indicates that cell type-specific transcription factors control sequential events of neuronal cell proliferation and differentiation in the early stages of developmental processes (1, 2). However, how the terminal maturation processes are programmed for functional neural network formation during development remains largely to be determined. In the developing cerebellum, granule cells proliferate, differentiate postmitotically in the external granular layer (EGL), and migrate inwardly into the internal granular layer (IGL), where they form refined synaptic connections with mossy fibers (3). In these developmental processes, the resting membrane potential of granule cells, as in many other developing neuronal cells, shifts significantly from a relatively depolarized state to a more hyperpolarized state (4, 5). This shift of the membrane potential enhances the responsiveness to excitatory neurotransmitters and regulates a set of the maturation gene battery characteristic of maturing granule cells (6, 7). The granule cells thus mature in an activity-dependent manner.
In primary cultures of the postnatal cerebellum, granule cells represent a predominant cell population (>90% of the cerebellum) and are capable of recapitulating many properties characteristic of maturing granule cells in vivo (8, 9). Using cultures of granule cells in combination with in vivo analysis, this study addressed how the maturation gene expression is regulated transcriptionally in an activity-dependent manner, namely, by either a common or a distinct set of transcription factors in the terminal maturation program of granule cells. Here we report that the Ets variant gene 1 (Etv1/Er81) transcription factor of the ETS family plays a key role in orchestrating the neural activity-dependent gene regulation for terminal maturation of granule cells.
Results
Activity-Dependent Up-Regulation of the Maturation Genes.
Granule cells were prepared from postnatal day 8 (PD 8) mice and cultured at the physiological KCl concentration (5 mM) for 96 h. When excitation of cultured granule cells was examined by loading these cells with the Fluo-4 Ca2+ indicator, they showed rapid oscillatory changes in intracellular Ca2+ levels (Fig. S1). This oscillatory response was abolished by incubation with tetrodotoxin (TTX) or the combined addition of an AMPA receptor-selective antagonist, 2, 3-dioxo-6-nitro-1, 2, 3, 4-tetrahydrobenzo [f] quinoxaline-7-sulfonamide (NBQX) and an NMDA receptor-selective antagonist, 3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP). Glutamate thus is released from granule cells in primary cultures and in turn excites both glutamate-released cells and their neighboring cells in a feed-forward or recycling manner.
Previous microarray analysis identified 21 genes that were up-regulated both in granule cells cultured at the physiological KCl concentration and also in the maturing IGL granule cells from PD 8 to PD 21 in vivo (7). We examined how the granule cell excitation is involved in up-regulation of the maturation gene expression by culturing granule cells in the presence or absence of TTX for 96 h. Microarray analysis indicated that, of the 21 previously identified genes, all but the testican gene were inhibited by TTX (Table S1). The testican gene turned out to be expressed mainly in stellate cells of the cerebellum. We selected 14 genes from the previous study and three additional TTX-suppressive genes which were identified in this study and expressed in the mature cerebellum (10–12). PCR analysis confirmed that the expression of all 17 genes was inhibited by TTX to different degrees, ranging from 15–85% (Fig. 1 and Fig. S2).
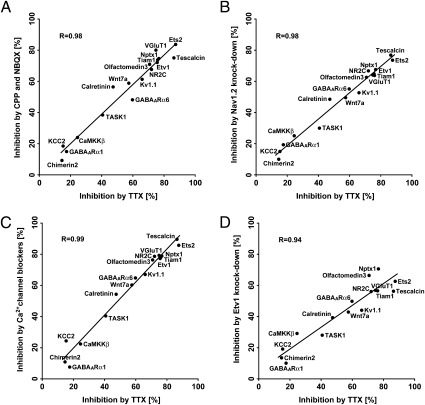
Fig. 1.
Unified signaling mechanisms of 17 up-regulated genes in maturing granule cells. (A–D) Granule cells were treated with TTX (5 μM), CPP and NBQX (100 μM each) (A), Nav1.2 siRNA (6 μg) (B), four types of Ca2+ channel blockers (3 μM each) (C), or Etv1 siRNA (6 μg) (D). mRNA levels were quantified by PCR. Data for TTX-treated and TTX-untreated cells are shown in Fig. S2 and used in A–D. Inhibition is expressed as the percentage of mRNA reduction in inhibitor-treated cells relative to untreated cells (n = 3). The inhibitory relationship was analyzed statistically, and its linearity is indicated by a straight line. VGluT1, vesicular glutamate transporter 1; Tiam1, T-cell lymphoma invasion and metastasis 1; Nptx1, neuronal pentraxin 1; Kv1.1, voltage-gated potassium channel, shaker-related subfamily, member 1; Wnt7a, wingless-related MMTV integration site 7A; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; TASK1, TWIK-related acid-sensitive potassium channel 1; KCC2, potassium-chloride cotransporter 2; GABAARα1, GABAA receptor α1.
The excitation of granule cells by AMPA and NMDA receptors evokes Na+ channel-mediated action potentials, which in turn promote Ca2+ entry via voltage-dependent Ca2+ channels (13). The roles of these signaling cascades in the up-regulation of the 17 maturation genes were investigated by inhibiting the respective signaling components in cultured granule cells. The glutamate receptors were blocked by combined addition of NBQX and CPP. Na+ channels were inhibited by siRNA against voltage-gated sodium channel type II (Nav1.2) mRNA, which was expressed predominantly in granule cells (14). This treatment abolished the action potential of cultured granule cells (Fig. S3 A–C). Ca2+ channels were inhibited by combined addition of four channel blockers, nifedipine, ω-agatoxin TK, ω-conotoxin GVIA, or NNC 55–0396, inhibiting L-type, P/Q-type, N-type, or T-type Ca2+ channels, respectively. Quantitative PCR analysis indicated that all these inhibitory treatments significantly reduced the up-regulated expression of the 17 maturation genes to various extents (Fig. 1). Importantly, this analysis disclosed not only a striking linear correlation but also a virtually identical order in the inhibitory extents of the 17 up-regulated genes between TTX treatment and the three other treatments (Fig. 1 A–C). These results demonstrate that the up-regulation of the 17 maturation genes is controlled by common and sequential mechanisms in which excitation of AMPA and NMDA receptors triggers the action potential via the activation of the Nav1.2 Na+ channel, leading to Ca2+ entry via the Ca2+ channels.
Unified Regulation of the Maturation Genes by the Etv1 Transcription Factor.
We previously reported that the Etv1 transcription factor of the ETS family is markedly up-regulated not only in cultured granule cells but also in the maturing IGL granule cells in vivo (7). Furthermore, this up-regulation was considerably inhibited by TTX in cultured granule cells (Fig. 1). We examined the regulatory role of Etv1 in up-regulation of the maturation gene expression (Fig. 1D). Two types of Etv1 siRNAs were prepared, one corresponding to the DNA-binding domain (siRNA-2) and the other to its upstream domain (siRNA-1). Both Etv1 siRNAs efficiently abrogated the expression of the Etv1 mRNA in the cultured granule cells (Fig. S3D). Then, treatment of the granule cells with either of these two Etv1 siRNAs, but not with scrambled siRNA (scRNA), inhibited all the maturation genes to comparable but different extents, depending on the genes analyzed (Fig. 1D). Remarkably, the inhibition by siRNA correlated well, in a linear fashion, with the TTX-mediated inhibition of the maturation genes (Fig. 1D). Etv1 siRNA inhibited the up-regulation of the E26 avian leukemia oncogene 2 (Ets2) mRNA of the ETS family (Fig. 1D), but the Ets2 siRNA failed to inhibit up-regulation of the Etv1 mRNA. The results thus indicate that Etv1 is the primary transcription factor that commonly directs up-regulation of the maturation genes in granule cells.
Promoter Regulation of the Maturation Genes by Etv1.
DNA sequence data analysis indicated that a number of Etv1-interacting motifs (Ets motifs), (G/C/A)GGA(A/T)(G/A) (15), are present in the promoter regions of the maturation genes. Therefore we performed a luciferase assay to test whether Etv1 would promote the transcriptional activity of representative maturation genes (Fig. 2A). The promoter sequences of 800–1,200 bp covering the transcription initiation site were attached to the luciferase gene and subjected to the luciferase assay in Neuro2A cells with or without the Etv1 gene. Although extents of the Etv1-mediated activation of the promoter activity were different (1.5- to 30-fold) among the promoter regions of the maturation genes analyzed, Etv1 significantly stimulated the promoter activity of all maturation genes (Fig. 2A). Because Etv1 was reported to be activated by protein phosphorylation (16), the potential phosphorylation sites at six residues of serine or threonine of Etv1 were converted to phosphoserine-mimic glutamic acid (Etv1act). This conversion activated more appreciably the promoter activity of all maturation genes analyzed by the luciferase assay (Fig. 2A). Notably, both Etv1 and Etv1act activated the Etv1 promoter, indicating that Etv1 autoregulates the Etv1 gene expression (Fig. 2A).
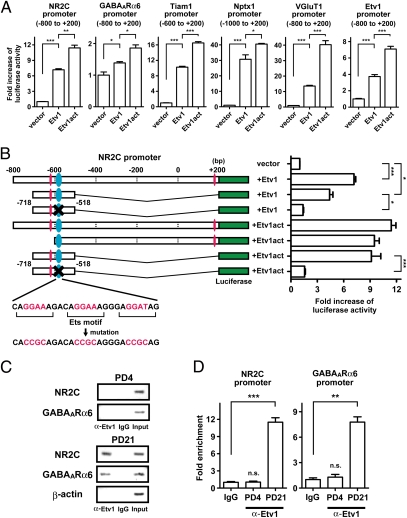
Fig. 2.
Regulation of the promoter function of the maturation genes by Etv1. (A) The Etv1- or Etv1act-dependent promoter activities of the indicated regions from the transcription initiation site (nucleotide residues are shown in parenthesis) were measured by a luciferase assay (n = 3). (B) A cluster of three Ets motifs is shown in blue ellipses. Other Ets motifs are shown in red ellipses. The core GGA(A/T) sequence of the Ets motifs was mutated as indicated and is marked by a cross. The promoter activities were measured by the luciferase assay (n = 3). (C) Protein–cross-linked chromatin was prepared from the cerebellum of PD 4 and PD 21 mice and reacted with anti-Etv1 antibody or control IgG. Immunoprecipitated DNA was subjected to PCR amplification and electrophoresed. (D) Enrichment of each promoter sequence by anti-Etv1 antibody was calculated by quantitative PCR (n = 3). Data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
We next examined whether the Ets motif was responsible for the activation of the promoter function of the NMDA glutamate receptor NR2C gene as a representative maturation gene. The NR2C gene possesses a cluster of three Ets motifs around 600 bp upstream of the transcription initiation site (Fig. 2B). When Etv1 or Etv1act was cotransfected, this region (−718 to −518 residues) was found to be sufficient to activate the promoter activity of the NR2C gene (Fig. 2B). Notably, the promoter activity was abrogated by mutations of the core GGA(A/T) sequence in the cluster of three Ets motifs (Fig. 2B). This analysis explicitly indicates that the Etv1 interaction with the Ets motifs is essential for the promoter function of the NR2C gene.
We next assessed the temporally regulated association of Etv1 with the promoters of the maturation genes by ChIP (Fig. 2 C and D). The Etv1 antibody significantly coimmunoprecipitated the promoter DNAs of the NR2C and GABAA receptor α6 (GABAARα6) genes in the PD 21 cerebellum but not in the PD 4 cerebellum. Neither nonimmunized IgG immunoprecipitated either gene from the PD 4 or PD 21 cerebellum, nor did the Etv1 antibody coimmunoprecipitate the β-actin promoter from the PD 21 cerebellum (Fig. 2C). Etv1 thus is associated with the promoter regions of the maturation genes in vivo in a developmentally regulated manner.
Critical Role of Etv1 in Granule Cell Maturation in Vivo.
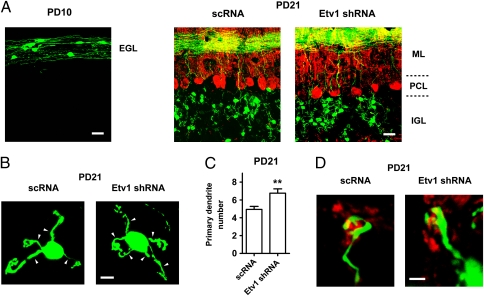
The late-maturing processes of granule cells are not easy to assess in vivo because the homologous Etv1-knockout mice die postnatally within 2–3 wk (17). Therefore, we used the electroporation method, which allows effective expression of the transgene in developing and maturing granule cells (18) (Fig. 3). Etv1 shRNA or scRNA was fused to the GFP DNA and directed by the CAG promoter (19). Upon electroporation into the cerebellum of PD 8 mice, GFP fluorescence was observed in immature granule cells in the EGL of PD 10 mice (Fig. 3A). Etv1 shRNA, but not scRNA, reduced Etv1 mRNA levels by about 80% in GFP+ granule cells on PD 21 (Fig. 4A). Despite the great reduction in Etv1 mRNA levels, shRNA-expressing granule cells, like scRNA-expressing cells, migrated vertically into the IGL and extended parallel fibers horizontally in the molecular layer (Fig. 3A). Expression of Etv1 shRNA had no effect on cell survival. Calbindin D-28K–immunoreactive Purkinje cells also were organized normally in a single layer with a well-polarized morphology on PD 21 (Fig. 3A).
Fig. 3.
Effects of expression of Etv1 shRNA on granule cell maturation in vivo. GFP-Etv1 shRNA or GFP-scRNA was electroporated into the cerebellum on PD 8. (A) Fluorescence visualization of coronal sections of GFP-expressing granule cells in the EGL on PD 10 and in the IGL on PD 21. Calbindin D-28K–immunoreactive Purkinje cells are shown in red. ML, molecular layer; PCL, Purkinje cell layer. (B and C) The distribution (B) and quantification (C) (n = 17 cells) of primary dendrites (arrowhead) of GFP-expressing granule cells on PD 21 are shown. Data are shown as mean ± SEM. **P < 0.01 vs. scRNA. (D) Immunoreactivity of presynaptic synaptophysin (red) was surrounded by a claw-like structure of both Etv1 shRNA-expressing and scRNA-expressing granule cells on PD 21. (Scale bars: 20 μm in A; 5 μm in B; 2 μm in D.)
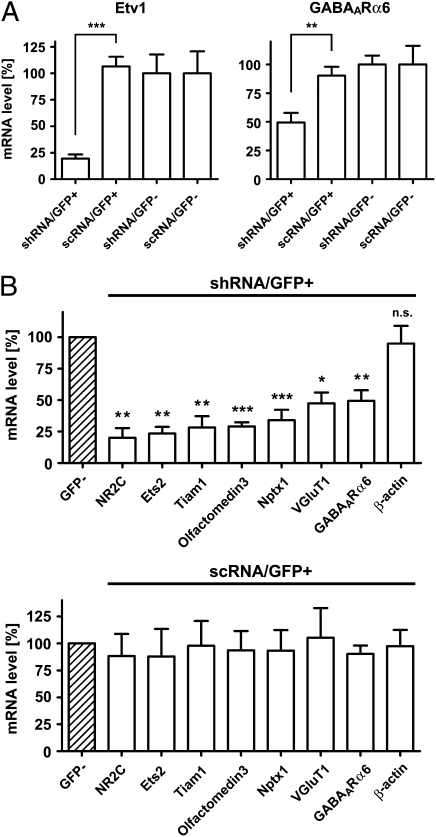
Fig. 4.
Etv1 shRNA prevents expression of maturation genes in granule cells in vivo. After electroporation on PD 8, GFP+/PI− and GFP−/PI− cells were isolated from the PD 21 cerebellum and purified by FACS. (A) mRNA levels of the Etv1 and GABAARα6 genes were quantified by PCR analysis and are presented relative to those in scRNA/GFP− cells (100%). n = 4–13. (B) mRNA levels of the indicated maturation genes were determined individually as in A. mRNA levels of Etv1 shRNA-expressing or scRNA-expressing GFP+ cells are presented relative to those in the corresponding GFP− cells (shaded) (100%). n = 4–14. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
In the terminally maturing phase of granule cells, immature dendritic processes are greatly retracted, and a claw-like structure is formed at the tip of the mature dendrites (3). Primary dendrites of Etv1 shRNA-expressing granule cells, like DNA-untransfected cells, were retracted during maturation in the IGL (Fig. 3B), but this number (6.8 ± 0.5 per cell) was slightly higher than that of scRNA-expressing cells (4.9 ± 0.3 per cell) (Fig. 3C). However, a claw-like structure was formed at the dendritic tips and encapsulated the synaptophysin-immunoreactive presynaptic terminal in the shRNA-expressing granule cells (Fig. 3 B and D). Therefore, consistent with the late up-regulation of Etv1 expression (7), the loss of the Etv1 activity did not perturb the differentiation and migration of developing granule cells or the axon projection pattern of these cells. Furthermore, the functional loss of Etv1 had no effect on the establishment of connections between granule cells and presynaptic axon terminals.
We then examined whether the reduction in Etv1 mRNA would inhibit the expression of the maturation genes in granule cells in vivo (Fig. 4). The cerebellum was isolated on PD 21 after electroporation on PD 8 in vivo, and granule cells were dissociated and stained with propidium iodide (PI), a marker of dead cells. The GFP+/PI− viable granule cells were purified to homogeneity by FACS with GFP and PI fluorometry. The GFP−/PI− cells also were pooled as DNA-untransfected granule cells. Quantitative PCR analysis showed that the reduction in the Etv1 mRNA level by Etv1 shRNA severely impaired the expression of GABAARα6 mRNA, but no such impairment was caused by expression of scRNA in granule cells (Fig. 4A). PCR analysis was extended to other maturation genes, and this analysis explicitly revealed that the expression of the maturation genes was inhibited severely by interference with the Etv1 expression in granule cells in vivo (Fig. 4B). Again, the expression of scRNA had no effect on any of the maturation genes in vivo (Fig. 4B). These results demonstrate that the Etv1 transcription factor plays a pivotal role in orchestrating the program of gene expression involved in the terminal maturation of granule cells.
Discussion
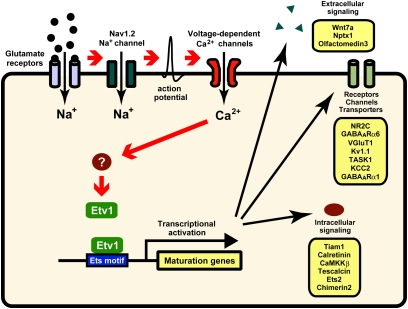
Neuronal cell development and maturation are controlled hierarchically not only by extracellular signaling molecules but also by the intrinsic properties of neuronal cells (1, 20). At the early postnatal period, the shift of resting membrane potential is observed commonly in many neuronal cell types including the lateral geniculate nucleus and layer 1 cortical neurons (21–23). In these neuronal cells, extensive modifications occur in their circuit formation at the early postnatal period (22, 23). Similarly, postmigratory granule cells shift the resting membrane potential from a depolarized state to a hyperpolarized one and markedly change their intrinsic properties (6, 21). The present study has revealed a strikingly unified mechanism for activity-dependent gene regulation responsible for the functional maturation of granule cells (Fig. 5). Glutamatergic excitation of granule cells leads to the Nav1.2 Na+ channel-mediated action potential, which in turn stimulates Ca2+ entry via voltage-dependent Ca2+ channels. This sequential signaling progressively induces Etv1 expression in the process of granule cell maturation (7). Importantly, the inhibition of Etv1 prevented the up-regulation of the maturation genes both in cultured granule cells and in the developing cerebellum in vivo. The Etv1 transcription factor thus plays a key role in orchestrating the activity-dependent gene expression in the terminal maturation program of granule cells.
Fig. 5.
Schematic model for terminal maturation of granule cells.
Although there is a good correlation in both the extent and the order of inhibition of maturation gene expression between TTX and Etv1 siRNA treatments, our several attempts failed to rescue the TTX-mediated inhibition of maturation gene expression by exogenously manipulated Etv1 DNA transfection in cultured cells. The exogenous expression of Etv1 alone thus was not sufficient to up-regulate the activity-dependent expression of the maturation genes. Etv1 is a member of the ETS family and undergoes multiple posttranslational modifications, including phosphorylation, acetylation, and sumoylation (24). The ETS transcription factors also interact with transcriptional partners to enhance transcriptional activation synergistically (25). The protein modifications or the functional protein assembly of Etv1 thus could be required for the Etv1-mediated regulation of the maturation gene expression. An interesting question for future study is how intracellular Ca2+ signaling and the subsequent activation of Etv1 could regulate the activity-dependent expression of the maturation genes.
The key role of Etv1 in the terminal maturation program has been reported in two other types of neuronal cells. Etv1/Er81 is essential for forming the connections between proprioceptive afferents and motor neurons in the assembly of sensory-motor circuitry in mice (26, 27). Etv1 (AST-1) also is a critical determinant that controls the expression of all dopamine pathway genes in Caenorhabditis elegans (15). In both cases, the functional deficit of the Etv1 gene disturbs a terminal step for the establishment of functional neural circuitry, but pan-neuronal features still remain expressed in the Etv1 mutants (15, 26, 28). Similar to these cases, the deficit of Etv1 prevented the expression of a gene battery characteristic of mature granule cells but had no effect on the gross morphology or positioning of the granule cells. Furthermore, consistent with the axogenesis of granule cells before their dendritic formation (29), in vivo knockdown of Etv1 expression showed no abnormality in the parallel fiber projection to Purkinje cells. Etv1 is capable of autoregulating its mRNA expression, and Etv1 mRNA levels increase progressively during granule cell maturation and are maintained throughout the life of granule cells (7). Importantly, Etv1 up-regulates a large number of maturation genes involved in synaptic transmission including NR2C, GABAARα6, and the TASK1 leak K+ channel genes. The coordinated regulation of these synaptic proteins is essential for functional synaptic transmission in the cerebellar circuit (30). For example, a switch of NMDA receptor subunits from NR2B to NR2C alters many properties of NMDA receptors and participates in the coordination of complex motor movements (31). The present study thus has demonstrated that the cell-intrinsic Etv1 regulation plays a key role both in directing the maturation program of the cerebellar circuit and in specifying the identity of the granule cell type.
Materials and Methods
Culture.
All procedures for animal handling were performed according to the guidelines of Osaka Bioscience Institute. Cultures containing ∼90% granule cells were prepared from PD 8 ICR mice. Granule cells were cultured for 24 h in medium containing serum and then for 96 h in serum-free medium containing a physiological concentration of KCl (5 mM KCl) in the presence or absence of the indicated inhibitors (7). The addition of the inhibitors used did not affect cell viability at least up to 96 h in culture (9, 32). TTX, CPP, NBQX, NNC 55–0396, and nifedipine were obtained from Tocris; ω-agatoxin TK and ω-conotoxin GVIA were obtained from Peptide Institute.
Microarray Analysis, Quantitative PCR, Immunochemical Analysis, and Electrophysiology.
Microarray analysis was performed twice by using an Affymetrix Mouse Genome 430 2.0 Array (7). Data were averaged and analyzed by using GeneSpring GX software (Agilent Technologies). Quantitative PCR analysis was performed using appropriate primers that gave rise to 60- to 150-bp DNA segments. GAPDH mRNA was used as an internal control for normalization of mRNA levels. Total RNA of FACS-purified granule cells was subjected to quantitative PCR analysis as described previously (33). For morphological characterization, GFP fluorescence and immunostained markers were visualized by confocal imaging (6). Anti–calbindin D-28K was obtained from Sigma-Aldrich, and anti-synaptophysin was obtained from Millipore Bioscience Research Reagents. For electrophysiological analysis, patch-clamp recordings were performed as described previously (6).
siRNA Analysis.
siRNAs were obtained from the following sources: Nav1.2 siRNA from Dharmacon (catalog no. J-066199-07); Etv1 siRNA-1 and siRNA-2 from Ambion (catalog nos. s65717 and s119233, respectively); Nav1.2 siRNA, Etv1 siRNA-1, and siRNA-2, corresponding to nucleotide residues 8,413–8,431, 821–839, and 1,042–1,060 from the translation initiation sites, respectively. siRNA or scRNA (6 μg each) was electroporated into dissociated granule cells with the use of a Mouse Neuron Nucleofector kit and Nucleofector device (Amaxa), and electroporated cells were cultured in medium containing serum for 24 h and in serum-free medium for 96 h. Cell viability was confirmed to be unaffected by siRNA treatment.
Luciferase Assay.
The promoter sequences were attached to the luciferase gene in the pGL4.10 vector (Promega). The Etv1act gene and the mutated NR2C promoter were generated by using a QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). DNAs were transfected into Neuro2A cells by using Lipofectamine LTX, and the luciferase activity was measured 24–36 h after DNA transfection by using the Dual-Glo luciferase assay system (Promega). Renilla luciferase was used as a transfection control.
ChIP Assay.
The ChIP assay was performed following the procedures described by Takahashi et al. (34). Briefly, the cerebellum was sliced (400-μm thickness) and placed in ice-cold PBS solution containing protease inhibitors. Genome-associated proteins were cross-linked by 1% formaldehyde. Slices were homogenized, and lysates were sonicated to yield DNA fragments 0.5–1.5 kbp in size. The genome-associated proteins were immunoprecipitated with anti-Etv1 antibody (Santa Cruz Biotechnology), and DNA samples were purified and subjected to PCR analysis. From the transcription initiation sites, the forward and reverse primers used were residues −611 to −591 and −491 to −471, respectively, for NR2C and residues −675 to −653 and −560 to −536, respectively, for GABAARα6.
In Vivo Electroporation and Cell Purification by FACS.
In vivo electroporation was performed according to the procedures described by Umeshima et al. (18). Briefly, on PD 8 pups were anesthetized, and 10 μg of plasmid DNA was injected by application of electric pulses (seven pulses of 75–80 V for 50-ms duration with 150-ms intervals). The Etv1 shRNA expression vector used was constructed by inserting in tandem the oligo DNA (residues 566–586 from the translation initiation site) into the pcDNA6.2-GW/EmGFP-miR vector (Invitrogen). The in-tandem shRNA oligo DNA then was transferred downstream of the GFP gene of the pCAGGS-GFP vector (6). GFP+/PI− and GFP−/PI− cells were isolated from the PD 21 cerebellum and purified by FACS as described previously (33).
Statistical Analysis.
Statistical analysis was conducted using unpaired student's t test or one-way ANOVA. Statistical significance and correlation coefficient were determined with GraphPad Prism software.
Supplementary Material
Acknowledgments
We thank H. Umeshima and M. Kengaku for technical advice. This work was supported by Grants KAKENHI 22220005 (to S.N.) and 21790294 (to M.O.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by a grant from the Research Fellowship of the Japan Society for the Promotion of Science (to H.A.), and by grants from the Takeda Science Foundation and the Suntory Institute for Bioorganic Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109940108/-/DCSupplemental.
References
- 1.Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 2.Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramón y Cajal S. In: Texture of the Nervous System of Man and the Vertebrates. Pasik P, Pasik T, editors. Vol II. Vienna: Springer; 2000. pp. 395–419. [Google Scholar]
- 4.Rossi P, De Filippi G, Armano S, Taglietti V, D'Angelo E. The weaver mutation causes a loss of inward rectifier current regulation in premigratory granule cells of the mouse cerebellum. J Neurosci. 1998;18:3537–3547. doi: 10.1523/JNEUROSCI.18-10-03537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J Neurosci. 2003;23:6074–6085. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazawa M, et al. Role of calcineurin signaling in membrane potential-regulated maturation of cerebellar granule cells. J Neurosci. 2009;29:2938–2947. doi: 10.1523/JNEUROSCI.5932-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato M, Suzuki K, Yamazaki H, Nakanishi S. A pivotal role of calcineurin signaling in development and maturation of postnatal cerebellar granule cells. Proc Natl Acad Sci USA. 2005;102:5874–5879. doi: 10.1073/pnas.0501972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: Electrical activity regulates the GABAA receptor α 6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci. 2005;25:9535–9543. doi: 10.1523/JNEUROSCI.2191-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez-Ford C, et al. Characterization of tescalcin, a novel EF-hand protein with a single Ca2+-binding site: Metal-binding properties, localization in tissues and cells, and effect on calcineurin. Biochemistry. 2003;42:14553–14565. doi: 10.1021/bi034870f. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci. 2003;17:2563–2572. doi: 10.1046/j.1460-9568.2003.02698.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolvetang EJ, et al. Overexpression of the chromosome 21 transcription factor Ets2 induces neuronal apoptosis. Neurobiol Dis. 2003;14:349–356. doi: 10.1016/s0969-9961(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 13.Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer Associates; 2001. pp. 95–306. [Google Scholar]
- 14.Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel α-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): Different expression patterns in developing rat nervous system. Brain Res Mol Brain Res. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- 15.Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458:885–889. doi: 10.1038/nature07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosc DG, Goueli BS, Janknecht R. HER2/Neu-mediated activation of the ETS transcription factor ER81 and its target gene MMP-1. Oncogene. 2001;20:6215–6224. doi: 10.1038/sj.onc.1204820. [DOI] [PubMed] [Google Scholar]
- 17.Cave JW, et al. Differential regulation of dopaminergic gene expression by Er81. J Neurosci. 2010;30:4717–4724. doi: 10.1523/JNEUROSCI.0419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc Natl Acad Sci USA. 2007;104:16182–16187. doi: 10.1073/pnas.0708047104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 20.Hébert JM, Fishell G. The genetics of early telencephalon patterning: Some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J Physiol. 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F-M, Hablitz JJ. Postnatal development of membrane properties of layer I neurons in rat neocortex. J Neurosci. 1996;16:1131–1139. doi: 10.1523/JNEUROSCI.16-03-01131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Launoit Y, et al. The Ets transcription factors of the PEA3 group: Transcriptional regulators in metastasis. Biochim Biophys Acta. 2006;1766:79–87. doi: 10.1016/j.bbcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Pei H, Watson DK. Regulation of Ets function by protein–protein interactions. Oncogene. 2000;19:6514–6523. doi: 10.1038/sj.onc.1204035. [DOI] [PubMed] [Google Scholar]
- 26.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 27.Patel TD, et al. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- 28.Hobert O. Regulatory logic of neuronal diversity: Terminal selector genes and selector motifs. Proc Natl Acad Sci USA. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M. The Cerebellum and Neural Control. New York: Lippincott-Raven; 1984. [Google Scholar]
- 30.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi S. Synaptic mechanisms of the cerebellar cortical network. Trends Neurosci. 2005;28:93–100. doi: 10.1016/j.tins.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Iijima K, Abe H, Okazawa M, Moriyoshi K, Nakanishi S. Dual regulation of NR2B and NR2C expression by NMDA receptor activation in mouse cerebellar granule cell cultures. Proc Natl Acad Sci USA. 2008;105:12010–12015. doi: 10.1073/pnas.0805574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamazaki H, Sekiguchi M, Takamatsu M, Tanabe Y, Nakanishi S. Distinct ontogenic and regional expressions of newly identified Cajal-Retzius cell-specific genes during neocorticogenesis. Proc Natl Acad Sci USA. 2004;101:14509–14514. doi: 10.1073/pnas.0406295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi Y, Rayman JB, Dynlacht BD. Analysis of promoter binding by the E2F and pRB families in vivo: Distinct E2F proteins mediate activation and repression. Genes Dev. 2000;14:804–816. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.