Abstract
Cannabinoids have been proposed to possess neuroprotective properties; though their mechanism of action remains contentious, they are posited to prevent neurodegenerative disorders, including Parkinson's disease, the pathogenesis of which has not been established. Recent studies have demonstrated that induction of proteasomal dysfunction in animal models results in a phenotype similar to Parkinson's disease. Here, we investigated the neuroprotective function of a synthetic cannabinoid-receptor agonist (WIN55.212.2) in dopaminergic neuronal death induced by a proteasomal synthase inhibitor (PSI), additionally testing the hypothesis that WIN55.212.2 modulates cytoplasmic accumulation of parkin and α-synuclein, a key feature of proteasomal dysfunction in Parkinson's. WIN55.212.2 protects PC12 cells from PSI-induced cytotoxicity, concomitantly inhibiting PSI-induced polyADP ribose polymerase expression and activation of caspase-3. While PSI induces cytoplasmic accumulation of α-synuclein and parkin, WIN55.212.2 counters these effects. Interestingly, however, while PSI induces the activation and nuclear translocalization of nuclear factor κB, WIN55.212.2 potentiates this effect. These data are suggestive that WIN55.212.2 might confer a neuroprotective benefit in PSI-induced proteasomal dysfunction, and could further protect against neuronal degeneration stemming from cytoplasmic accumulation of α-synuclein and parkin. These results indicate that WIN55.212.2 may be a candidate for treatment of neurodegenerative diseases, including Parkinson's disease.
Keywords: Cannabinoid-receptor agonist, PC12 cells, Proteasomal inhibitor, Alpha-synuclein, NF-kappa B
Introduction
Cannabinoids (CBs) from the Cannabis sativa L. plant, including tetrahydrocannabinol, the principal psychoactive component of marijuana, produce euphoria and relaxation and also impair motor coordination, perception of time, and short-term memory [1]. The principal actions of CBs are mediated by activation of their cognate receptors on presynaptic nerve ends [2]. Various types of cannabinoid receptors, including the orphan G-protein coupled receptors CB1 and CB2, are found in blood vessels, the central nervous system, and immune cells [3-5]. While CB1 is expressed abundantly in several areas in the brain as well as in peripheral tissues, CB2 is primarily expressed in the immune system [6, 7], although it was recently detected at low levels in peripheral nerve endings, microglial cells, and astrocytes, as well as in the cerebellum and brain stem [8]. CB1 receptor activation is involved in the control of neural cell fate [9] and mediates neuroprotectivity in different in vivo models of brain injury, including excitotoxicity and ischemia [10, 11].
In recent years, the capacity of CBs to effect neuroprotec tion and neurotoxicity has received increasing attention. Evidence of possible neuroprotective effects has accumulated in vitro from models of neurodegenerative diseases, including Alzheimer's and Parkinson's diseases and multiple sclerosis, as well as from in vivo clinical trial data [12-14]. These compounds are also able to decrease inflammation by acting on glial cells that influence neuronal survival [12]. The molecular mechanisms underlying cannabinoid-mediated neuroprotection are still poorly understood, but may include the direct activation of neuronal survival signaling pathways through cannabinoid receptors [15] or indirect effects mediated by microglial CB2-receptor stimulation [16].
The ubiquitin-proteasome system (UPS) is the primary pathway effecting degradation of unwanted intracellular soluble proteins, including those that are mutant, misfolded, damaged, or mislocalized, and can operate in the cytoplasm, nucleus, and secretory system of eukaryotic cells [17, 18]. Ubiquitin-conjugated target proteins are routed to the proteasome, a 2,000 kDa ATP-dependent proteolytic complex, for degradation [18, 19]. Failure of ubiquitylation results in protein accumulation and cell death [20, 21]. Recent studies have demonstrated that ubiquitylation-mediated degradation or modulation of protein activity is a common regulatory mechanism in apoptosis [22, 23].
An increasing body of evidence suggests that UPS dysfunction may play an important role in the pathogenesis of neurodegenerative diseases, especially Parkinson's disease. Indeed, synthetic proteasomal inhibitors induce dopaminergic neuronal cell death and the appearance of cytoplasmic Lewy-body like eosinophilic inclusions, two primary features of Parkinson's [24]. However, there is currently no proof that CBs affect proteasomal degradation. Nuclear factor κB (NF-κB), a key transcription factor that regulates many genes involved in inflammatory processes, cell differentiation, and cell death [25], can also be induced by proteasomal inhibition. Interestingly, treatment with the CB1-receptor agonist WIN55.212.2 is associated with inhibition of NF-κB activity and cell death [26].
In this report, we demonstrate that WIN55.212.2 inhibits PC12 dopaminergic neuronal cell apoptosis by proteasomal inhibition as well as NF-κB activation. In addition, we show that it can prevent neuronal accumulation of α-synuclein and parkin. We further discuss the importance of these findings with regard to regulation of the UPS and neuronal response to CBs.
Materials and Methods
Cell culture
PC12 dopaminergic neuronal cells were obtained from the Korean Cell Line Bank (KCLB, Korea) and were cultured in RPMI-1640 with heat-inactivated fetal bovine serum, 2 mM glutamine, and 100 units/ml penicillin/streptomycin (Gibco-BRL, San Diego, CA, USA). Cells were incubated at 37℃ in 5% CO2 under conditions of 85-95% humidity; after 48 hours, the medium was changed and cells were exposed for 24 hours to proteasomal synthase inhibitor (PSI; Sigma, St. Louis, MO, USA) and/or a cannabinoid-receptor agonist (WIN55.212.2, Sigma) dissolved in dimethyl sulfoxide (DMSO).
Preparation of small molecules
PSI (Z-Ile-Glu(OtBu)-Ala-Leu-al) and WIN55.212.2 were prepared as 1 mM or 50 mM stock solutions, respectively, in DMSO, and were diluted in culture medium prior to addition to cells. Thiazolyl Blue Tetrazolium Bromide (MTT; Sigma) was prepared as a 2 mg/ml stock solution in phosphate buffered saline (PBS).
Cell viability assays
Cell viability was determined using the MTT assay. Approximately 5×103 cells were plated in each well of a 96-well plate and allowed to attach to the substrate for 24 hours. The cells were then exposed to 1 µM PSI with 1, 5, or 10 µM WIN55.212.2 for a further 24 hours. Subsequently, 50 µl of MTT stock solution (see above) were added to each well and absorbance measured at 570 nm with a Bio-Tek microplate reader (Winooski, VT, USA). Reduction in cell viability was expressed relative to that of untreated cells.
Western blotting
After 24-hour exposure to 1 µM PSI with 10 µM WIN55.212.2, PC12 cells were resuspended in 200 µl lysis buffer (1.0 mM PMSF, 1.0 mM EDTA, 1 µM pepstatin A, 1 µM leupeptin, 1 µM aprotinin) and incubated on ice for 45 minutes. Lysates were centrifuged at 20,000 ×g for 20 minutes at 4℃, and supernatant stored at -70℃ prior to sodium dodecyl sulfate polyacrylamide gel electrophoresis. Protein concentrations were determined using the Bradford assay. All steps of protein preparation were carried out at 4℃.
Supernatant aliquots containing 60 µg total protein were added to an equal volume of 2× sample buffer; samples were boiled for 5 minutes and loaded onto 12% polyacrylamide gels for electrophoresis. Samples were transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After blocking with 5% skim milk, membranes were incubated with polyclonal antibodies against nuclear polyADP ribose polymerase (PARP), NF-κB, or caspase-3 (all 1 : 1,000, Cell Signaling Technology, Berverly, MA, USA). Antibodies against α-synuclein (1 : 1,000) and extracellular signal regulated kinase 2 (1 : 10,000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protein was visualized on X-ray film using PicoWest Chemiluminescent Substrate (Pierce, Rockford, IL, USA). All experiments were performed at least in triplicate. Statistical analysis was performed using the Student's t-test (P<0.05).
Immunocytochemistry
For double immunolabeling of tyrosine hydroxylase (TH), parkin, NF-κB, and cleaved caspase-3, PC12 cells treated with PSI and WIN55.212.2 were fixed and incubated with primary antibody in PBS containing 1% normal goat serum and 0.1% Triton X-100 at 4℃ overnight. The primary antibodies used were mouse anti-TH (1 : 200), mouse anti-NF-κB (1 : 200), mouse anti-parkin (1 : 200) and rabbit anti caspase-3 (1 : 500), all of which were purchased from Santa Cruz Biotechnology. Primary antibody binding was detected with donkey CY3-conjugated anti-rabbit IgG and donkey CY5-conjugated antimouse IgG fluorescently labeled secondary antibodies (1 : 200, Jackson Immunoresearch Laboratory, West Grove, PA, USA). Labeled cells were imaged using a standard fluorescence microscope (Nikon, Tokyo, Japan) and images captured with a digital camera attachment. Additional fluorescent images were obtained via confocal microscopy (LSM META 510, Carl Zeiss, Jena, Germany).
Results
WIN55.212.2 protects PC12 dopaminergic neuronal cells from death due to proteasomal inhibition
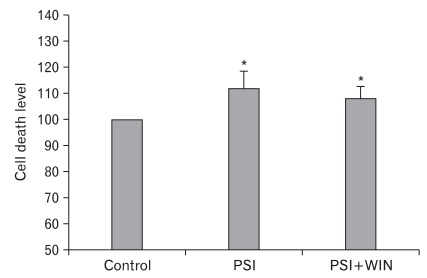
We first wished to ascertain the influence of the synthetic cannabinoid-receptor agonist WIN55.212.2 on cell viability. By MTT assay, PC12-cell viability was reduced significantly by exposure to PSI, but this effect was reversed by WIN55.212.2 (Fig. 1). These results demonstrate that WIN55.212.2 inhibits cell death in PC12 cells by counteracting the activity of a proteasomal inhibitor.
Fig. 1.
MTT assay. Administration of proteasomal synthase inhibitor (PSI) induces PC12-cellular death. Co-administration of WIN55.212.2 protects PC12 cells from cell death. Note that there are statically significant decreases (*) in cytotoxicity in cells co-administered PSI and WIN55.212.2 (P<0.05; data represent mean and standard deviation).
WIN55.212.2 blocks the caspase-3 activation induced by PSI treatment of PC12 cells
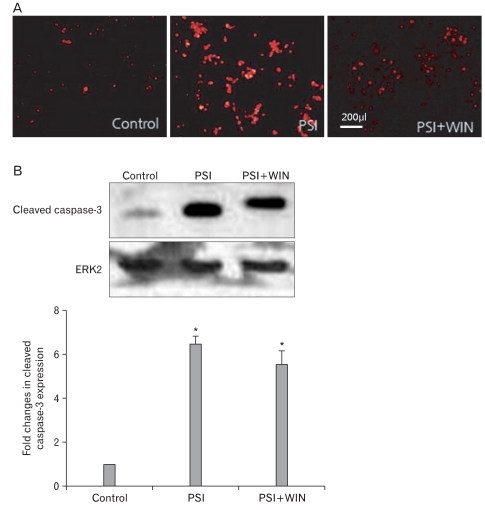
To elucidate the mechanism of this WIN55.212.2-mediated PC12-cell survival, we assessed activation of the apoptotic effector protein caspase-3. Western-blotting analyses revealed that PSI treatment in the absence of WIN55.212.2 increased levels of cleaved caspase-3, but this effect was reversed by co-administration of the compounds. We also performed immunofluorescent staining of PC12 cells (Fig. 2), which likewise revealed that expression of cleaved caspase-3 increased with PSI addition but decreased with WIN55.212.2 and PSI co-administration. These data suggest that WIN55.212.2 inhibits PC12-cell apoptosis via modulation of caspase-3 activation and by promotion of cellular survival behavior.
Fig. 2.
WIN55.212.2 inhibits proteasomal synthase inhibitor (PSI)-induced activation of caspase-3. (A) Representative immunofluorescent micrographs of PC12 cells cultured in the presence of PSI and WIN55.212.2 and stained for activated caspase-3. Note the decreased staining in cultures where PSI and WIN55.212.2 were co-administered, while staining was increased in cultures treated with PSI alone. (B) Upper panel: representative Western blots for cleaved caspase-3 in lysates of PC12 cells cultured in the presence or absence of WIN55.212.2 and PSI (normalized for expression of extracellular signal-regulated kinase 2 [ERK2]). Lower panel: quantitation of cleaved caspase-3 expression in PC12 cells as described above. Note that there are statically significant changes(*) in cleaved caspase-3 expression in cultures treated with PSI alone and in those treated with both PSI and WIN55.212.2 (P<0.05; data represent mean and standard deviation).
WIN55.212.2 suppresses PSI-induced expression of α-synuclein and parkin
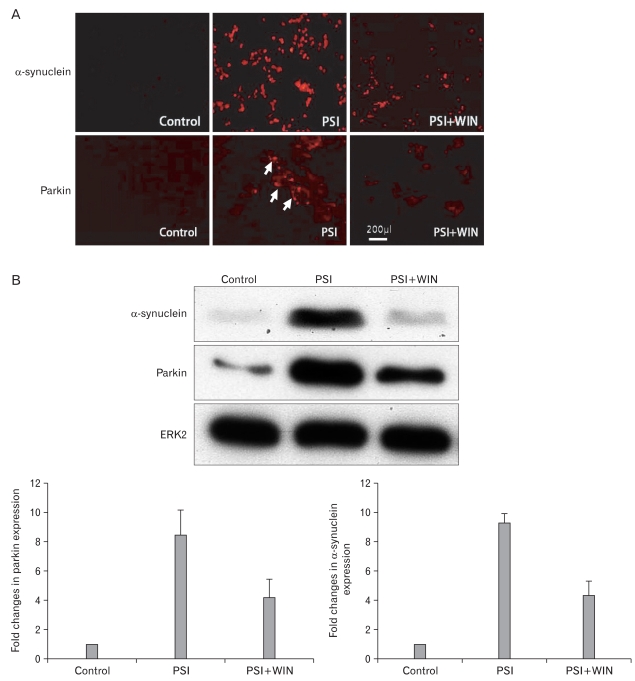
As inhibition of the proteasome elicits an increase in cellular levels of ubiquitylated proteins, as well as inducing the appearance of parkin- and α-synuclein positive perinuclear structures, we asked if proteasomal dysfunction could also lead to the formation of discrete cytoplasmic inclusions in dopaminergic PC12 cells, and if WIN55.212.2 could reduce the numbers of parkin- and α-synuclein-positive structures (Fig. 3). We examined the existence of α-synuclein and parkin-positive structures in PC12 cells incubated with PSI by immunofluorescence. While α-synuclein and parkin levels were augmented following the addition of PSI, co-administration of WIN55.212.2 and PSI resulted in a significant reduction in expression of either protein. These results suggest that proteasomal function is modulated by the addition of WIN55.212.2.
Fig. 3.
WIN55.212.2 inhibits the proteasomal synthase inhibitor (PSI)-induced accumulation of α-synuclein and parkin. (A) Representative immunofluorescent micrographs of PC12 cells cultured in the presence of PSI and WIN55.212.2 and stained for α-synuclein and parkin (arrows). Note the decreased staining in cultures treated with both PSI and WIN55.212.2 relative to that in cultures treated with PSI alone. (B) Upper panel: representative Western blots for α-synuclein and parkin in lysates of PC12 cells cultured in the presence or absence of WIN55.212.2 and PSI. Lower panel: quantitation of α-synuclein and parkin expression in PC12 cells as described above (arrows). Note that there are statically significant changes in α-synuclein and parkin expression in cultures treated with PSI alone compared to those treated with both PSI and WIN55.212.2 (P<0.05; data represent mean and standard deviation). ERK2, extracellular signal-regulated kinase 2.
WIN55.212.2 modulates the NF-κB signaling pathway following treatment with PSI
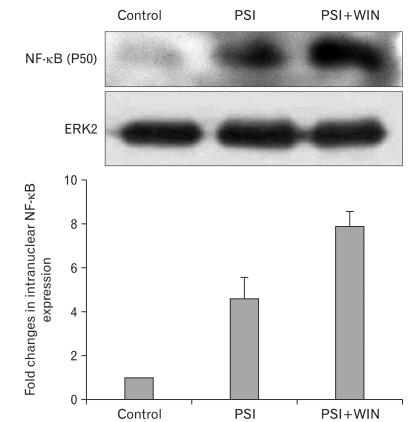
NF-κB is an apoptotic effector operating independent of the mitochondrial apoptosis pathway. To evaluate the effect of proteasomal dysfunction on NF-κB's proapoptotic activities, we performed Western blots to detect its expression in PC12-cell lysates in the presence of PSI and WIN55.212.2. NF-κB expression was enhanced by addition of PSI; interestingly, this increase was potentiated through co-administration of PSI and WIN55.212.2 (Fig. 4).
Fig. 4.
WIN55.212.2 increases intranuclear expression of nuclear factor κB (NF-κB). Upper panel: representative Western blots for NF-κB in nuclear lysates of PC12 cells cultured in the presence or absence of WIN55.212.2 and proteasomal synthase inhibitor (PSI). Lower panel: quantitation of nuclear NF-κB expression in PC12 cells as described above. Note that there are statistically significant changes in intranuclear NF-κB expression in cultures treated with PSI alone compared to those treated with both PSI and WIN55.212.2 (P<0.05; data represent mean and standard deviation). ERK2, extracellular signal-regulated kinase 2.
NF-κB is activated by nuclear translocation. To study this activation, we separately isolated the cytoplasm and nucleus from cultured PC12 cells and assayed protein expression in each fraction. By Western blotting, we found that the level of NF-κB expression in cytoplasmic lysates did not change with the addition of PSI and WIN55.212.2. In contrast, that in nuclear lysates increased with the addition of PSI, and co-administration of WIN55.212.2 with PSI potentiated this effect. These results suggest that WIN55.212.2 modulates apoptosis induced by proteasomal inhibition, in part through its regulation of NF-κB activity.
Discussion
The primary manifestation of Parkinson's disease pathology is degeneration of dopaminergic neurons in the substantia nigra pars compacta, coupled with the appearance of cytoplasmic inclusions known as Lewy bodies. Together, these result in loss of the nigrostriatal pathway and a reduction of dopamine levels in the striatum [27]. The mechanism responsible for neurodegeneration in Parkinson's disease has not been conclusively demonstrated, although there is some evidence of apoptotic cytotoxicity initiated concomitant with a cascade of events including oxidative stress, mitochondrial dysfunction, inflammation, and excitotoxicity [28].
Recent studies have suggested that a defect in the UPS plays a major role in the pathogenesis of some hereditary and sporadic forms of Parkinson's disease [24, 29]. Patients with sporadic Parkinson's disease were observed to exhibit a significant reduction in levels of α-subunits of the 20S proteasome, along with a diminution in the compensatory activities of the PA700 and PA28 proteasomal activators. Inhibition of proteasomal function in cultured cells can also lead to selective dopaminergic cell degeneration in conjunction with formation of inclusion bodies [30].
Synthetic proteasomal inhibitors may likewise cause UPS dysfunction, recapitulating features of Parkinson's. PSI induces dopaminergic neuronal-cell death and the emergence of cytoplasmic Lewy-body like eosinophilic inclusions containing α-synuclein and parkin [24]. Additionally, the administration of PSI induces the expression and accumulation of α-synuclein and parkin in primary cultured neurons. These results indicate that proteasomal inhibitors play at least a partial role in neurodegeneration in the mouse model for Parkinson's disease [31].
In the present study, PSI treatment resulted in increased expression of α-synuclein in PC12-cell lysates. Interestingly, we found that the CB receptor agonist WIN55.212.2 could modulate the UPS in this cell-type. Moreover, WIN55.212.2 altered neuronal accumulation of α-synuclein, a Lewy-body component in Parkinson's disease. While pharmacological proteasomal inhibition increases PC12-cell death, administration of WIN55.212.2 confers a protective effect. To determine the signaling pathway responsible for this neuroprotection, we used Western blotting to assess activation of caspase-3 in response to co-administration of WIN55.212.2 and PSI. We found that PSI triggered the apoptotic pathway through caspase-3 activation, but that WIN55.212.2 rather inhibited this activation.
The data are still controversial concerning the effects of CBs on neural cell survival. CBs have been demonstrated to induce apoptosis in several neural or neuronal cell lines, as well as in primary neurons [10, 32]; at the same time, however, numerous reports are indicative of neuroprotective properties of CBs through inhibition of apoptosis and necrosis in vitro and in vivo [26, 33-35].
A cannabinoid-receptor 2 ligand can protect against apoptosis effected through the mitochondrial pathway in an auditory cell line, blocking the activation of a series of caspases, including caspase-3, as well as cleavage of PARP and cytochrome c release [36]. In another report, however, the synthetic cannabinoid delta(9)-tetrahydrocannabinol induced apoptosis in vitro in neonatal rat brain cultures via CB receptor 1. A synthetic cannabinoid has also been demonstrated to activate the stress-activated protein kinase c-jun N-terminal kinase, as well as the pro-apoptotic protease caspase-3. In a separate study of cultured cortical neurons, tetrahydrocannabinol activated caspase-3 [37], and CB1 receptor activation was also found to exert a neuroprotective effect in different in vivo models of brain injury including ischemia [11, 38]. However, in vivo administration of delta(9)-tetrahydrocannabinol in adult rats was not associated with apoptotic pathway induction in the cerebral cortex.
To determine the degree of PC12-cell apoptosis after administration of PSI and WIN55.212.2, we assessed cell viability and levels of activated PARP and caspase-3. We found that administration of WIN55.212.2 improved cellular survival via caspase-3 inactivation. Importantly, however, the duration of WIN55.212.2 exposure is a determinant of toxicity. Whereas in previous reports cells were treated only briefly with the compound, for periods ranging from 30 minutes to 2 hours, in our study, PC12 cells were exposed to PSI and WIN55.212.2 for two days, and thus our results indicate that prolonged exposure may be protective.
Recently, CBs have been identified as therapeutic candidates for amelioration of Parkinson's disease symptoms. WIN55.212.2 may attenuate the action of dopaminergic drugs and modulate release of neurotransmitters in the striatum, with consequent improvement of motor function in Parkinson's patients [39]. However, the results of the present study suggest that WIN55.212.2's therapeutic importance may lie in directly blocking dopaminergic neuronal death and α-synuclein accumulation.
The transcription factor NF-κB regulates numerous biological functions; the mechanisms that ensure selective termination of its activity are not fully understood. However, whereas proteasomal activity contributes to the shutoff of NF-κB dependent gene expression, proteasomal inhibition prevents termination of NF-κB activity, increasing expression of NF-κB as well as its target genes [40]. We find that PSI induces the expression of NF-κB subunits.
WIN55.212.2 and other CB-receptor agonists modulate NF-κB dependent transcription. In general, NF-κB functions to counteract apoptosis. In dendritic cells, phosphorylation of inhibitor of κB-α and the activation and intranuclear localization of NF-κB are enhanced by a 30-minute exposure to WIN55.212.2 [26], suggesting that WIN55.212.2 may participate in immunoregulation through NF-κB activation. In other reports, 2-hour treatment with WIN55.212.2 inhibited PC12-cell activation of NF-κB [41]. Our findings show that activation of NF-κB is induced by longterm exposure to WIN55.212.2 and PSI. WIN55.212.2 appears not only to modulate the mitochondrial apoptosis pathway, but also to protect PC12 cells from PSI-induced cell death through NF-κB mediated gene induction.
Given our findings that WIN55.212.2 restricts caspase activity in PC12 cells undergoing proteasomal inhibition, it is likely that WIN55.212.2 protects neurons from cell death due to proteasomal dysfunction. WIN55.212.2 modulates the UPS through an unknown mechanism. Thus, a more complete understanding of the complex, dynamic interactions between the UPS and CBs appears warranted if CBs are to be considered candidates as therapeutic agents for neurodegenerative diseases such as Parkinson's that involve underlying dysfunction of the proteasomal machinery.
References
- 1.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 2.Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142:1209–1218. doi: 10.1038/sj.bjp.0705881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mechoulam R, Hanus L, Fride E. Towards cannabinoid drugs: revisited. Prog Med Chem. 1998;35:199–243. doi: 10.1016/s0079-6468(08)70037-7. [DOI] [PubMed] [Google Scholar]
- 4.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 8.Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci STKE. 2002;2002:re5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- 10.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, Greenberg DA. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 12.Sagredo O, García-Arencibia M, de Lago E, Finetti S, Decio A, Fernández-Ruiz J. Cannabinoids and neuroprotection in basal ganglia disorders. Mol Neurobiol. 2007;36:82–91. doi: 10.1007/s12035-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 13.Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pryce G, Giovannoni G, Baker D. Mifepristone or inhibition of 11beta-hydroxylase activity potentiates the sedating effects of the cannabinoid receptor-1 agonist Delta(9)-tetrahydrocannabinol in mice. Neurosci Lett. 2003;341:164–166. doi: 10.1016/s0304-3940(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 15.Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Yu X. Regulation of apoptosis: the ubiquitous way. FASEB J. 2003;17:790–799. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
- 19.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 20.Colell A, García-Ruiz C, Roman J, Ballesta A, Fernández-Checa JC. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. FASEB J. 2001;15:1068–1070. doi: 10.1096/fj.00-0574fje. [DOI] [PubMed] [Google Scholar]
- 21.McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 23.Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Chang M, Li H, Hou S, Zhang Y, Hu Y, Han W, Hu L. Proteomic changes of PC12 cells treated with proteasomal inhibitor PSI. Brain Res. 2007;1153:196–203. doi: 10.1016/j.brainres.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 25.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 26.Jüttler E, Potrovita I, Tarabin V, Prinz S, Dong-Si T, Fink G, Schwaninger M. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B) Neuropharmacology. 2004;47:580–592. doi: 10.1016/j.neuropharm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003;53(Suppl 3):S61–S70. doi: 10.1002/ana.10489. [DOI] [PubMed] [Google Scholar]
- 29.Marshansky V, Wang X, Bertrand R, Luo H, Duguid W, Chinnadurai G, Kanaan N, Vu MD, Wu J. Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells. J Immunol. 2001;166:3130–3142. doi: 10.4049/jimmunol.166.5.3130. [DOI] [PubMed] [Google Scholar]
- 30.Jenner P, Olanow CW. The pathogenesis of cell death in Parkinson's disease. Neurology. 2006;66(10 Suppl 4):S24–S36. doi: 10.1212/wnl.66.10_suppl_4.s24. [DOI] [PubMed] [Google Scholar]
- 31.Yang SJ, Kim MJ, Jeong HJ, Kim GC, Gil YG, Kim KR, Kim H. Effects of hypoxia on the ubiquitin-proteasome system in primary cortical neuronal cell cultures. Korean J Phys Anthropol. 2008;21:21–29. [Google Scholar]
- 32.Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of delta9-tetrahydrocannabinol. J Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 34.van der Stelt M, Veldhuis WB, Bär PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J Neurosci. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abood ME, Rizvi G, Sallapudi N, McAllister SD. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci Lett. 2001;309:197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- 36.Jeong HJ, Kim SJ, Moon PD, Kim NH, Kim JS, Park RK, Kim MS, Park BR, Jeong S, Um JY, Kim HM, Hong SH. Antiapoptotic mechanism of cannabinoid receptor 2 agonist on cisplatin-induced apoptosis in the HEI-OC1 auditory cell line. J Neurosci Res. 2007;85:896–905. doi: 10.1002/jnr.21168. [DOI] [PubMed] [Google Scholar]
- 37.Downer EJ, Gowran A, Campbell VA. A comparison of the apoptotic effect of delta(9)-tetrahydrocannabinol in the neonatal and adult rat cerebral cortex. Brain Res. 2007;1175:39–47. doi: 10.1016/j.brainres.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 38.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papa SM. The cannabinoid system in Parkinson's disease: multiple targets to motor effects. Exp Neurol. 2008;211:334–338. doi: 10.1016/j.expneurol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Geng H, Wittwer T, Dittrich-Breiholz O, Kracht M, Schmitz ML. Phosphorylation of NF-kappaB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 2009;10:381–386. doi: 10.1038/embor.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]