Abstract
Objective
We evaluate presentation and outcome of patients with metastatic RCC to the gallbladder from our institution and published literature.
Methods
Patients with a history of gallbladder metastasis from RCC were selected from our institution’s prospective database. A systematic PubMed search was performed to identify articles describing patients with metastatic RCC to the gallbladder. The final cohort included 33 patients: 4 from our institution and 29 from 28 previously published cases. Survival analysis was conducted using LogRank Kaplan-Meier analysis.
Results
Median patient age was 63 years and the majority of patients were male. Most patients were asymptomatic and diagnosed with gallbladder metastasis on imaging performed for surveillance or staging. The median time to gallbladder metastasis following nephrectomy was 4 years. Metastasis to the gallbladder occurred both synchronously (33%) and metachronously (67%). Of the patients with available histology, all had clear cell RCC (n=28). Of all patients, 13 (39%) only had metastasis to the gallbladder, while 20 (61%) had additional sites of metastasis. The most common sites of additional metastasis were contralateral kidney (30%), pancreas (21%), lung (18%), adrenal (18%), and lymph nodes (9%). All patients underwent cholecystectomy. At a median follow up time of 1.5 years after cholecystectomy, 54% of patients had no evidence of disease, 14% were alive with metastasis, 23% had died from metastatic RCC, and 9% died from causes unrelated to their cancer.
Conclusion
Gallbladder metastasis from RCC is a rare event that may occur synchronously or metachronously with most patients being asymptomatic. Clear cell carcinoma appears to be the primary pathology associated with gallbladder metastasis. High rates of bilateral RCC and pancreatic metastasis suggest novel associations in patients with RCC and gallbladder metastasis.
Keywords: renal cell carcinoma, bilateral RCC, metastatic kidney cancer, gallbladder metastases, pancreatic metastases
Introduction
As many as one third of patients with newly diagnosed renal cell carcinoma (RCC) will have metastatic disease at presentation. Additionally, nearly 25–50% will develop metastatic disease metachronously after surgical treatment of the primary renal mass [1–3]. While the most common sites of metastasis are lung, bone, liver, adrenal, and brain, some unusual sites of metastasis have been reported [4,5]. Among uncommon sites for metastasis, gallbladder involvement with RCC has been reported at a rate of less than 1% [6,7].
In the past year, we have treated two unrelated patients in our clinic with unique histories of RCC metastasis. Both patients underwent previous resection of the gallbladder for RCC metastasis and later developed RCC metastasis to the pancreas. Almost all prior reports of RCC metastasis to the gallbladder are limited to single cases, and prior reviews have not completely addressed pathology, metastatic patterns, and outcomes in these patients. In this paper we report on cases seen at our institution and review published literature to better understand the pattern of RCC metastasis to the gallbladder. We evaluate the influence of specific RCC histology on the development of metastatic disease of the gallbladder, associations of the gallbladder with other sites of metastasis, and outcomes of these patients.
Materials and Methods
A systematic PubMed search was performed to identify all articles in any language describing patients with metastatic RCC to the gallbladder (Table 1). When available, data was extracted to focus on 6 parameters: demographics, histology of primary renal tumor, timing and clinical presentation of metastasis to the gallbladder, concomitant metastasis, and outcomes of these patients. We excluded two articles: one which we could not translate and another that did not discuss history of primary renal tumor, timing and clinical presentation of metastasis to the gallbladder, and concomitant metastasis.
Table 1.
List of Previously Published Cases
| Author | Year | # Pts | PubMed ID |
|---|---|---|---|
| Botting et al. | 1963 | 1 | 14014295 |
| Fullarton et al. | 1991 | 1 | 1877140 |
| Golbey et al. | 1991 | 1 | 1742682 |
| Satoh et al. | 1991 | 1 | 2007370 |
| Gonzalez et al. | 1994 | 1 | 8192507 |
| Nagler et al. | 1994 | 1 | 7956618 |
| Coskun et al. | 1995 | 1 | 7900494 |
| King et al. | 1995 | 1 | 7495130 |
| Pagano et al. | 1995 | 1 | 7747378 |
| Finkelstein et al. | 1996 | 1 | 8936450 |
| Lombardo et al. | 1996 | 1 | 8887246 |
| Furakawa et al. | 1997 | 1 | 9353490 |
| Sparwasser et al. | 1997 | 1 | 9253132 |
| Uchiyama et al. | 1997 | 1 | 9028147 |
| Celebi et al. | 1998 | 1 | 9624563 |
| Bissen et al. | 1999 | 1 | 10431276 |
| Kechrid et al. | 2000 | 1 | 18209350 |
| Aoki et al. | 2002 | 2 | 11871827 |
| Limani et al. | 2003 | 1 | 12768871 |
| Park et al. | 2003 | 1 | 12728482 |
| Ishizawa et al. | 2006 | 1 | 16877212 |
| Pandey et al. | 2006 | 1 | 16877838 |
| Hellenthal et al. | 2007 | 1 | 17308877 |
| Mujahid et al. | 2008 | 1 | 18774665 |
| Nojima et al. | 2008 | 1 | 18392717 |
| Ricci et al. | 2008 | 1 | 18689184 |
| Kucukakin et al. | 2009 | 1 | 19732538 |
| Sand et al. | 2009 | 1 | 19258219 |
| Chung et al. (current) | 2009 | 4 | |
| Total | 33 | 29 |
Patients with a history of gallbladder metastasis from RCC were selected from a prospective Urologic Oncology Branch database at the National Cancer Institute (NCI). Data for each patient was collected retrospectively via review of medical history, operative reports, and pathology (Table 2). Survival analysis was conducted using LogRank Kaplan-Meier analysis and SigmaPlot software (Systat Software Inc, Version 11).
Table 2.
NIH Patients
| Age | Sex | Renal Pathology |
Site | Stage | Time for gallbladder metastasis following nephrectomy (mo) |
Symptomatic/ Radiographic |
Imaging used to detect gallbladder mass |
Sites of synchronous metastasis |
Intervention | Size of gallbladder tumor (cm) |
Polypoid or pedunculated shaped tumor |
Future metastasis |
Adjuvant therapy |
Last Follow-up (mo) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | F | clear cell histology | L | 3 | 120 | radiographic | US, CT | - | cholecystectomy | 3.5×2×1.5 | Y | contralateral kidney, lung, brain, pancreas | N | 84 | alive |
| 52 | F | clear cell histology | L | 4 | 0 | radiographic | US, CT | - | cholecystectomy | 1.8×1.6×1.4 | Y | contralateral kidney, lung, pancreas | N | 60 | alive |
| 51 | M | clear cell histology | L | 4 | 0 | radiographic | US, CT | lungs, bone, contralateral kidney | cholecystectomy | 1.7×1.0.7 | Y | ethmoid, orbit, cribiform | Y | 132 | dead from metastasis |
| 42 | M | clear cell histology | R/L | 4 | 0 | radiographic | US, CT | - | cholecystectomy | n/a | Y | contralateral kidney, spine | N | 6 | dead from metastasis |
Results
The final cohort included a total of 33 patients, 4 from our institution and 29 from 28 previously published cases (Table 1). The clinical characteristics of patients evaluated at the NCI are indicated in Table 2, while the characteristics of the entire cohort of the present study are shown in Table 3. Median patient age was 63 years. Of the patients with available histology, all had clear cell RCC (n=28). The majority of the patients were male (n=27, 85%) and had Stage 4 disease (n=14, 56%) at time of nephrectomy. Twelve of 14 Stage 4 patients presented with synchronous gallbladder metastases. Eighteen patients (55%) underwent nephrectomy for a right renal mass, while 15 patients (45%) underwent nephrectomy for a left renal mass (Table 3).
Table 3.
Patient Characteristics
| Parameter | # Pts | # Studies |
|---|---|---|
| Median age, years (range) | 63 (39–84) | 29 |
| Gender | 29 | |
| Male (%) | 27 (82) | |
| Female (%) | 6 (18) | |
| Pathology | 29 | |
| Clear cell RCC (%) | 28 (85) | |
| RCC (histoloy not available) (%) | 5 (15) | |
| Laterality of RCC | 28 | |
| Right (%) | 18 (55) | |
| Left (%) | 15 (45) | |
| Size of RCC Tumor, cm (range) | 6.5 (3–13) | 13 |
| Stage of RCC at Presentation | 22 | |
| 1 (%) | 3 (12) | |
| 2 (%) | 3 (12) | |
| 3 (%) | 5 (20) | |
| 4 (%) | 14 (56) |
The median time for metachronous gallbladder metastasis following nephrectomy was 4 years (range 0.2–27). Most patients (n=16, 55%) were diagnosed with gallbladder metastasis incidentally on imaging performed for surveillance or staging. Ultrasound (n=23, 74%) and computer tomography (n=24, 77%) were the most commonly used modalities to identify gallbladder masses. Patients who presented symptomatically (n=13, 45%) often reported nonspecific abdominal pain or symptoms of acute cholecystitis or cholangitis. On pathology, all gallbladder tumors were consistent with RCC metastasis, and the median tumor size was 3.0 cm (range 1.1–7.5). All tumors were intraluminal and pedunculated or polypoid in shape. Radiologic imaging and pathology showed that most gallbladders did not contain stones (Table 4).
Table 4.
Presentation and Characteristics of Gallbladder Metastasis
| Parameters | # Pts | # Studies |
|---|---|---|
| Median time for metachronous gallbladder metastasis following nephrectomy, years (range) |
4.0 (0.2–27) | 21 |
| Median size of gallbladder tumor, cm (range) | 3.0 (1.1–7.5) | 23 |
| Symptomatic (%) | 13 (45) | 28 |
| Radiographic (%) | 16 (55) | 28 |
| Imaging used to detect gallbladder mass | 28 | |
| US (%) | 23 (74) | |
| CT (%) | 24 (77) | |
| MRI (%) | 2 (6) | |
| Cholangiography (%) | 4 (13) | |
| Angiography (%) | 2 (6) | |
| Presence of stones (%) | 4 (20) | 19 |
| Polypoid or pedunculated shaped tumor (%) | 24 (100) | 23 |
RCC metastasis to the gallbladder occurred both synchronously (n=11, 33%) and metachronously (n=22, 67%). Of all patients, 13 (39%) only had metastasis to the gallbladder, while 20 (61%) had additional sites of metastasis. The most common sites of additional metastasis were contralateral kidney (n=10, 30%), pancreas (n=7, 21%), lung (n=6, 18%), adrenal (n=6, 18%), and lymph nodes (n=3, 9%) (Table 5). All patients, including those with evidence of metastasis elsewhere, underwent cholecystectomy. Only a few patients received adjuvant therapy (n=8, 24%).
Table 5.
Presentation of All Sites of Metastasis
| Parameters | # Pts | # Studies |
|---|---|---|
| Synchronous | 29 | |
| Gallbladder only (%) | 6 (18) | |
| Gallbladder with other metastasis (%) | 5 (15) | |
| Metachronous | 29 | |
| Gallbladder only (NED after nephrectomy) (%) | 7 (21) | |
| Gallbladder with other metastasis (%) | 15 (46) | |
| Metastasis to only gallbladder (%) | 13 (39) | 29 |
| Sites of other metastasis | 29 | |
| Contralateral kidney (%) | 10 (30) | |
| Pancreas (%) | 7 (21) | |
| Lung (%) | 6 (18) | |
| Adrenal (%) | 6 (18) | |
| Lymph node (%) | 3 (9) | |
| Skin (%) | 2 (6) | |
| Ethmoid (%) | 2 (6) | |
| Spine (%) | 2 (6) | |
| Thyroid (%) | 1 (3) | |
| Deltoid (%) | 1 (3) | |
| Hamstring (%) | 1 (3) | |
| Liver (%) | 1 (3) | |
| Brain (%) | 1 (3) | |
| Orbit (%) | 1 (3) | |
| Cribiform (%) | 1 (3) | |
| Hernia (%) | 1 (3) |
At a median follow up time of 1.5 years (range 0.2–11) after cholecystectomy, 54% of patients had no evidence of disease, 14% were alive with metastasis, 23% had died from metastasis, and 9% died from causes unrelated to their cancer (Table 6).
Table 6.
Intervention, Follow up, and Outcome
| Parameter | # Pts | # Studies |
|---|---|---|
| Intervention | ||
| Cholecystectomy (%) | 33 (100) | 29 |
| Adjuvant therapy (%) | 8 (24) | 8 |
| Median follow up time, years (range) | 1.5 (0.2–11) | 18 |
| Available outcome | 22 | 18 |
| No evidence of disease | 12 (54) | |
| Alive with metastasis | 3 (14) | |
| Death from metastasis | 5 (23) | |
| Non-cancerous death | 2 (9) |
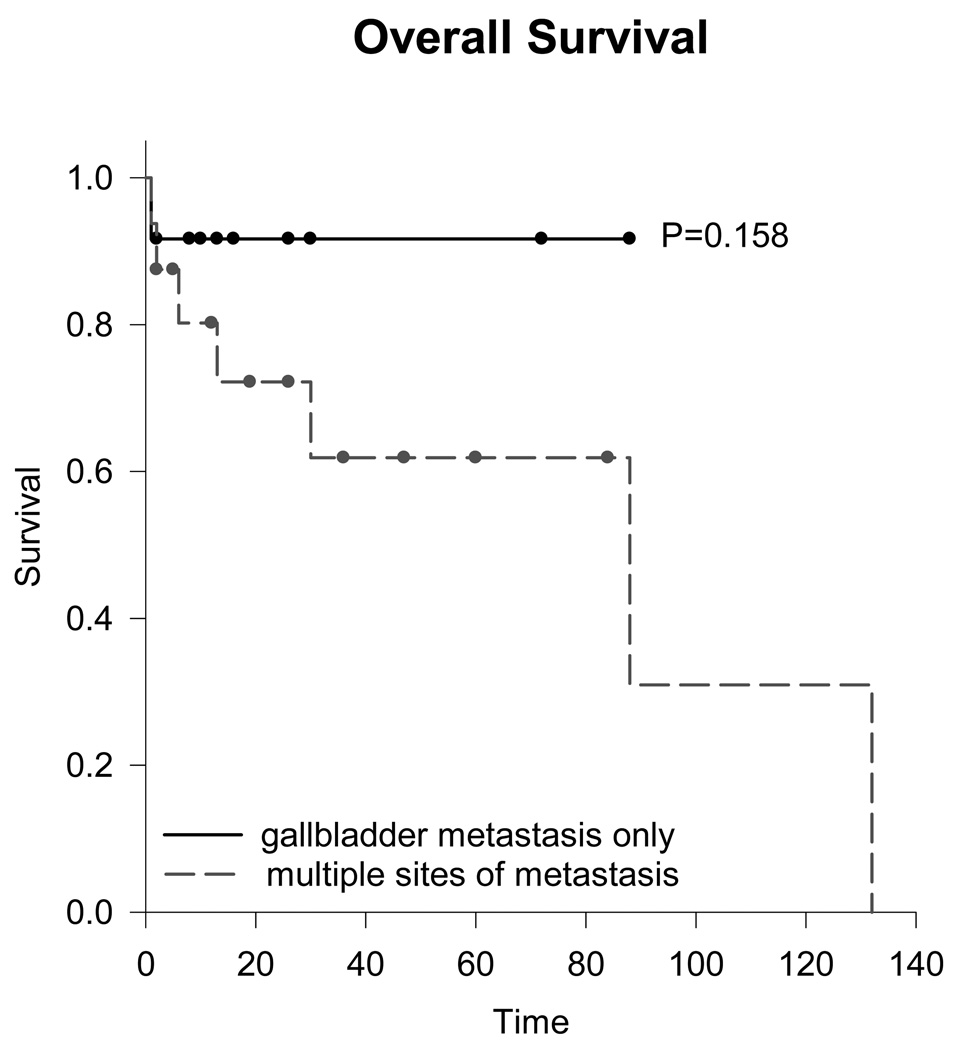
Kaplan-Meier analysis was conducted to compare survival between patients with metastasis to only the gallbladder and patients with multiple sites of metastasis (Figure 1). Competing risks were not considered in analysis. Eight of 13 patients with metastasis to only the gallbladder had recorded follow up. At a median time of 1.1 years (range 0.1–6) after cholecystectomy, all living patients had no evidence of disease. One patient died of a cause other than cancer. Fourteen of the 20 patients with multiple sites of metastasis had survival data available for analysis. At a median follow up of 2 years (range 0.2–11) after cholecystectomy, 8 (57%) were alive, 5 (36%) died from cancer, and 1 (7%) died of a cause other than cancer.
Figure 1.
Kaplan-Meier analysis of overall survival for gallbladder metastasis only versus multiple sites of metastasis.
Discussion
In our attempt to review the literature of gallbladder metastasis from RCC, we appreciated the relative paucity of data on this subject. Nevertheless, we searched the literature as well as our own institution’s database to congregate the largest reported patient group with gallbladder metastasis from RCC to date. Despite the small number of patients in this review, we were able to learn several intriguing facts about patients with gallbladder metastasis from RCC.
First, the most common histology for the development of gallbladder metastasis was clear cell RCC. It is not definitive whether this finding is a true reflection of the inherent biology of clear cell RCC to metastasize to the gallbladder. Our findings may be explained by the predominant frequency of clear cell RCC in the general population. The small size of our cohort may not have allowed for other histologies to contribute to the total number of metastatic events to the gallbladder. Nevertheless, this study spanned a period of over 45 years, and not one report documented the presence of non-clear cell metastasis to the gallbladder.
Second, most patients with gallbladder metastasis were diagnosed incidentally on follow up imaging for RCC. Of note, the majority of patients were asymptomatic. Some have suggested that although isolated presentations of primary RCC and primary gallbladder can occur in the same patient, the preoperative diagnosis of gallbladder metastasis should be considered in patients with a history of RCC [8,9]. As of today, specific postoperative screening for gallbladder metastasis in asymptomatic patients with a history of RCC would be inadvisable, since the number needed to screen to identify a single gallbladder metastasis would be in the thousands; however, when cross-sectional imaging is performed pre- or post-operatively, a “closer look” at the gallbladder is reasonable.
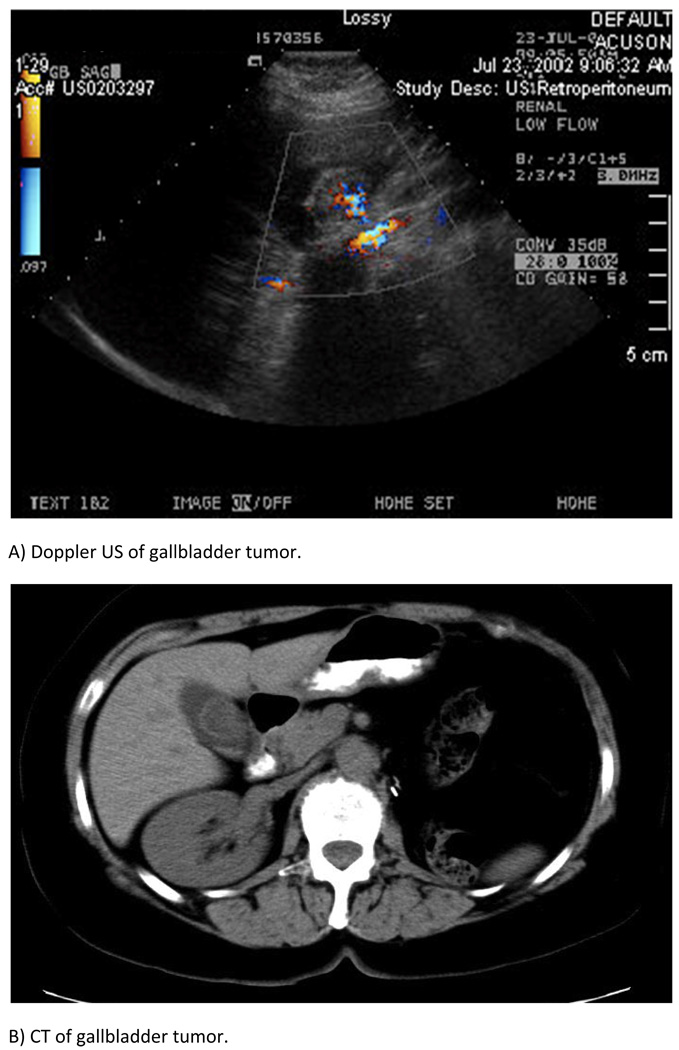
Third, metastasis to the gallbladder appeared to be a truly systemic disease, rather than due to direct involvement of the renal tumor. This is supported by the following observations. A) In those patients that developed gallbladder metastasis metachronously from RCC, the median time for gallbladder involvement following nephrectomy was 4 years. B) The metastatic lesion is present on the luminal surface of the gallbladder, rather than the serosal surface (Figure 2). C) There was no predilection of laterality of the original renal tumor for development of metastasis in the gallbladder (55% right vs. 45% left). D) Only 39% of patients metastasized to only the gallbladder, while 61% metastasized to additional sites. Other investigators have also attributed metastasis to the gallbladder from RCC to hematogenous spread [10,11].
Figure 2.
Fourth, the presence of concomitant metastasis is quite different in patients with gallbladder involvement. The most common site of metastasis seems to be the contralateral kidney (30%). Compared to the documented rate of contralateral renal metastasis of 5%, the 30% contralateral kidney involvement observed in our cohort is intriguing [12]. It is unclear whether these were indeed metastasis or primary tumors in patients with multifocal, bilateral RCC that developed gallbladder metastasis. If the latter were true, it may raise a question of predilection of multifocal RCC for gallbladder metastasis. Interestingly, three of our four patients had bilateral RCC, although the population seen at our institution may explain this observation.
Fifth, while pancreatic metastasis has been reported to occur in less than 1% of RCC cases, it was the second most common site of distant disease in our cohort [13]. Seven patients (21%) had pancreatic involvement, surpassing even the frequency of lung metastasis (18%). Lung is usually the site of highest rate metastasis from RCC, occurring in up to 60% of patients [14]. Conversely, in a review of pancreatic metastasis from RCC, only 1 of 72 patients had metastasis to the gallbladder [15]. No strong associations between gallbladder and pancreatic metastasis from other cancers have been previously described. In our search of the literature, only two isolated cases of gallbladder and pancreatic metastases were found: one from metastatic lobar carcinoma of the breast and another from malignant melanoma [16,17].
Of the seven patients in our cohort with pancreatic metastasis, 4 metastasized synchronously with gallbladder metastasis, while 3 metastasized following cholecystectomy. This high incidence of gallbladder metastasis occurring synchronously or metachronously with pancreatic metastasis may suggest that the gallbladder may be a leading site for the pancreatic involvement through hematogenous or biliary spread. Biliary spread was previously disregarded since there is no presence of tumor invasion in the liver or extrahepatic ducts [10,11]. An anomalous junction of the pancreaticobiliary duct (AJPBD) may increase the risk of primary gallbladder carcinoma by allowing pancreatic secretions to reflux into the biliary tree [18]. Perhaps a similar reflux event may allow biliary secretions from the gallbladder to reflux into the pancreas. The absence of knowledge about the exact location of the pancreatic metastasis precludes us from making any definitive statements. Nevertheless, the association of pancreatic metastasis in patients with gallbladder metastasis is first reported here. These findings also suggest that there should be closer surveillance of the pancreas with follow up imaging in patients with gallbladder metastasis.
Sixth, outcomes for patients with solitary gallbladder metastasis that underwent cholecystectomy appear to be similar to patients with RCC and a solitary metastatic site treated with metastasectomy. The 5-year survival rate following resection of solitary metastasis from RCC is 35–50% [19]. Although follow up from cases in our study was not long enough to state equivalence of survival with the solitary lung and bone metastasis reported previously, cholecystectomy may increase survival for patients presenting with only gallbladder metastasis and may serve as curative resection. Among 8 of 13 patients with available follow up (median 1.1 years) following cholecystectomy and only gallbladder metastasis, all had no evidence of disease. Among 14 of the 20 patients with available follow up (median 2 years) following cholecystectomy and multiple sites of metastases, 8 (57%) were alive, 5 (36%) died from cancer, and 1 (7%) died from unrelated cause. The rates of survival in patients with gallbladder and other site of metastases in our cohort were greater than the 2-year survival of 63% for single metastasis and 23% for multiple metastases reported by Han et al. [20]. This may be explained by the assignment of multifocal, bilateral RCC as metastatic disease or differences in other sites of metastasis.
The identification of primary versus secondary gallbladder cancer can be challenging. Primary gallbladder cancer predominantly affects women greater than 65 years of age. Histology is most often adenocarcinoma and is highly correlated with gallstone disease. Presentation is frequently nonspecific, but can often be identified on imaging as a space occupying mass with increased vasculature (Figure 2) [21]. Secondary gallbladder cancer is rare, with the most frequent primary sources being malignant melanoma, colon, breast, pancreas, and kidney [10]. Our cohort of patients with secondary metastasis to the gallbladder from RCC had a median age of 63 years and included 82% males. This high proportion of males is in contrast to primary gallbladder cancer, but is not surprising for RCC since men are more commonly affected than women.
Whether the gallbladder tumor is primary or secondary, cholecystectomy may provide the best survival outcome in either case. The 5-year prognosis for all stages of primary gallbladder cancer is about 5% [21]. Gallbladder lesions with diameters greater than 1.0 cm are usually managed with cholecystectomy because they are at greater risk for malignancy than smaller lesions [22]. The median gallbladder tumor size from our cohort was 3.0 cm (range 1.1–7.5). When following recommendations for gallbladder cancer, all gallbladder tumors from our cohort would be managed with cholecystectomy. With further support from possible increased survival from cholecystectomy for both gallbladder metastasis alone and additional sites of metastasis, aggressive surgical management of gallbladder metastasis is reasonable.
We acknowledge numerous shortcomings of this review: small patient number, incomplete and retrospective data, and absence of centralized pathologic or radiologic review. The cohort of patients in this review may not reflect a much greater number of patients that may be encountered in clinical practices throughout the world. Hesitation of people to publish another case report may explain the paucity of literature, although we do understand that gallbladder is indeed a rare site of metastatic disease. Nevertheless, to our knowledge, this is the most comprehensive review of RCC metastatic gallbladder. We provide new information to patients and treating physicians about histology, associated metastases, and prognosis of patients with gallbladder metastasis.
Conclusions
Gallbladder metastasis from RCC is a rare event that may occur synchronously or metachronously; with most patients being asymptomatic. Clear cell carcinoma appears to be the primary pathology associated with gallbladder metastasis. High rates of bilateral RCC and pancreatic metastasis suggest novel associations in patients with RCC and gallbladder metastasis. Survival for patients with gallbladder metastasis may be similar or better when compared to those with common sites of metastatic RCC.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28:1165–1177. doi: 10.1002/1097-0142(1971)28:5<1165::aid-cncr2820280513>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206–212. doi: 10.1200/JCO.1994.12.1.206. [DOI] [PubMed] [Google Scholar]
- 3.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 4.Saito J, Yamanaka K, Sato M, Mori N, Sekii K, Yoshioka T, Itatani H, Nakatsuka S. Four cases of advanced renal cell carcinoma with pancreatic metastasis successfully treated with radiation therapy. Int J Clin Oncol. 2009;14:258–261. doi: 10.1007/s10147-008-0833-8. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer. 1981;48:1487–1491. doi: 10.1002/1097-0142(19810915)48:6<1487::aid-cncr2820480635>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Bennington JL, Kradjan RM. Renal Carcinoma. Philadelphia: Saunders; 1967. Distribution of metastases from renal carcinoma; pp. 156–170. [Google Scholar]
- 7.Weiss L, Harlos JP, Torhorst J, Gunthard B, Hartveit F, Svendsen E, Huang WL, Grundmann E, Eder M, Zwicknagl M. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605–612. doi: 10.1007/BF00398185. [DOI] [PubMed] [Google Scholar]
- 8.Andoh H, Kurokawa T, Yasui O, Shibata S, Sato T. Resection of a solitary pancreatic metastasis from renal cell carcinoma with a gallbladder carcinoma: report of a case. Surg Today. 2004;34:272–275. doi: 10.1007/s00595-003-2680-6. [DOI] [PubMed] [Google Scholar]
- 9.Morelli L, Piscioli F, Cudazzo E, Del Nonno F, Licci S. Simultaneous occurrence of metastasizing carcinoid tumour of the gallbladder and chromophobe renal cell carcinoma in a young man. Acta Gastroenterol Belg. 2007;70:371–373. [PubMed] [Google Scholar]
- 10.Satoh H, Iyama A, Hidaka K, Nakashiro H, Harada S, Hisatsugu T. Metastatic carcinoma the gallbladder from renal cancer presenting as intraluminal polypoid mass. Dig Dis Sci. 1991;36:520–523. doi: 10.1007/BF01298886. [DOI] [PubMed] [Google Scholar]
- 11.Golbey S, Gerard PS, Frank RG. Metastatic hypernephroma masquerading as acute cholecystitis. Clin Imaging. 1991;15:293–295. doi: 10.1016/0899-7071(91)90123-d. [DOI] [PubMed] [Google Scholar]
- 12.Blute ML, Thibault GP, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Multiple ipsilateral renal tumors discovered at planned nephron sparing surgery: importance of tumor histology and risk of metachronous recurrence. J Urol. 2003;170:760–763. doi: 10.1097/01.ju.0000081422.47894.e6. [DOI] [PubMed] [Google Scholar]
- 13.Machado NO, Chopra P. Pancreatic metastasis from renal carcinoma managed by Whipple resection. A case report and literature review of metastatic pattern, surgical management and outcome. JOP. 2009;10:413–418. [PubMed] [Google Scholar]
- 14.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335 doi: 10.1056/NEJM199609193351207. 865875. [DOI] [PubMed] [Google Scholar]
- 15.Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96:579–592. doi: 10.1002/bjs.6606. [DOI] [PubMed] [Google Scholar]
- 16.Pappo I, Feigin E, Uziely B, Amir G. Biliary and pancreatic metastases of breast carcinoma: is surgical palliation indicated? J Surg Oncol. 1991;46:211–214. doi: 10.1002/jso.2930460318. [DOI] [PubMed] [Google Scholar]
- 17.Hatanaka N, Miyata M, Kamiike W, Okumura K, Hashimoto T, Yamaguchi T, Kishino Y, Sakurai M, Matsuda H. Radical resection of primary malignant melanoma of the gallbladder with multiple metastases: report of a case. Surg Today. 1993;23:1023–1026. doi: 10.1007/BF00308983. [DOI] [PubMed] [Google Scholar]
- 18.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671–681. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 19.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 20.Han KR, Pantuck AJ, Bui MH, Shvarts O, Freitas DG, Zisman A, Leibovich BC, Dorey FJ, Gitlitz BJ, Figlin RA, Belldegrun AS. Number of metastatic sites rather than location dictates overall survival of patients with node-negative metastatic renal cell carcinoma. Urology. 2003;61:314–319. doi: 10.1016/s0090-4295(02)02163-5. [DOI] [PubMed] [Google Scholar]
- 21.Gourgiotis S, Kocher HM, Solaini L, Yarollahi A, Tsiambas E, Salemis NS. Gallbladder cancer. Am J Surg. 2008;196:252–264. doi: 10.1016/j.amjsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77:363–364. doi: 10.1002/bjs.1800770403. [DOI] [PubMed] [Google Scholar]