Abstract
Here we report a human intellectual disability disease locus on chromosome 14q31.3 corresponding to mutation of the ZC3H14 gene that encodes a conserved polyadenosine RNA binding protein. We identify ZC3H14 mRNA transcripts in the human central nervous system, and we find that rodent ZC3H14 protein is expressed in hippocampal neurons and colocalizes with poly(A) RNA in neuronal cell bodies. A Drosophila melanogaster model of this disease created by mutation of the gene encoding the ZC3H14 ortholog dNab2, which also binds polyadenosine RNA, reveals that dNab2 is essential for development and required in neurons for normal locomotion and flight. Biochemical and genetic data indicate that dNab2 restricts bulk poly(A) tail length in vivo, suggesting that this function may underlie its role in development and disease. These studies reveal a conserved requirement for ZC3H14/dNab2 in the metazoan nervous system and identify a poly(A) RNA binding protein associated with a human brain disorder.
Keywords: polyadenylation, RNA processing, zinc-finger, mental retardation
Unraveling the complex networks underlying brain function is a challenging problem for both basic and medical science. One way to understand brain function is to identify and characterize genes that, when mutated, impair normal human intellectual development. Intellectual disability (ID), previously referred to as mental retardation, is characterized by limited intellectual capacities reflected by an intelligence quotient (IQ) below 70 and major constraints in adaptive behavior (1). Therapeutic options for the treatment of ID are extremely limited, and its comparatively high prevalence of about 2% renders this disorder a major socioeconomic burden (1).
During the course of a large-scale systematic study to identify autosomal recessive ID (ARID) causing genetic defects in large Iranian families with intellectually disabled children born from blood-related parents (2, 3), we identified a locus for unspecific or nonsyndromic ARID (NS-ARID) on chromosome 14q31.3 corresponding to mutation of the ZC3H14 gene in two independent families. ZC3H14 encodes an evolutionarily conserved Cys3His tandem zinc finger polyadenosine RNA binding protein (4, 5). The founding member of this protein family, Saccharomyces cerevisiae Nab2, is essential for viability and required for proper 3′-end formation and poly(A) RNA export from the nucleus (6, 7). Although multiple tissue-specific splice variants of human ZC3H14 have been described (5), their function in multicellular organisms has not been examined.
To better understand ZC3H14/Nab2 function in metazoans, we exploited Drosophila melanogaster as a model for the developmental consequences of ZC3H14 loss in humans. Loss of the putative Drosophila ZC3H14 ortholog, dNab2, disrupts normal development and impairs neural function. Using tissue-specific depletion, we identify a pan-neuronal requirement for dNab2 in normal behavior. Biochemical and genetic analyses indicate that dNab2 restricts bulk RNA poly(A) tail length in vivo and suggest that this conserved function may underlie the effect of dNab2 loss on development and behavior. Taken together, these studies reveal a conserved requirement for ZC3H14/dNab2 in the metazoan nervous system and identify a poly(A) RNA binding protein associated with a human brain disorder.
Results
ZC3H14 Gene Is Mutated in NS-ARID Patients.
To identify molecular causes of NS-ARID, we performed a large-scale autozygosity mapping and linkage analysis in a cohort of more than 200 consanguineous Iranian families (3). This analysis identified an NS-ARID locus on chromosome 14q31.3-q32.12 in a family with three affected males (Fig. 1A and Table S1). The linkage interval had the maximum attainable LOD [logarithm (base 10) of odds of linkage] score of 2.7 (Fig. S1B), and no other autosomal linkage intervals were observed according to the one LOD down rule (8). A second significant interval was identified on chromosome Xp22.11-p11.4 (LOD = 1.2), but this interval contained no sequence changes in protein coding regions (Fig. S1 B, D, and E). Sequencing of all protein coding regions within the 14q31.3-q32.12 locus (Fig. S1 F and G) identified a homozygous nonsense mutation (R154X) in exon 6 of the ZC3H14 gene (Fig. 1B and Fig. S2A), which cosegregated with the disease. This mutation was absent in 1,864 chromosomes from healthy individuals, including 1,184 chromosomes from ethnically matched controls, 310 chromosomes from German controls, and 370 chromosomes from the 1,000 genome pilot projects 1 and 2 (9). Moreover, screening of the entire gene in a subset of 330 chromosomes from the Iranian controls and the 370 chromosomes from the 1,000 genome project detected no deleterious mutations.
Fig. 1.
ZC3H14 is mutated in NS-ARID patients. (A) Pedigree of Family-1. (B) Schematic of four ZC3H14 splice variants indicating exons encoding the N-terminal PWI-like domain and C-terminal Cys3His zinc-finger (ZnF) RNA binding motif (CCCH) domain. Positions of patient mutations are indicated by red stars. (C) Anti-ZC3H14 immunoblot of two control lymphoblast lines and one derived from a Family-1 R154X patient. The ZC3H14 antibody (5) recognizes ZC3H14 isoforms 1 and 2/3. Anti-PABPN1 is shown as a loading control (20). (D) Immunofluorescent detection of ZC3H14 (green) in control or patient (R154X) fibroblasts using a commercial ZC3H14 antibody (Abcam) directed against the common ZnF domain. DAPI (blue) marks nuclei. (Scale bar: 10 μm.)
The ZC3H14 gene encodes a poly(A) RNA binding protein with similarity to S. cerevisiae Nab2 (4, 5). As shown in Fig. 1B, ZC3H14 is alternatively spliced to encode four ZC3H14 protein isoforms (5). The R154X mutation is predicted to disrupt the ubiquitously expressed longer isoforms 1–3 but not the shorter brain- and testes-enriched isoform 4. Immunoblot analysis using an anti-ZC3H14 antibody raised against the N-terminal proline-tryptophan-isoleucine (PWI)-like domain of isoforms 1–3, which exclusively recognizes isoforms 1–3 (5), confirmed that R154X patient-derived lymphoblasts lack ZC3H14 isoforms 1–3 (Fig. 1C). Parallel staining of R154X patient fibroblasts with a commercial ZC3H14 antibody that recognizes all four ZC3H14 isoforms (Abcam) revealed no detectable nuclear ZC3H14 (isoforms 1–3), whereas the cytoplasmic pool of protein, corresponding to isoform 4 (5), was still present (Fig. 1D, Lower). Subsequent sequencing of ZC3H14 in a second family showing NS-ARID and a linkage interval with maximum attainable LOD score (2.5) at the same chromosome 14 locus revealed a 25-bp deletion located 16 bp downstream of the 3′-end boundary of the annotated common exon 16 of ZC3H14 (Fig. 1B, Figs. S1 A, C, and H and S2 B–D, and Table S1). This mutation cosegregated with the patient phenotype and was not found to be homozygous in 831 control individuals.
ZC3H14 Protein Is Expressed in the CNS and Colocalizes with Poly(A) mRNA in Hippocampal Neurons.
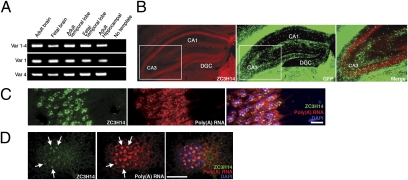
We confirmed that ZC3H14 is expressed in the brain. ZC3H14 transcripts were readily detected in adult and fetal human brain samples by RT-PCR (Fig. 2A). Immunostaining of sections of adult mouse brain revealed that ZC3H14 protein is enriched in hippocampal neurons relative to glia (Fig. 2B). Furthermore, poly(A) RNA-specific FISH combined with anti-ZC3H14 immunostaining using the anti–PWI-like domain antibody showed that ZC3H14 and poly(A) RNA colocalize in nuclear speckles in both the pyramidal layer of mouse CA1 (Fig. 2C) and cultured rat hippocampal neurons (Fig. 2D).
Fig. 2.
ZC3H14 is expressed in vertebrate hippocampal neurons. (A) RT-PCR analysis of ZC3H14 splice variants: variants 1–4 (Top), variant 1 (Middle), or variant 4 (Bottom) from indicated tissues. For B–D, ZC3H14 was detected with the N-terminal antibody that recognizes ZC3H14 isoforms 1 and 2/3 (5). (B) Immunofluorescent detection of ZC3H14 protein (red) in a mouse hippocampal section expressing oligodendroglia-GFP (green) (21). Cornu ammonis fields 1 and 3 (CA1 and CA3, respectively) and dentate gyrus granular cells (DGC) regions of the hippocampus are indicated. The white boxes indicate the zoom-in region in the merge. (C) Adolescent mouse brain sections probed with an oligo-dT FISH probe to detect poly(A) RNA (red) and costained for ZC3H14 (green). ZC3H14 appears in poly(A) RNA-positive nuclear speckles in hippocampal pyramidal neurons. DAPI (blue) marks nuclei. (Scale bars: 20 μm.) (D) Poly(A) RNA FISH (red) and indirect immunofluorescence in cultured rat embryonic hippocampal neurons reveal colocalization of ZC3H14 protein (green) with poly(A) RNA speckles in the nucleus (blue). (Scale bar: 5 μm.)
dNab2 Is a Putative D. melanogaster Ortholog of ZC3H14 and a Member of an Evolutionarily Conserved Class of Zinc Finger Polyadenosine RNA Binding Proteins.
We next exploited Drosophila as a system to understand tissue-specific roles and requirements for ZC3H14 in metazoans. Based on sequence similarity and domain conservation, we identified the uncharacterized gene CG5720 (Flybase.org) as the putative Drosophila ZC3H14/Nab2 ortholog (dNab2) (Fig. 3A). The conserved dNab2 C-terminal tandem Cys3His zinc finger (ZnF) domain (Fig. 3A), which mediates polyadenosine RNA binding in other species (4, 10), showed preferential binding to polyadenosine RNA in vitro (Fig. 3B). The intracellular localization of dNab2 mirrored the localization of both Nab2 (7) and ZC3H14 (isoforms 1–3) (5), because immunostaining for dNab2 revealed nuclear expression throughout development in all tissues examined, including the nervous system (Fig. S3 A and B).
Fig. 3.
dNab2 is a putative D. melanogaster ortholog of ZC3H14. (A) Domain alignment of S. cerevisiae Nab2, Drosophila dNab2, and human ZC3H14. The conserved N-terminal PWI-like fold, Q-rich, RGG/predicted nuclear localization signal (NLS), and C-terminal tandem Cys3His ZnF RNA binding motif (CCCH) domains are indicated (5, 10). Amino acid alignment of the five Cys3His tandem ZnFs from fly dNab2 and human ZC3H14 shows conserved spacing and intervening basic and aromatic residues (underlined) that are required for RNA binding in S. cerevisiae Nab2 (10). (B) RNA binding properties of dNab2 analyzed by RNA electrophoretic mobility shift assay. GST-dNab2 ZnF (ZnFs 1–5) but not GST binds to polyadenosine 25-mer (pA25) RNA. The top arrow indicates a shift. Unlabeled pA25 but not randomized polyN 25-mer (pN25) RNA competitor oligonucleotide competes efficiently for binding to dNab2 ZnF. (C) Schematic of the dNab2 locus indicating the location of the EP3716 and EY08422 elements (inverted triangles) and the five imprecise excision alleles (ex1–ex5). (D) Quantitative real-time RT-PCR analysis of dNab2 transcript levels in adults flies. All genotypes were analyzed in triplicate and normalized to dNab2 transcript levels in w1118 control animals (set to 1.0). β-tub is an internal control. Error bars = SD. (E–O) Light microscopic images of adult flies of indicated genotypes. (E and F) The majority of dNab2ex3 and dNab2ex3/Df [Df(3R)Exel8178] animals die at pharate adult stage, often as partially emerged adults. (G and H) The remainder emerge with a wings held-out phenotype that is (I) absent in controls (p-ex). (J–O) Front and side views of the thorax showing thoracic bristles. dNab2ex3 (J and K) and dNab2ex3/Df (L and M) mutants show bent major thoracic bristles (arrowheads in J and L are enlarged in J Inset and L Inset, respectively) and disorganized minor thoracic bristles (arrows in J and L) compared with p-ex controls (N and O).
dNab2 Is Essential for Normal Development.
To determine whether dNab2 contributes to development or function of the nervous system, we created dNab2 alleles by imprecise excision of a P-element (P{EPgy2}EY08422) located upstream of the dNab2 gene (Fig. 3C). Five excisions of EY08422 (ex1-ex5) were recovered with genomic deletions ranging from 0.9 to 1.5 kb that extend into the dNab2 gene (Fig. 3C); all of these alleles failed to express dNab2 mRNA and protein (Fig. 3D and Fig. S3C). The dNab2ex3 null allele was used for all subsequent experiments. Through mid-pupal development, dNab2ex3 homozygous mutants showed no evidence of morphological or behavioral defects, reduced viability, or developmental delay. However, a majority of the dNab2ex3 homozygotes died during late pupal phase and displayed eclosion defects (Fig. 3E); the few remaining dNab2ex3 mutants (∼3–5%) emerged completely, but they exhibited a shortened lifespan (∼1.5 wk) and morphological defects reminiscent of other mutants in RNA binding proteins, including defects in neuronal function (11, 12). These phenotypes include wings held-out, in which flies fail to fold their wings together over the dorsal surface of the thorax and abdomen (Fig. 3G), and disorganization and bending of thoracic bristles (Fig. 3 J and K). All of these phenotypes were also present in animals carrying dNab2ex3 in trans to genomic deletions (deficiencies) that completely remove the dNab2 gene (Fig. 3 F, H, L, and M and Fig. S3 D–K), but they were absent in control flies that are homozygous for a precise excision (p-ex) of the EY08422 element (Fig. 3 I, N, and O). Embryos lacking germline contribution of dNab2 died early in embryogenesis, indicating that dNab2 is required for both embryonic viability and development.
dNab2 Is Required in Neurons for Normal Drosophila Behavior.
In addition to morphological defects, dNab2ex3 mutant flies or those flies carrying dNab2ex3 in trans to an uncovering deficiency displayed severely compromised flight behavior and poor locomotor activity (Fig. 4A, Fig. S4 A and B, and Movies S1 and S2). However, zygotic loss of dNab2 caused no changes in expression patterns of the neuronal marker Elav or the presynaptic active zone marker Nc82 that were evident at the level of the whole brain (Fig. S3 L–O). Similarly, loss of dNab2 had no detectable effect on gross synaptic structure based on analysis of synaptic bouton number and organization at the larval muscle 6/7 neuromuscular junction (Fig. S3P).
Fig. 4.
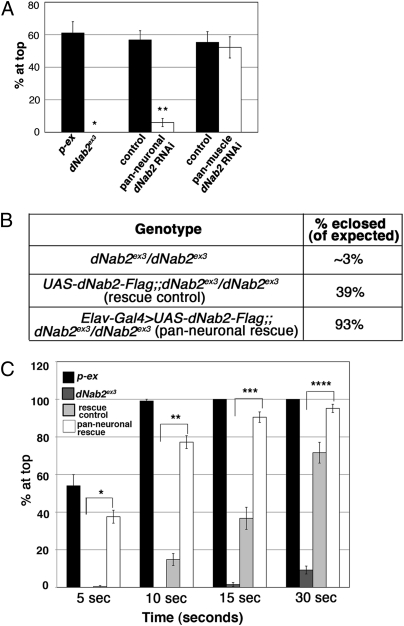
A neuronal-specific requirement for dNab2 in normal behavior. (A) Locomotor phenotypes of genomic alleles and tissue-specific RNAi of dNab2. Data are presented as the average percentage of flies that reach the top of a cylinder after 5 s across all trials. Groups of 10 5-d-old flies were tested for at least 10 independent trials per genotype (*P = 8.36 × 10−9 and **P = 8.38 × 10−9 in a two-tailed t test). Error bars = SEM. (B and C) Pan-neuronal expression of dNab2 rescues both eclosion and locomotor defects. (B) Table summarizing the percentage of flies eclosed (of expected) for indicated genotypes. (C) For the locomotor assay, data are presented as the average percentage of flies that reaches the top of a cylinder after indicated time points across all trials. Groups of 10 2-d-old flies were tested for at least 10 independent trials per genotype (*P = 5.12 × 10−13, **P = 6.59 × 10−13, ***P = 1.97 × 10−7, and ****P = 0.002 in a two-tailed t test). Error bars = SEM.
To examine tissue-specific requirements for dNab2, we used an inverted repeat (IR) dNab2 RNAi transgene, which reduced dNab2 protein levels in vivo (Fig. S4D), to deplete dNab2 from specific tissues. Pan-neuronal knockdown of dNab2 (Elav-Gal4 > Dcr2, IR) caused flight defects, indicating that dNab2 is required in neurons for normal flight behavior (Fig. S4A). Furthermore, pan-neuronal knockdown of dNab2 strongly recapitulated the locomotor defect of the dNab2 genomic null allele (Fig. 4A and Movies S3 and S4). A modest decline in locomotor activity was also observed in flies depleted of dNab2 specifically in motor neurons (OK6-Gal4 > IR) (Fig. S4C), suggesting that dNab2 acts within multiple types of neurons to support normal locomotor behavior. In contrast, pan-muscle dNab2 knockdown flies generated by using two independent muscle-specific Gal4 drivers (Mef2-Gal4 or Mhc-Gal4) showed a negative geotaxis response indistinguishable from controls (Fig. 4A and Fig. S4E), revealing that dNab2 may be specifically required in neurons for normal locomotor activity.
To confirm the neuronal requirement for dNab2, we tested whether a transgene expressing WT dNab2 (UAS-dNab2-Flag) only in neurons could rescue the dNab2ex3 mutant phenotype. The UAS-dNab2 transgene alone mildly rescued eclosion rates and locomotor defects among dNab2 mutants in the absence of a Gal4 driver (rescue control) (Fig. 4 B and C). Consistent with this rescue, a low level of leaky expression from the transgene was confirmed by immunoblotting. Importantly, restoring dNab2 expression pan-neuronally (Elav-Gal4) in dNab2 null flies completely rescued the eclosion and locomotor defects (Fig. 4 B and C and Movies S5 and S6). In control experiments, ubiquitous overexpression of dNab2 was lethal to WT flies, but those flies overexpressing dNab2 from a pan-neuronal driver (Elav-Gal4) were viable and performed normally in locomotor assays (Fig. S4 F and F′), indicating that rescue by neuronal expression of dNab2 was not caused by enhanced performance. Thus, expression of dNab2 only in neurons seems to be sufficient to rescue viability and behavior in animals otherwise lacking dNab2.
dNab2 Is Required for Proper Control of Poly(A) Tail Length.
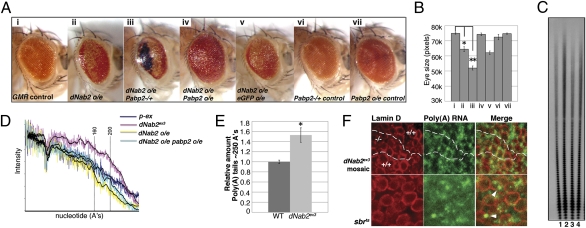
To begin to understand the molecular role of dNab2 in the nervous system, we used a genetic-modifier approach to screen a small collection of alleles of select genes for their ability to modify a rough-eye phenotype produced by overexpressing WT dNab2 in the differentiating neurons of the eye (Fig. 5A, i and ii). Although most alleles tested showed little or no effect, we observed robust and fully penetrant genetic interactions between dNab2 and two components of the polyadenylation machinery (Table S2): the poly(A) polymerase hiiragi (hrg) (13) and Pabp2, the Drosophila ortholog of the nuclear poly(A) binding protein PABPN1/PABP2 that promotes polyadenylation of mRNAs (14). Heterozygosity for an hrg loss of function allele, hrg10 (15), strongly enhanced the dNab2-driven adult eye phenotype, but it had no dominant effect on eye morphology in a WT background. Similarly, the Pabp255 loss of function allele dominantly enhanced the dNab2 overexpression phenotype, resulting in a smaller, more disorganized, and blackened eye (Fig. 5A, iii). Reciprocally, overexpression of Pabp2 using the EP2264 allele (16) significantly suppressed the dNab2-driven rough-eye phenotype (Fig. 5A, iv), whereas expression of a control UAS-eGFP transgene had no effect (Fig. 5A, v). This qualitative modification of the dNab2-driven rough, small-eye phenotype by Pabp2 alleles was confirmed by 2D quantification of eye size (Fig. 5B).
Fig. 5.
dNab2 regulates poly(A) tail length. (A) Light microscopic images of adult fly eye (A) and (B) quantification of eye size (pixels) for the following genotypes: (A, i) GMR-Gal4/+ (GMR control), (A, ii) GMR-Gal4/+;dNab2EP3716/+ (dNab2 o/e), (A, iii) GMR-Gal4/Pabp255;dNab2EP3716/+ (dNab2 o/e Pabp2−/+), (A, iv) GMR-Gal4/Pabp2EP2264;dNab2EP3716/+ (dNab2 o/e Pabp2 o/e), (A, v) GMR-Gal4/+;dNab2EP3716/UAS-eGFP (dNab2 o/e eGFP o/e), (A, vi) GMR-Gal4/Pabp255 (Pabp2−/+ control), and (A, vii) GMR-Gal4/Pabp2EP2264 (Pabp2 o/e control). o/e, overexpression. *P = 0.0006 and **P = 0.0014 in a two-tailed t test (n = 5 per genotype). Error bars = SEM. (C) Bulk poly(A) tail length measurements in heads of (1) control (p-ex), (2) dNab2ex3, (3) GMR-Gal4/+;dNab2EP3716/+, and (4) GMR-Gal4/Pabp2EP2264;dNab2EP3716/+. (D) Densitometric quantification of poly(A) tracts (Image J) from C showing poly(A) tail length profiles of the indicated genotypes (highlighted in colored lines). (E) Bulk poly(A) tail length from whole flies as analyzed by densitometric quantification of poly(A) tracts (Image J) of ∼250 nt normalized to poly(A) tracts of ∼100 nt for WT (w1118) and dNab2ex3. w1118 control was set to 1.0. (*P < 0.04; n = 3, two-tailed t test). Error bars = SD. (F) Poly(A) RNA localization was analyzed in wing disc cells subjected to FISH to visualize poly(A) RNA (green) and costained with anti-Lamin D (red) to visualize the nuclear periphery. Upper shows a dNab2ex3 mosaic larval wing disc; dotted lines denote boundaries between WT (+/+) and dNab2ex3 mutant clones (−/−). Lower shows a wing disc homozygous for the sbrts allele (17) shifted to 33 °C. Arrowheads indicate nuclei accumulating poly(A) RNA.
To determine whether the genetic interactions between dNab2 and Pabp2 in the adult eye reflect a role for dNab2 in poly(A) tail length control, bulk RNA poly(A) tail length was measured and quantified in adult heads (Fig. 5 C and D). As described for S. cerevisiae nab2 mutants (6, 10), poly(A) tail length was increased in dNab2ex3 mutant heads (Fig. 5C, lane 2, and D) relative to p-ex controls (Fig. 5C, lane 1, and D). Similar data were obtained from analysis of RNA isolated from whole flies (Fig. 5E). Reciprocally, overexpression of dNab2 in the eye shortened bulk poly(A) tail length relative to p-ex control, and consistent with the genetic modification data, this molecular effect was rescued by cooverexpression of Pabp2 (Fig. 5 C and D). These biochemical effects, thus, parallel the genetic relationship between dNab2 and Pabp2, and they argue strongly that these proteins have antagonistic effects on bulk poly(A) tail length in neuron-enriched adult tissues such as the eye.
In contrast to the effect of dNab2 on polyadenylation, no obvious change in poly(A) RNA localization was apparent in clones of dNab2ex3 mutant larval wing disc cells (−/−) generated in the background of WT control cells (+/+) (Fig. 5F, Upper). As a control, cells mutant for the mRNA export receptor sbr (17) showed nuclear accumulation of poly(A) RNA (arrowheads in Fig. 5F, Lower). Thus, loss of dNab2 seems to dysregulate poly(A) tail length and cellular function without perturbing bulk poly(A) RNA export from the nucleus.
Discussion
In an effort to better understand the molecular and cellular processes that underlie normal brain function, we have sought to identify mutations that lead to ID in the human population. Here, we identify mutations in the human ZC3H14 gene in human patients with NS-ARID and create a tractable genetic model that recapitulates key phenotypic elements of the human disease. We show that the Drosophila ZC3H14 ortholog dNab2 regulates RNA poly(A) tail length and that loss of dNab2 leads to extended RNA poly(A) tails. The effect of dNab2 on RNA poly(A) tail length coupled with neuronal-specific behavioral phenotypes seen in dNab2 mutant flies and ZC3H14-associated NS-ARID patients provide evidence that dNab2-mediated control of RNA poly(A) tail length is required for normal neuronal function.
Loss of dNab2 in Drosophila or NAB2 in budding yeast causes an increase in bulk RNA poly(A) tail length (6), but the mechanism by which these hyperadenylated mRNAs contribute to neuronal dysfunction or possibly, to human disease is not established. Likely consequences of hyperadenylated mRNAs could include altered transcript stability, titration of critical poly(A) RNA binding proteins, and/or bypass of cytoplasmic polyadenylation necessary for activity-dependent translation of neuronal mRNAs. Individually or combined, these defects could disrupt spatiotemporal control of gene expression needed for development of the nervous system and higher-order brain function. Thus, we speculate that ZC3H14/dNab2 could play critical roles in neurons such as ensuring that transcripts are properly targeted to sites of localized translation. This hypothesis is consistent with a report that budding yeast Nab2 aids in targeting transcripts to the bud site (18). Alternatively, ZC3H14 and dNab2 may regulate a set of mRNAs that play key roles in neurons, such that ZC3H14/dNab2 loss disproportionately affects this cell type. These mechanisms could explain why mutation of ubiquitously expressed posttranscriptional regulatory factors such as dNab2 and ZC3H14 leads to neuronal defects in flies and more critically, to NS-ARID in humans.
Although the NAB2 and dNab2 genes are essential (7), loss of the corresponding forms of the ZC3H14 protein (isoforms 1–3) in humans seems to selectively impair brain function, because patients display nonsyndromic intellectual disability. At this early stage of investigation, it is unclear whether ZC3H14 is simply not essential in humans or whether the remaining cytoplasmic isoform of the protein, isoform 4, suffices in all tissues except the brain. Alternatively, we cannot rule out the possibility that a protein that is functionally redundant with ZC3H14 exists; however, the human genome does not encode any apparent sequence orthologs of ZC3H14. Because isoform 4 is only expressed in mammals (5), future studies exploiting mammalian model systems will be required to address the functional requirements for specific isoforms of ZC3H14 as they relate to human intellectual disability.
The identification of ZC3H14 mutations in NS-ARID places ZC3H14/dNab2 among several other RNA binding proteins implicated in human diseases that impact neural function (19). However, this study identifies a direct link between a poly(A) RNA binding protein and a human brain disorder and thus provides insight into the molecular basis of intellectual disability and brain function.
Materials and Methods
Subjects.
All patients from both families suffer from either mild–moderate (Family-1) or severe (Family-2) NS-ARID (Table S1). Sample collection and clinical evaluation were carried out as previously described (2) with the informed written consent of the parents.
Genetic Analyses.
All affected members, parents, and healthy siblings from both families were genotyped using the Affymetrix GeneChip Human Mapping 10K Arrays. All subsequent analyses were carried out using the standard methods described in SI Materials and Methods.
Drosophila Analyses.
All genetic manipulations, behavioral assays, and immunostaining experiments were performed using the standard methods described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the H.-H.R., A.W.K., K.H.M., A.H.C., G.J.B., Y.F., and S.S. laboratories for helpful discussion and advice. We are grateful to M. Schlicht, B. Lipkowitz, and S. Freier for technical support, A. V. Mortimer for fly behavioral assays, A. Locke for statistical analysis, M. Simonelig, I. Davis, and K. Moses for reagents, and C. Goswami for confocal microscopy. We appreciate the contributions of S. Arzhangi, S. Banihashemi, S. G. Firouzabadi, and M. Falah from the Genetics Research Center. We particularly thank the patients and their families. Special thanks to Dr. S. Nakhai, head of the University of Social Welfare and Rehabilitation Sciences. Financial support was provided by the Max Planck Innovation Fund (to H.-H.R.), German Federal Ministry of Education and Research Grant MRNET 01GS08161-2 (to H.-H.R.), the Iranian National Science Foundation (to H.N.), and National Institutes of Health Grant GM058728-09A1S109 (to A.H.C. and K.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107103108/-/DCSupplemental.
References
- 1.Ropers HH. Genetics of intellectual disability. Curr Opin Genet Dev. 2008;18:241–250. doi: 10.1016/j.gde.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Najmabadi H, et al. Homozygosity mapping in consanguineous families reveals extreme heterogeneity of non-syndromic autosomal recessive mental retardation and identifies 8 novel gene loci. Hum Genet. 2007;121:43–48. doi: 10.1007/s00439-006-0292-0. [DOI] [PubMed] [Google Scholar]
- 3.Kuss AW, et al. Autosomal recessive mental retardation: Homozygosity mapping identifies 27 single linkage intervals, at least 14 novel loci and several mutation hotspots. Hum Genet. 2011;129:141–148. doi: 10.1007/s00439-010-0907-3. [DOI] [PubMed] [Google Scholar]
- 4.Kelly SM, et al. Recognition of polyadenosine RNA by zinc finger proteins. Proc Natl Acad Sci USA. 2007;104:12306–12311. doi: 10.1073/pnas.0701244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung SW, et al. Splice variants of the human ZC3H14 gene generate multiple isoforms of a zinc finger polyadenosine RNA binding protein. Gene. 2009;439:71–78. doi: 10.1016/j.gene.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hector RE, et al. Dual requirement for yeast hnRNP Nab2p in mRNA poly(A) tail length control and nuclear export. EMBO J. 2002;21:1800–1810. doi: 10.1093/emboj/21.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: A yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suthers GK, Wilson SR. Genetic counseling in rare syndromes: A resampling method for determining an approximate confidence interval for gene location with linkage data from a single pedigree. Am J Hum Genet. 1990;47:53–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly SM, et al. Recognition of polyadenosine RNA by the zinc finger domain of nuclear poly(A) RNA-binding protein 2 (Nab2) is required for correct mRNA 3′-end formation. J Biol Chem. 2010;285:26022–26032. doi: 10.1074/jbc.M110.141127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil GP, Schroeder AJ, Roberts MA, Jackson FR. Genetic analysis of functional domains within the Drosophila LARK RNA-binding protein. Genetics. 2001;159:229–240. doi: 10.1093/genetics/159.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YQ, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 13.Juge F, Zaessinger S, Temme C, Wahle E, Simonelig M. Control of poly(A) polymerase level is essential to cytoplasmic polyadenylation and early development in Drosophila. EMBO J. 2002;21:6603–6613. doi: 10.1093/emboj/cdf633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerwitz Y, et al. Stimulation of poly(A) polymerase through a direct interaction with the nuclear poly(A) binding protein allosterically regulated by RNA. EMBO J. 2003;22:3705–3714. doi: 10.1093/emboj/cdg347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata T, et al. The hiiragi gene encodes a poly(A) polymerase, which controls the formation of the wing margin in Drosophila melanogaster. Dev Biol. 2001;233:137–147. doi: 10.1006/dbio.2001.0205. [DOI] [PubMed] [Google Scholar]
- 16.Benoit B, et al. An essential cytoplasmic function for the nuclear poly(A) binding protein, PABP2, in poly(A) tail length control and early development in Drosophila. Dev Cell. 2005;9:511–522. doi: 10.1016/j.devcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Wilkie GS, et al. Small bristles, the Drosophila ortholog of NXF-1, is essential for mRNA export throughout development. RNA. 2001;7:1781–1792. [PMC free article] [PubMed] [Google Scholar]
- 18.van den Bogaart G, Meinema AC, Krasnikov V, Veenhoff LM, Poolman B. Nuclear transport factor directs localization of protein synthesis during mitosis. Nat Cell Biol. 2009;11:350–356. doi: 10.1038/ncb1844. [DOI] [PubMed] [Google Scholar]
- 19.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet. 2008;24:416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Apponi LH, et al. Loss of nuclear poly(A)-binding protein 1 causes defects in myogenesis and mRNA biogenesis. Hum Mol Genet. 2010;19:1058–1065. doi: 10.1093/hmg/ddp569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.