Abstract
Human natural killer (NK) lymphocytes are able to destroy tumor cells and virally-infected cells. Interference with their function can leave an individual with increased susceptibility to cancer development and/or viral infection. We have shown that the tumor destroying (lytic) function of NK cells can be dramatically decreased by exposure to the environmental contaminant tetra-bromobisphenol A (TBBPA). TBBPA is a flame retardant used in a variety of materials including circuit boards, carpeting, and upholstery and has been found in human blood samples. TBBPA interferes with NK cell lytic function, in part, by decreasing the ability of NK cells to bind to target cells. This study examines the effects of exposures to concentrations of TBBPA (i.e., that were able to decrease the binding capacity of NK cells) on the expression of cell-surface proteins (CD2, CD11a, CD16, CD18, and CD56) that are needed for NK cells to bind target cells. NK cells were exposed to TBBPA for 24 hr, 48 hr, and 6 d or for 1 hr followed by 24 hr, 48 hr, and 6 d in TBBPA-free media. Twenty-four hr exposures to 5 µM TBBPA caused decreases in four of the cell surface proteins examined. CD16 was decreased by > 35%. The decreases in cell surface proteins after a 48 hr exposure were similar to those seen after 24 hr. The results indicate that TBBPA exposures that decrease the binding function of human NK cells do so by decreasing the expression of cell surface proteins needed for attachment of NK cells to targets cells.
Keywords: NK cells, Tetrabromobisphenol A, CD16, CD18, CD56
Introduction
Human natural killer (NK) cells are lymphocytes that are capable of targeting and destroying tumor cells, virally infected cells, and antibody-coated cells. NK cells are a crucial immune defense against malignant neoplasms and viral infections (Lotzova, 1993; Vivier et al., 2004). They limit the spread of blood borne metastases, as well as the development of primary tumors (Kiessling and Haller, 1978; Hanna, 1980). Their role is vital in protecting individuals against viral infections. This is demonstrated by increased incidences of viral infection in individuals that lack NK cells (Fleisher et al., 1982; Biron et al., 1989). Unlike T-lymphocytes, NK cells lyse appropriate target cells without prior sensitization making them a frontline immune defense.
Brominated flame-retardants (BFRs) are compounds used to prevent fires in plastics, textiles, and electronic equipment (Birnbaum and Staskal, 2004). They may bioaccumulate and are regarded as a potential environmental health problem (de Wit, 2002; de Boer et al., 2002). Tetrabromobisphenol A (TBBPA) has been used as a replacement for the polybrominated diphenylethers (PBDEs), whose persistence in the environment and potential for negative effects on health have prompted concerns (Sjodin et al., 1999; Eriksson et al, 2001). Its main use is as a reactive flame retardant in epoxy resin circuit boards (IPCS/WHO, 1995; HSDB, 2001). However, it is also applied to carpeting and upholstery (Gain, 1997). In 1998, the annual United States production of TBBPA was > 1 million pounds, with estimates ranging between 100–500 million pounds (U.S. EPA, 2000a; Environ Defense, 2001). Studies have shown that this compound may leak into the environment from treated products (Sellstroem and Jansson, 1995) and several recent reports on TBBPA in human and wildlife samples have shown the presence of this compound. It is a highly hydrophobic compound and would be expected to absorb onto suspended solids and sediment in streams (U.S. EPA, 2002b). Its half-life in water is expected to be 48–84 days (U.S. EPA, 2000b).
Levels of TBBPA as high as 0.24–0.71 ng/g lipid were reported in serum samples from Norway (≈ 1.8 – 5.3 pM, using a conversion factor of 400 mg lipid/100ml serum) (Thomsen et al., 2002) and averaged 4.5 ng/g lipid (≈33.8 pM) in a Japanese study (Nagayama et al., 2001). A toxicokinetic study following a single oral dose of 300 mg/kg TBBPA to adult humans showed a peak plasma concentration of 103 µM within 3 hr that declined, with an elimination half-life of 13 hr (Schauer et al., 2006). The presence of this compound in commercial drinking water that was stored in polycarbonate containers (Peterman et al., 2000) and in seafood (IPCS/WHO, 1995), as well as dust inhalation (Peterman et al., 2000), may account for the levels seen in human serum. TBBPA has been shown to increase the activity of glutathione reductase in rain-bow trout (Ronisz et al., 2004) and in exposed mice it causes decreases in serum proteins and red blood cells as well as increases in spleen weight (IPCS/WHO, 1995). Newborn rats exposed to TBBPA developed polycystic kidney lesions (Fukuda et al., 2004). A study in Wistar rats indicated that TBBPA exposures affected circulating thyroid hormone (T4) levels in Wistar rats (Van der Ven et al., 2008) but had no effect on the function of splenic NK cells in the exposed rats or their offspring. An in vitro study indicated that TBBPA was able to compete with T4 for binding to human transthyretin (thyroid hormone transport protein) (Meerts et al., 2000).
Our previous studies have shown that exposures to TBBPA can cause very significant losses of NK lytic function, which are accompanied by decreases in the ability of NK cells to bind to targets (Kibakaya et al., 2009). Thus, TBBPA has the capacity to increase the risk of viral infection and tumor formation by interference with NK function. In the current study, TBBPA was examined for its potential to disrupt the cell surface protein expression of NK cells. TBBPA concentrations and lengths of exposure previously shown to be able to decrease binding function (Kibakaya et al., 2009) were examined for any alteration in cell surface protein expression. Five cell surface proteins that are important in NK cells binding and/or lysis of targets, CD2, CD11a, CD16, CD18, and CD56, were analyzed via flow cytometry to determine whether TBBPA interferes with cell surface protein expression. CD2, an NK cell adhesion molecule, has been implicated in activation of the cytotoxic signaling response (Lotzova, 1993). CD11a/CD18 form the functional LFA-1 adhesion complex shown to be required for NK binding to tumor targets (Nitta et al., 1989). CD56, a cognate of the neural cell adhesion molecule, has also been shown to be important in NK binding to targets (Nitta et al., 1989; Lotzova, 1993). CD16 has a role as activating receptor of the NK lytic process with antibody-coated (Lotzova, 1993) and tumor targets (Mandelboim et al., 1999).
Materials and Methods
Isolation of NK cells
Peripheral blood from healthy adult (male and female) volunteer donors was used for this study. Buffy coats (source leukocytes) obtained from Key Biologics, LLC (Memphis, TN) were used to prepare NK cells. Consent was obtained by Key Biologics.
Highly-purified NK cells were obtained using a rosetting procedure; this is a negative selection technique. Buffy coats were mixed with 0.6 ml of RosetteSep human NK cell enrichment antibody cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) per 45 ml of buffy coat. The mixture was incubated for 20 min at room temperature (~25°C). Following the incubation, 7–8 ml of the mixture was layered onto 4 ml of Ficoll-Hypaque (1.077 g/ml; MP Biomedicals, Irvine, CA) and centrifuged at 1200 × g for 30–40 min. The cell layer was then collected and washed twice with phosphate-buffered saline (PBS; pH 7.2) and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated bovine calf serum [BCS], 2 mM L-glutamine, and 50 U penicillin G\50 µg streptomycin/ml) at 1 million cells/ml (Whalen et al., 2002). The resulting cell preparation was ~80% CD16+, ~0% CD3+, and ~90% CD56+ by flow cytometry.
Chemical preparation
TBBPA (purchased from Fisher Scientific, 97% pure) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) to yield a 100 mM stock solution. Desired concentrations of TBBPA were then prepared in complete media. The final concentration of DMSO in any of the TBBPA exposures did not exceed 0.01%.
Cell Viability
Cell viability was determined by trypan blue exclusion. Cell numbers and viability were assessed at the beginning and end of each exposure. Viability was determined at each TBBPA concentration for each exposure period. The viability of treated cells was then compared to that of control cells at each length of exposure (Whalen et al., 2003). Only those concentrations where viability was unaffected were used at a given length of exposure. Viability data at the concentrations and time points used in the study are given in Table 1.
Table 1.
Effect of 24 hr, 48 hr, and 6 d TBBPA or 1 hr followed by 24 hr, 48 hr, or 6 d periods in TBBPA-free media on human NK cell viability.
| % NK cell viability of TBBPA Exposed NK cells |
|||||
|---|---|---|---|---|---|
| Length of exposure to TBBPA |
Concentration of TBBPA in exposure | ||||
| 10 µM | 5 µM | 2.5 µM | 1 µM | control | |
| 24 hr | 89 ± 11 | 97 ± 0 | 97±1 | ||
| 48 hr | 96 ± 0 | 91 ± 16 | 96±2 | ||
| 6 d | 83 ± 10 | 89±3 | |||
| 1 hr, then 24 hr | 95 ± 1 | 94 ± 4 | 94 ± 3 | 96±5 | |
| 1 hr, then 48 hr | 93 ± 2 | 95 ± 0 | 95 ± 0 | 95±5 | |
| 1 hr, then 6 d | 88 ± 0 | 82 ± 6 | 87 ± 1 | 88±4 | |
Flow Cytometry
NK cells, prepared as described above, were exposed to TBBPA as follows: vehicle (control), 2.5, or 5 µM TBBPA for 24 hr; control, 1, or 2.5 µM TBBPA for 48 hr; control or 1 µM TBBPA for 6 d. DMSO controls appropriate to the various concentrations of TBBPA were tested. Each experiment was repeated four time using cells prepared from different blood donors. Additionally, NK cells were exposed to control, 2.5, 5, or 10 µM TBBPA for 1 hr after which the vehicle- or TBBPA-containing solution was removed and the cells were washed twice with fresh media, and then re-suspended in TBBPA-free media for 24 hr, 48 hr, and 6 d incubations. The studies of effects from 1-hr exposures to TBBPA followed by varying times in TBBPA-free media were repeated three times using cells from different donors.
Following the exposures to TBBPA or the post-TBBPA exposure/incubation in TBBPA-free media, the cells were prepared for analysis on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Briefly, the cells were washed twice with ice-cold PBS and a 100 µl aliquot of cell suspension (250,000–500,000 cells) was placed into a 12×75 mm polystyrene tube to which 10 µl of one of the following antibodies, anti-CD2, anti-CD11a, anti-CD16, anti-CD18, or anti-CD56 (all from Pharmingen, San Diego, CA) was then added. Anti-CD2, anti-CD11a, anti-CD16, and anti-CD18 were fluorescein isothiocyanate (FITC)-conjugated; anti-CD56 was phycoerythrin (PE)-conjugated. This is a direct staining protocol, with appropriate FITC- and PE-conjugated isotype control antibodies used as negative controls (Givan, 2000). Each anti-body-containing cell suspension was then incubated a minimum of 30 min on ice in the dark. Thereafter, the cells were washed twice with ice-cold PBS (1 ml) and suspended in 500 µl ice-cold 1% paraformaldehyde in PBS. Each antibody was a monoclonal antibody (mouse IgG) specific for the stated human surface protein.
Samples were ultimately analyzed using a FACSCalibur flow cytometer from Becton Dickinson Immunocytometry Systems, Inc (BDIS, San Jose, CA). Instrument performance was standardized weekly using Calibrite beads (BDIS) and the same instrument settings were used for all acquisitions. The assays were sufficiently uniform to use the same forward scatter (FSC), side scatter (SSC), and fluorescence (FL) settings. The sensitivity of the instrument was constant. The acquisition and analysis software for flow cytometry data was CELLQuest Pro from BDIS running on an Apple computer. A minimum of 10,000 events/sample was analyzed.
Statistical Analysis
Statistical analysis of the data was carried out utilizing ANOVA and Student's t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA was followed by pair wise analysis of control versus exposed data using Student’s t test, a p value of less than 0.05 was considered significant.
Results
Cell surface protein expression in NK cells exposed to TBBPA for 24 hr, 48 hr, and 6 d
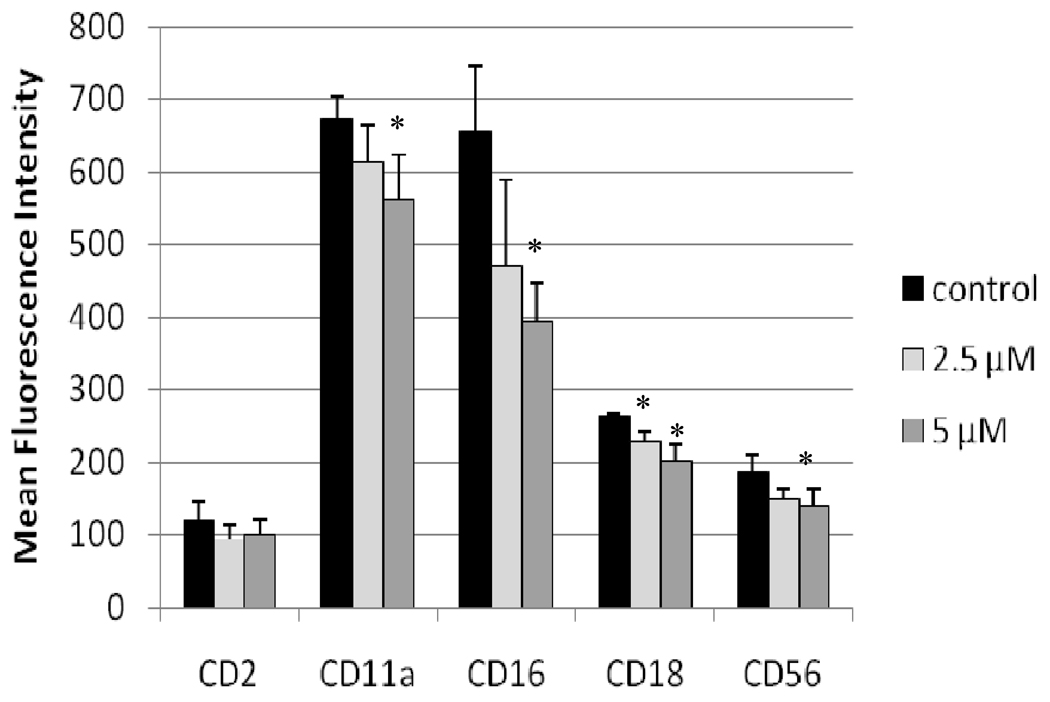
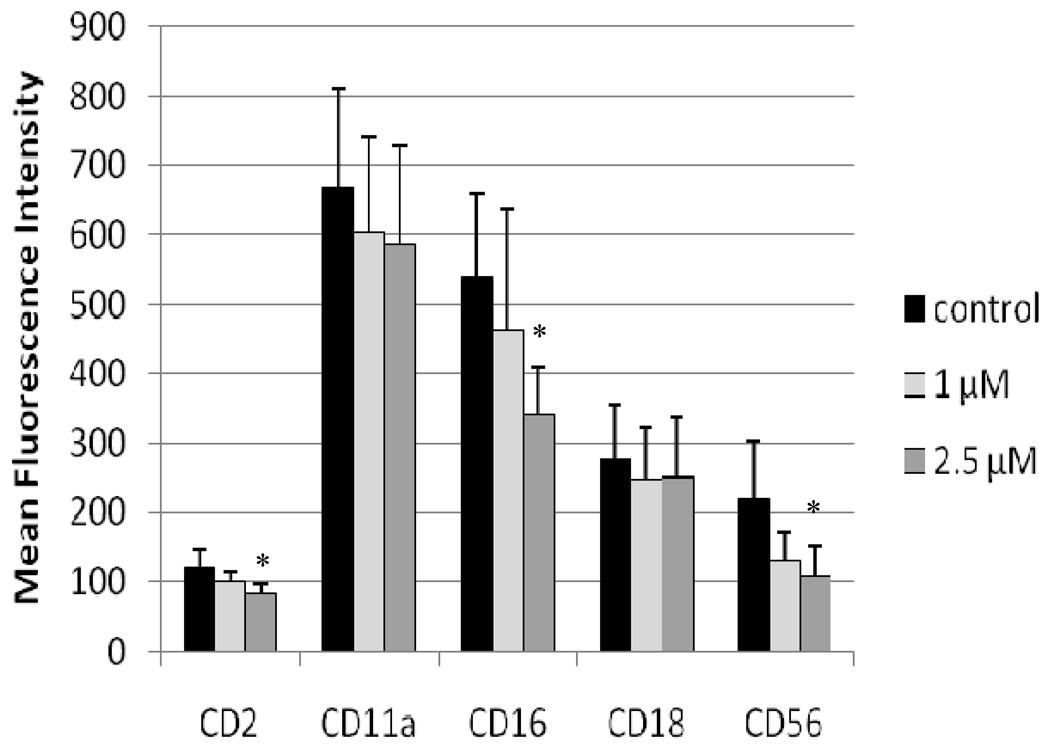
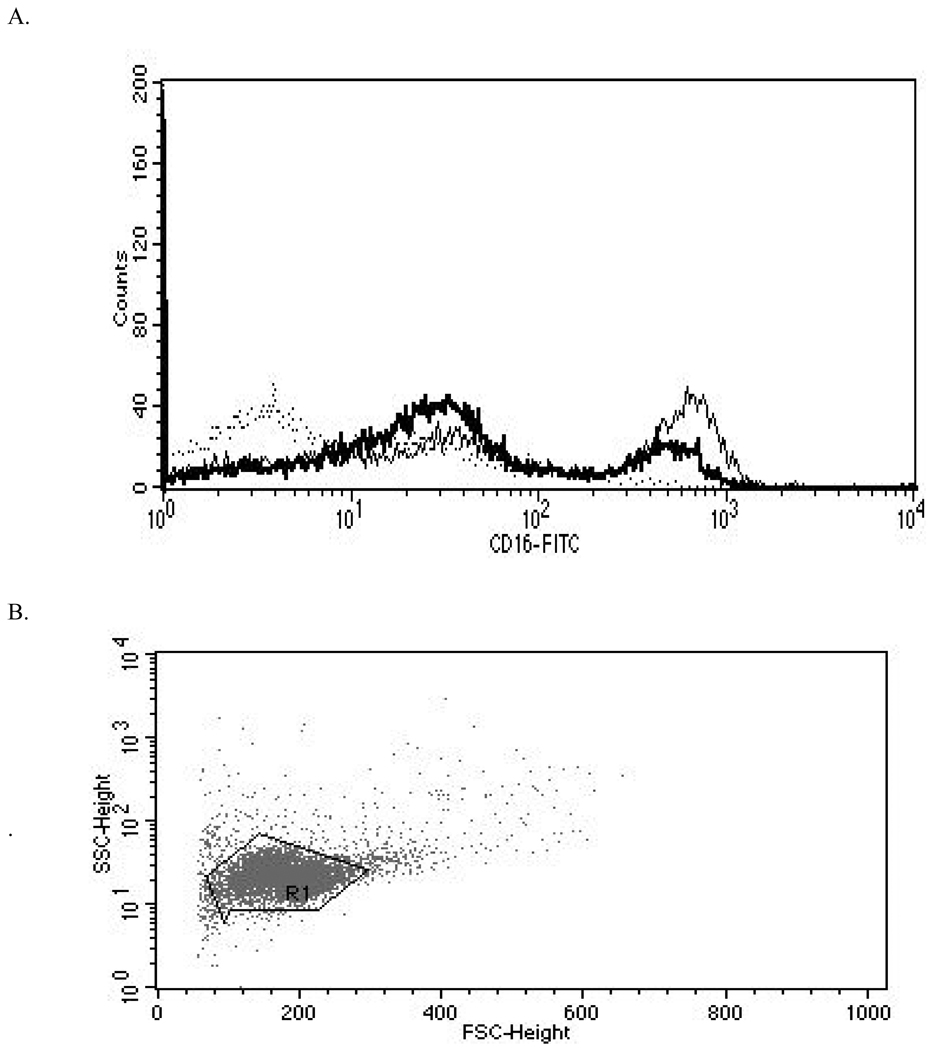
The effects of TBBPA on expression of cell surface proteins important to the binding of NK cells to their target cells were examined after 24 hr exposures to vehicle (control), 2.5 µM TBBPA, or 5 µM TBBPA. The cell surface proteins examined were CD2, CD11a, CD16, CD18, and CD56. Figure 1 shows the effects of these exposures on the expression (mean fluorescence intensity [MFI]) of these proteins on the cell surface. Exposures to 5 µM TBBPA for 24 hr caused significant decreases in expression of CD11a, CD16, CD18, and CD56. Expression of CD11a was decreased an average of 16%, while those of both CD18 and CD56 were decreased by ≈ 20% compared to control cells. The greatest decrease was seen in CD16 levels which were decreased by 35%. A 24-hr exposure to 2.5 µM TBBPA also caused significant decreases (i.e., of 14%) in CD18 expression compared to levels on control cells. Figure 2 shows the shift in peak fluorescence intensity for a representative experiment for each of the proteins whose expression was decreased after treatment with 5 µM TBBPA.
Figure 1. Effects of 24-hr TBBPA exposures on NK cell surface protein expression.
Values are the mean (± SD) of mean fluorescence intensity (MFI) combined from four different experiments each using a different donor (n = 4). Control cells were vehicle treated. *Statistically significant decrease (p < 0.05) analyzed as described in the materials and methods section.
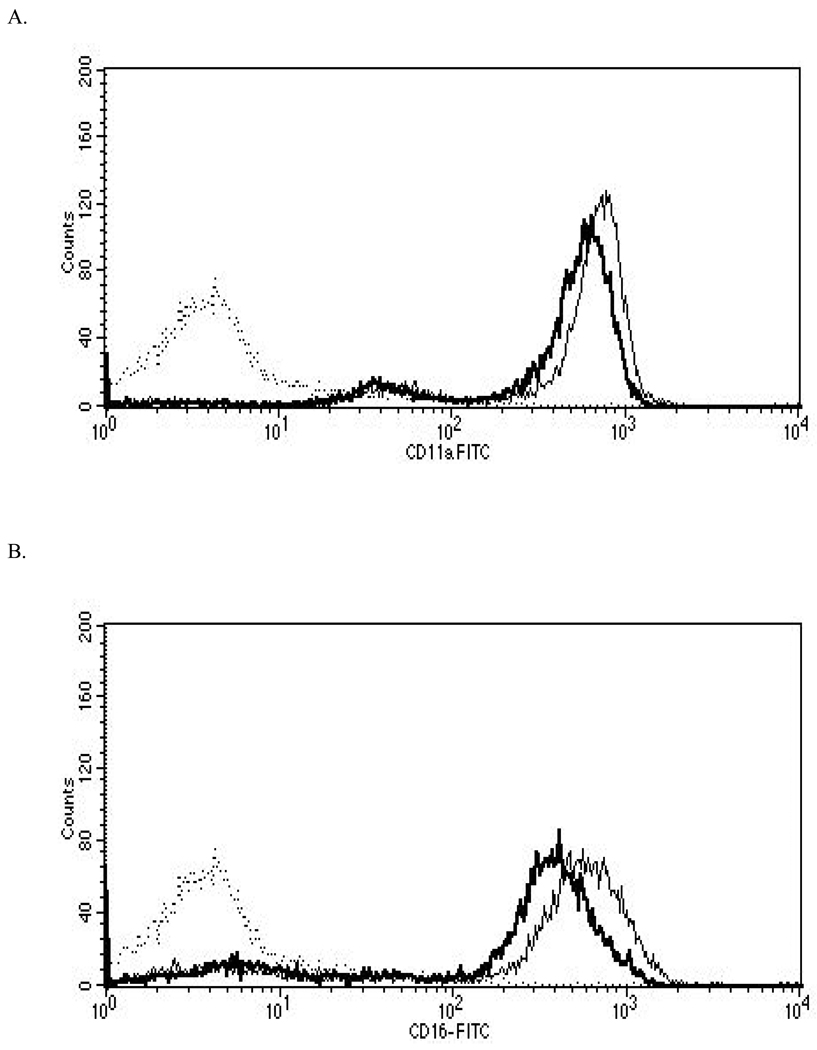
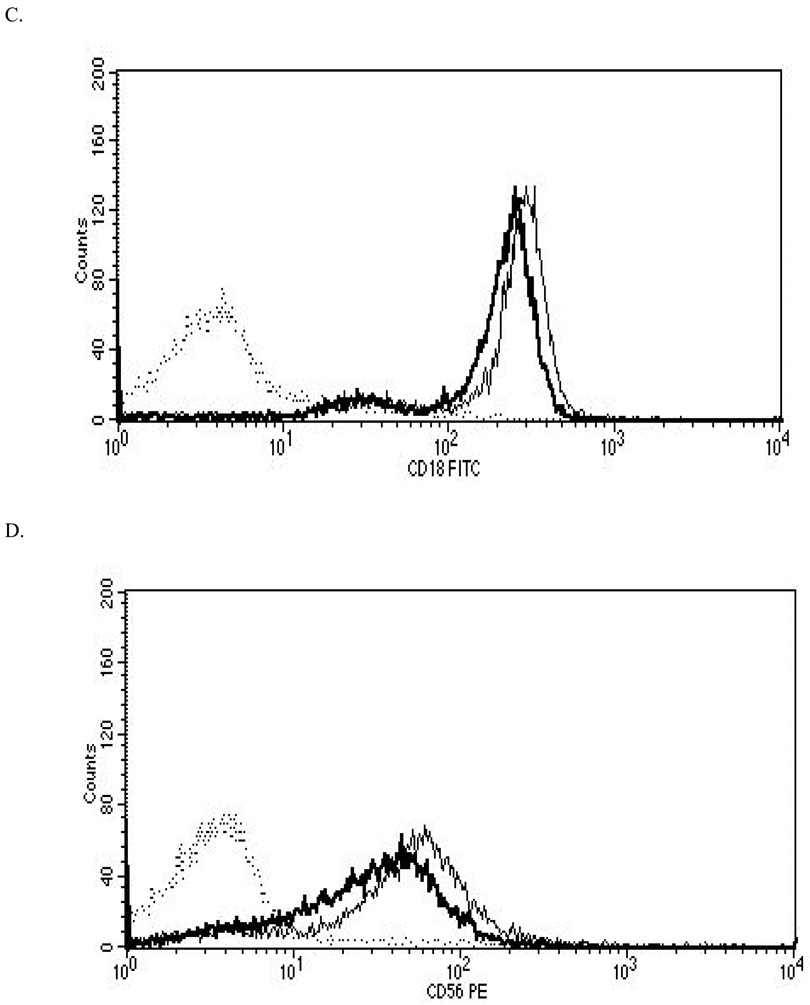
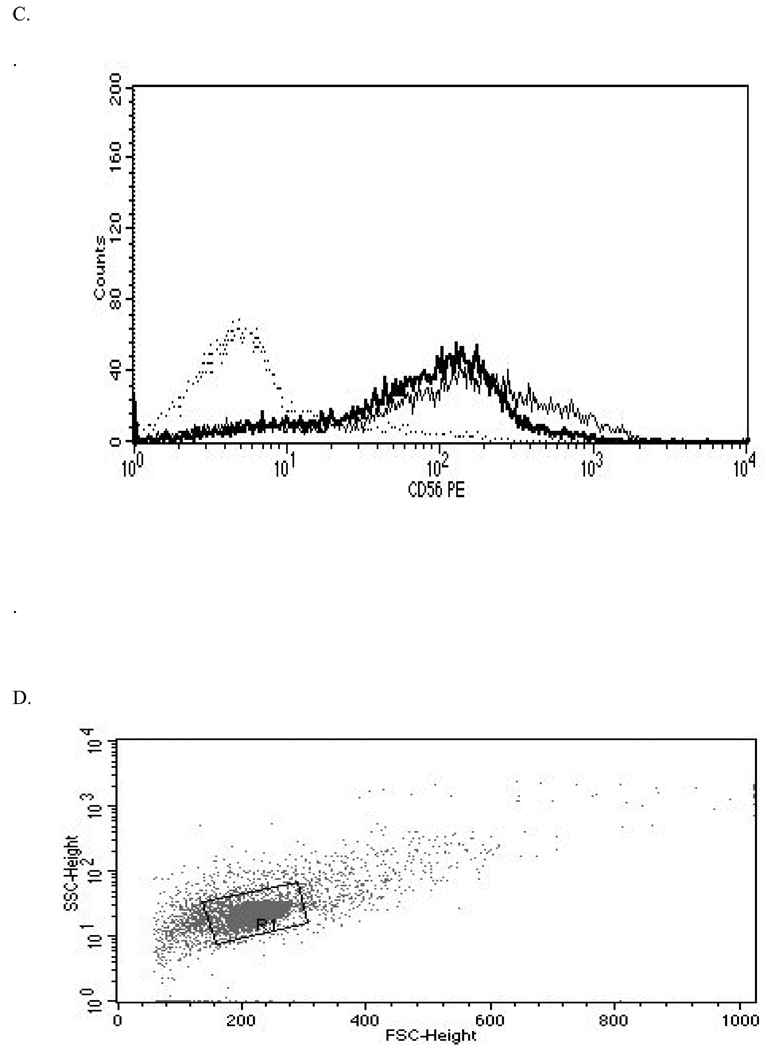
Figure 2. Representative histograms from studies of effects of 24-hr exposures to 5 µM TBBPA on NK cell surface protein expression.
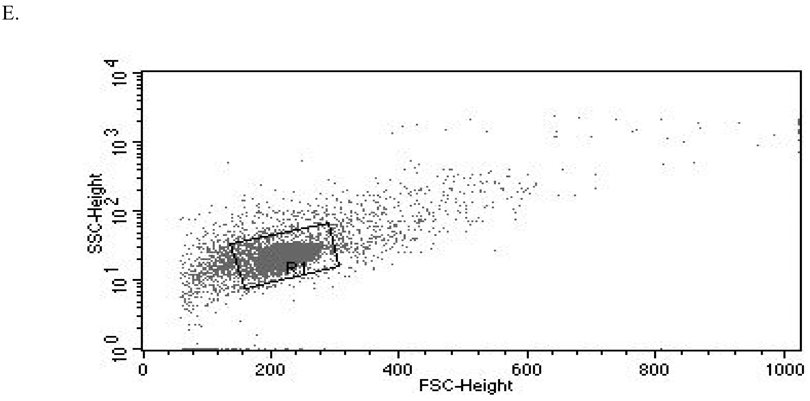
(A) CD11a (control mean fluorescence intensity (MFI) = 643.32, TBBPA MFI = 512.52); (B) CD16 (control MFI = 580.52, TBBPA MFI = 390.5); (C) CD18 (control MFI = 259.63, TBBPA MFI = 210.54); and, (D) CD56 (control MFI = 68.54, TBBPA MFI = 46.54). Dotted line = IgG control; thin solid line = control NK cells + appropriate antibody; bold line = TBBPA-exposed cells + appropriate antibody. Y-axis = cell number; X-axis = fluorescence intensity. (E) Dot-plot of cell preparation used in experiment. Shifts in fluorescence intensity were essentially the same whether gated (R1) or un-gated cell populations were used.
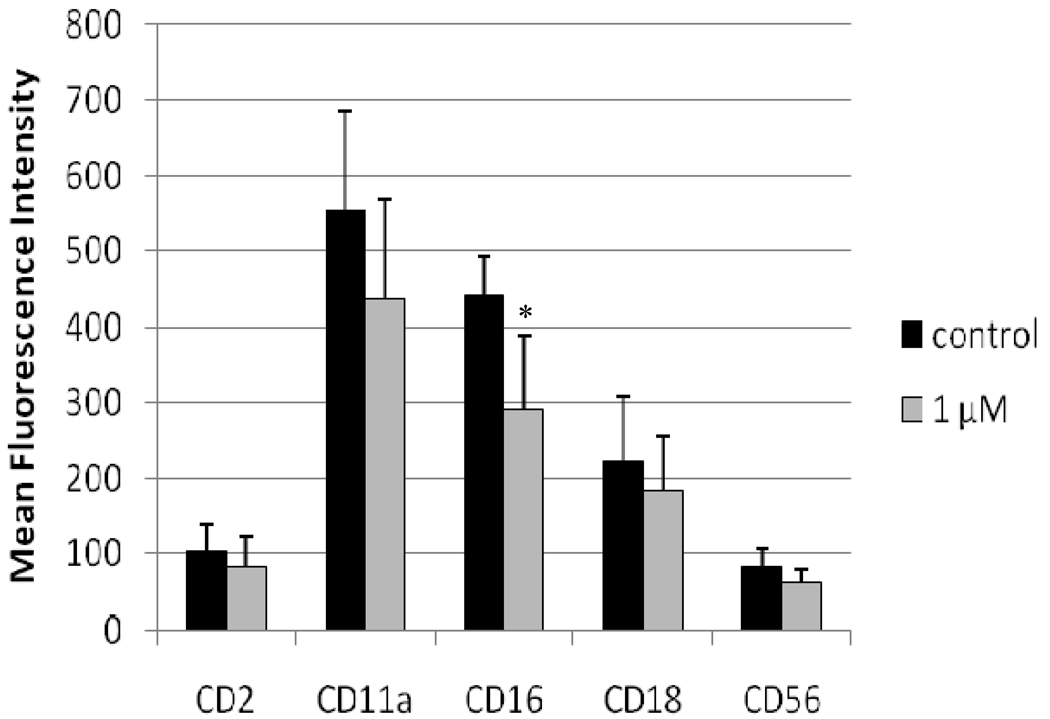
NK cells exposed to vehicle, 1 µM TBBPA, or 2.5 µM TBBPA for 48 hr were also assessed for expression of these surface proteins (Figure 3). Statistically significant decreases in expression (MFI) compared to in control cells were seen for CD2 (32%), CD16 (36%), and CD56 (46%) (Figure 3). There was no significant decreases in expression of any of the cell surface proteins of interest at 1 µM TBBPA. Figure 4 shows the shift in peak fluorescence intensity for a representative experiment for each of the proteins whose expression was significantly decreased at 2.5 µM TBBPA.
Figure 3. Effects of 48-hr TBBPA exposures on NK cell surface protein expression.
Values are the mean±S.D. of mean fluorescence intensity (MFI) combined from four different experiments each using a different donor (n = 4). *Statistically significant decrease (p < 0.05).
Figure 4. Representative histograms from studies of effects of 48-hr exposures to 2.5 µM TBBPA on NK cell surface protein expression.
(A) CD2 (control MFI = 123.71, TBBPA MFI = 79.36); (B) CD16 (control MFI = 678.42, TBBPA MFI = 442.22); and, (C) CD56 (control MFI = 277.39, TBBPA MFI = 154.74). Dotted line = IgG control; thin solid line = control NK cells + appropriate antibody; bold line = TBBPA-exposed cells + appropriate antibody. Y-axis = cell number; X-axis = fluorescence intensity. (D) Dot-plot of cell preparation used in experiment.
NK cells were also exposed to 1 µM TBBPA for 6 d and then examined for changes in expression of cell surface proteins that have a role in NK binding to target cells (Figure 5). CD16 was the only protein that showed a statistically significant decrease in expression (MFI) after a 6-d exposure to 1 µM TBBPA. Expression of this protein was decreased by 35% compared to on control cells (Figure 5). Figure 6 shows the shift in peak fluorescence intensity for a representative experiment examining CD16 expression after a 6-d exposure to 1 µM TBBPA.
Figure 5. Effects of 6-d TBBPA exposures on NK cell surface protein expression.
Values shown are the mean (± SD) of mean fluorescence intensity combined from four different experiments each using a different donor (n = 4). *Statistically significant decrease (p < 0.05).
Figure 6. Representative histograms from studies of effects of 6-d exposures to 2.5 µM TBBPA on CD16 expression in NK cells.
(A) CD16 (control MFI = 392.05, TBBPA MFI = 182.58). Dotted line = IgG control; thin solid line = control NK cells + appropriate antibody; bold line = TBBPA-exposed cells + appropriate antibody. Y-axis = cell number; X-axis = fluorescence intensity. (B) Dot-plot of cell preparation used in experiment.
Cell-surface protein expression in NK cells exposed to TBBPA for 1 hr, followed by 24 hr, 48 hr, and 6 d in TBBPA-free medium
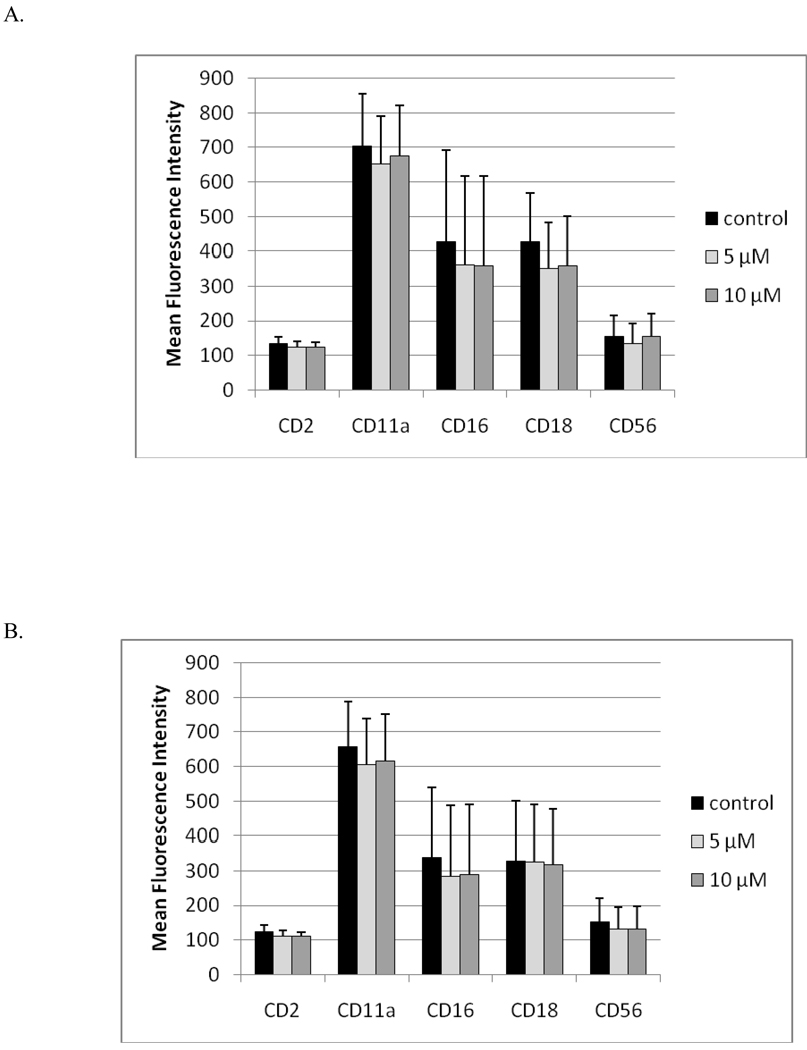
Exposures of NK cells to 10 µM TBBPA for 1 hr, followed by 24 hr, 48 hr, or 6 d incubations in TBBPA-free medium, caused no significant decreases in expression of any of the cell surface proteins that were examined in these studies (Figure 7).
Figure 7. Effects of 1 hr exposures to TBBPA - followed by 24 hr, 48 hr, or 6 d incubations in TBBPA-free media - on NK cell surface protein expression.
(A) 1 hr exposures to 10 µM and 5 µM TBBPA followed by 24 hr incubation in TBBPA-free media. (B) 1 hr exposures to 10 µM and 5 µM TBBPA followed by 48 hr incubation in TBBPA-free media. (C) 1 hr exposures to 10 µM, 5 µM, and 2.5 µM TBBPA followed by 6 d incubation in TBBPA-free media. Values shown are the mean± (± SD) of mean fluorescence intensity (MFI) combined from three different experiments each using a different donor (n = 3).
Discussion
Our previous studies showed that NK cells exposed to 2.5 and 5 µM TBBPA for 24 hr showed very significant reductions in NK cell lytic function of 76% and 96%, respectively (Kibakaya et al., 2009). Concentrations of TBBPA as low as 0.5 µM caused a greater than 23% decrease in lytic function. The data also indicated that the ability of NK cells to bind targets was diminished at 2.5 µM (19%) and 5 µM (70%) TBBPA (Kibakaya et al., 2009). A 48 hr exposure to 2.5 µM TBBPA decreased lytic function by 86%. Binding function was decreased by 50% at 2.5 µM TBBPA after 48 hr compared to the 19% loss seen after 24 hr. Six-day exposures to 1 µM TBBPA decreased lytic function by 89%. Binding function decreased 33% at the 1 µM concentration at 6 d (Kibakaya et al., 2009). The effects of a brief (1 hr) exposure to TBBPA followed by 24 hr, 48 hr, and 6 d periods in TBBPA-free media on lytic and binding function showed that while a brief exposure to 2.5–10 µM TBBPA caused persistent loss of lytic function and there was a loss of binding function only after 6 d at the highest concentration of TBBPA (Kibakaya et al., 2009). Thus, loss of lytic function can be (but is not always) accompanied by a loss of the ability of the NK cell to bind to its target.
As mentioned earlier, CD11a/CD18 form the functional LFA-1 adhesion complex shown to be required for NK binding to tumor targets (Nitta et al., 1989). CD11a - along with CD2 and CD16 - has been shown to activate NK cell cytotoxic signaling responses, including tyrosine phosphorylation of the zeta (ζ) chain (Lotzova, 1993; O’Shea et al., 1991). CD16 has also been shown to have a role in NK lysis of and tumor targets (Mandelboim et al., 1999).
The data presented here indicate that concentrations of TBBPA that caused a loss of binding function in NK cells also caused decreases in cell surface proteins needed for binding. There appears to be a greater effect of a given concentration of TBBPA as the length of exposure increases, as evidenced by the fact that 2.5 µM TBBPA caused no statistically significant decrease in CD56 expression after 24 hr as compared to a 46% decrease after 48 hr. A 1-µM TBBPA exposure for 48 hr caused no decreases in CD16 while a 6-d exposure to that same concentration caused a decrease of 35% (relative to value on control cells). The results also indicate there is a dose-response relationship in the loss of the surface proteins examined. For example, treatment with 2.5 µM TBBPA for 48 hr caused expression decreases of 36% and 46% in CD16 and CD56, while a 48-hr exposure to 1 µM TBBPA caused no decrease in either protein. The greater decreases in CD16 and CD56 compared to the expression of the other proteins with most TBBPA treatments suggest that the intracellular molecular targets of TBBPA may be involved in pathways that differentially regulate these proteins.
Previous studies have shown that a variety of environmental contaminants cause decreases in NK cell-binding function that were accompanied by decreases in expression of cell surface proteins. These include another brominated flame retardant, hexabromocylododecane (HBCD) (Hinkson and Whalen, 2010), the carbamate pesticide ziram (Taylor and Whalen, 2009), as well as the organotin compounds tributyltin (TBT) and dibutyltin (DBT) (Whalen et al., 2002; Odman-Ghazi et al., 2003). The effects of HBCD, ziram, TBT, and DBT on the expression of cell surface proteins have in common very significant decreases in CD16 on NK cells under conditions where binding was decreased (Whalen et al., 2002b; Odman-Ghazi et al., 2003; Taylor and Whalen, 2009; Hinkson and Whalen, 2010). This is similar to what was seen here with the TBBPA exposures. Thus, loss of CD16 may be at least in part responsible for the loss of binding seen with TBBPA as well as the other contaminants that have been shown to interfere with NK lytic and binding function. Each of these compounds, like TBBPA, was able to dramatically decrease the lytic function of NK cells (Wilson et al., 2004; Dudimah et al., 2007a,b; Hinkson and Whalen, 2009).
The negative effects of TBBPA on NK cells in our previous studies suggest that TBBPA may have the potential to inhibit NK function in vivo. The importance of NK cells in preventing neoplasm in humans has been illustrated by the fact that individuals whose NK cells are unable to lyse target cells have a predisposition to development of certain tumors (Ortaldo et al., 1992). While there are no direct in vivo studies of NK cell function in response to TBBPA, there is a study in mice indicating that TBBPA exposure increased respiratory syncytial virus infection (Watanabe et al., 2010); such an outcome could at least in part be due to suppressed NK function (although this possibility was not addressed in that study).
Levels of TBBPA in human serum of 4.5 ng/g lipid (33.8 pM, using 400 mg lipid/100 ml serum) have been reported (Nagayama et al., 2001) in humans with no occupational TBBPA exposure. Computer Technicians, who work with components that contain TBBPA, were examined for TBBPA levels. Their levels of TBBPA were higher than other workers in the same location but not higher than levels reported by the Nagayama group (Jakobsson et al., 2002). Thus, the levels of TBBPA appear to be considerably lower in the general population than those levels wherein effects on NK function were noted. However, those involved in the production of TBBPA and its application to epoxy resins, carpeting, and upholstery may have exposures that are considerably higher possibly even approaching the concentrations where NK function was affected. Studies of these workers have not been carried out. The concentrations where our laboratory has seen effects on lytic function, binding function (Kibakaya et al., 2009), and cell-surface proteins were much lower than those in a study examining TBBPA effects on cell proliferation in a human cell line (Strack et al., 2007). Studies in rats have shown that at plasma levels of TBBPA ranging from 6 – 94 µM, there were decreases in plasma levels of thyroxine (T4) and increases in triiodothyronine (T3) (Van der Ven et al., 2008). These levels of TBBPA are mostly above the highest levels tested in the current study. Additionally, TBBPA may have additive/synergistic effects with other compounds that have been shown to block NK function (and are also present in human blood), such as the organotin and carbamate compounds and HBCD mentioned above. This interaction could make even very low concentrations of TBBPA harmful (Wilson et al., 2004; Dudimah et al., 2007a,b; Hinkson and Whalen, 2009).
In summary, the data indicate that exposure of NK cells to TBBPA can cause very significant losses of cell surface proteins needed by NK cells, most especially CD16 and CD56, to bind to targets. This is a pattern seen when NK cells are exposed to a variety of other environmental contaminants. The current study shows that part of the mechanism by which TBBPA interferes with the essential immune function of NK cells is by decreasing the presence/availability of some important cell surface proteins.
Acknowledgements
This research was supported by Grant 2S06GM-08092-35 from the National Institutes of Health.
Footnotes
Declaration of Interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
References
- Birnbaum LS, Staskal DF. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl. J. Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- de Boer J, Allchin C, Zegers B, Boon JP, Brandsma SH, Morris S, Kruijt AW, van der Hesselingen JM, Haftka JJ. HBCD and TBBP-A in Sewage Sludge, Sediments and Biota, Including Inter-laboratory Study. CO33/02. Wageningen: The Netherlands: RIVO-Netherlands Institute for Fisheries Research; 2002. p. 40. [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on the ATP levels in human natural killer cells: Relationship to TBT-induced decreases in NK function. J. Appl. Toxicol. 2007a;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Gibson C, Whalen MM. Effect of dibutyltin (DBT) on ATP levels in human natural killer cells. Environ. Toxicol. 2007b;22:117–123. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- Environ Defense. 2,2',6,6'-Tetrabromo-4,4'-isopropylidenediphenol - Industrial uses. 2001 http://www.scorecard.org/chemical-profiles/uses.tcl?edf_substance_id=79%2d94%2d7. [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment. Environ. Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Bar virus infections. J. Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Ito Y, Yamaguchi M, Mitumori K, Koizumi M, Hasegawa R, Kamata E, Ema M. Unexpected nephrotoxicity induced by Tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004;150:145–155. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gain B. Flame retardants’ Albemarle boosts capacity. Chem. Week. 1997:88. (July 2, 1997, Full text available from PROMT 97:369076. [Google Scholar]
- Givan AL. Chapter 2: The Basics of Staining for Cell Surface Proteins. In: Diamond R, DeMaggio S, editors. In Living Color: Protocols in Flow Cytometry and Cell Sorting. Berline: Springer; 2000. pp. 142–164. [Google Scholar]
- Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural-killer cell mediated cytotoxicity. Int. J. Cancer. 1980;26:675–690. doi: 10.1002/ijc.2910260521. [DOI] [PubMed] [Google Scholar]
- Hinkson NC, Whalen MM. Hexabromocyclododecane decreases the lytic function and ATP levels of human natural killer cells. J. Appl. Toxicol. 2009;29:656–661. doi: 10.1002/jat.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkson NC, Whalen MM. Hexabromocyclododecane decreases tumor-cell-binding capacity and cell-surface protein expression of human natural killer cells. J. Appl. Toxicol. 2010;30:302–309. doi: 10.1002/jat.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank) 2,2´,6,6´-Tetrabromobisphenol A. Bethesda, MD: National Library of Medicine; 2001. http://www.toxnet.nlm.nih.gov/cgi-bin/sis/search. [Google Scholar]
- IPC/WHO (International Program on Chemical Safety/World Health Organization) Environmental Health Criteria 172: Tetrabromobisphenol A and Derivatives. Geneva: World Health Organization; 1995. [Google Scholar]
- Jakobsson K, Thuresson K, Rylander L, Sjodin A, Hagmar L, Bergman A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Kibakaya EC, Stephen K, Whalen MM. Tetrabromobisphenol A has immunosuppressive effects on human natural killer cells. J. Immunotoxicol. 2009;6:285–292. doi: 10.3109/15476910903258260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Haller O. Natural killer cells in the mouse, an alternative surveillance mechanism? Contemp. Top. Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Lotzova E. Definition and function of natural killer cells. Nat. Immunol. 1993;12:177–193. [PubMed] [Google Scholar]
- Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc. Natl. Acad. Sci. USA. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Nagayama J, Takasuga T, Tsuji H, editors. Human Levels and Trends, Part 4. 2001. Contamination levels of brominated flame retardants, dioxins, and organochlorine compounds in the blood of Japanese adults; pp. 218–221. Located at http://www.kemi.se/aktuellt/BFR/bfr_del4.pdf. [Google Scholar]
- Nitta T, Yagita H, Sato K, Okomura K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer-target cell interaction. J. Exp. Med. 1989;170:1757–1761. doi: 10.1084/jem.170.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odman-Ghazi SO, Hatcher F, Whalen MM. Expression of functionally-relevant cell surface markers in dibutyltin-exposed human natural killer cells. Chem.-Biol. Interact. 2003;146:1–18. doi: 10.1016/s0009-2797(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Ortaldo JR, Glenn GM, Young HA, Frey JL. Natural killer (NK) cell lytic dysfunction and putative NK cell receptor expression abnormality in members of a family with chromosome 3p-linked von Hippel-Lindau disease. J. Natl. Cancer Inst. 1992;84:1897–1903. doi: 10.1093/jnci/84.24.1897. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Weissman AM, Kennedy IC, Ortaldo JR. Engagement of the natural killer IgG Fc receptor results in tyrosine phosphorylation of the ζ chain. Proc. Natl. Acad. Sci. USA. 1991;88:350–354. doi: 10.1073/pnas.88.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman PH, Orazio CE, Gale RW. Detection of tetrabromobisphenol A and formation of brominated [13C]-bisphenol A’s in commercial drinking water stored in reusable polycarbonate containers. ACS Div. Environ. Chem. Extended Abstr. 2000;40:431–433. [Google Scholar]
- Ronisz D, Farmen Finne E, Karlsson H, Forlin L. Effects of the brominated flame retardants hexabromocyclododecane (HBCDD), and Tetrabromobisphenol A (TBBPA), on hepatic enzymes and other biomarkers in juvenile rainbow trout and feral eelpout. Aquat. Toxicol. 2004;69:229–245. doi: 10.1016/j.aquatox.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Schauer UM, Volkel W, Dekant W. Toxicokinetics of Tetrabromobisphenol A in humans and rats after oral administration. Toxicol. Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- Sellstroem U, Jansson B. Analysis of Tetrabromobisphenol A in a product and environmental samples. Chemosphere. 1995;31:3085–3092. [Google Scholar]
- Sjödin A, Hagmar L, Klasson-Wehler E, Kronholm-Diab K, Jakobsson E, Bergman A. Flame retardant exposure: Polybrominated diphenyl ethers in blood from Swedish workers. Environ. Health Perspect. 1999;107:643–648. doi: 10.1289/ehp.107-1566483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Detzel T, Wahl M, Kuch B, Krug HF. Cytotoxicity of TBBPA and effects on proliferation, cell cycle and MAPK pathways in mammalian cells. Chemosphere. 2007;67:S405–S411. doi: 10.1016/j.chemosphere.2006.05.136. [DOI] [PubMed] [Google Scholar]
- Taylor TR, Whalen MM. Effects of ziram on tumor-cell-binding capacity, cell-surface marker expression, and ATP levels of human natural killer cells. Cell Biol. Toxicol. 2009;25:447–455. doi: 10.1007/s10565-008-9098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environ. Sci. Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Washington, DC: U.S. EPA, Office of Pollution Prevention and Toxics (OPPT); 2000a. Non-confidential Production Volume Information Submitted by Companies for Chemicals Under the 1998 Inventory Update Rule (IUR). CAS no. 79947. Internet: http://www.epa.gov/cgi-bin/iursrch3.cgi. [Google Scholar]
- U.S. EPA. Washington, DC: Office of Environmental Information; 2000b. Emergency Planning and Community Right-To-Know Act Section 313 Reporting Guidance for Rubber and Plastics Manufacturing. EPA 745-B-00-017; pp. 2–16. Internet: http://www.epa.gov/tri/00rubber.pdf. [Google Scholar]
- Van der Ven LT, Van de Kuil T, Verhoef A, Verwar CM, Lilienthal H, Leonards PE, Schauer UM, Canton RF, Litens S, de Jong FH, Visser TJ, Dekant W, Stern N, Hakansson H, Slob W, Van den Berg M, Vos JG, Piersma AH. Endocrine effects of Tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one–generation reproduction study and a subacute toxicity study. Toxicology. 2008;245:76–89. doi: 10.1016/j.tox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Watanabe W, Shimizu T, Sawamura R, Hino A, Konno K, Hirose A, Kurokawa M. Effects of tetrabromobisphenol A, a brominated flame retardant, on the immune response to respiratory syncytial virus infection in mice. Int. Immunopharmacol. 2010;10:393–397. doi: 10.1016/j.intimp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Ghazi S, Loganathan BG, Hatcher F. Expression of CD16, CD18 and CD56 in tributyltin-exposed human natural killer cells. Chem.-Biol. Interact. 2002;139:159–176. doi: 10.1016/s0009-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Yamashita N, Saito T. Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chem.-Biol. Interact. 2003;145:311–319. doi: 10.1016/s0009-2797(03)00027-9. [DOI] [PubMed] [Google Scholar]
- Wilson S, Dzon L, Reed A, Pruitt M, Whalen MM. Effects of extended in vitro exposures to low levels of organotin and carbamate pesticides on human natural killer cell cytotoxic function. Environ. Toxicol. 2004;19:554–563. doi: 10.1002/tox.20061. [DOI] [PubMed] [Google Scholar]