Abstract
In an attempt to delay the progression of osteoarthritis from an index injury, early intervention via repair of injured musculoskeletal soft tissue has been advocated. Despite the development of a number of scaffolds intended to treat soft tissue defects, information about their functional performance is lacking. The goal of this study was to consolidate a suite of in vitro and in vivo models into a pre-clinical test platform to assess the functional performance of meniscal repair scaffolds. Our objective was to assess the ability of a scaffold (Actifit™; Orteq, UK) to carry load without detrimentally abrading against articular cartilage. Three test modules were used to assess the functional performance of meniscal repair scaffolds. The first module tested the ability of the scaffold to carry load in an in vitro model designed to measure the change in normal contact stress magnitude on the tibial plateau of cadaveric knees after scaffold implantation. The second module assessed the in vitro frictional coefficient of the scaffold against cartilage to assess the likelihood that the scaffold would destructively abrade against articular cartilage in vivo. The third module consisted of an assessment of functional performance in vivo by measuring the structure and composition of articular cartilage across the tibial plateau 12 months after scaffold implantation in an ovine model. In vitro, the scaffold improved contact mechanics relative to a partly meniscectomized knee suggesting that, in vivo, less damage would be seen in the scaffold implanted knees vs. partly meniscectomized knees. However, there was no significant difference in the condition of articular cartilage between the two groups. Moreover, in spite of the high coefficient of friction between the scaffold and articular cartilage, there was no significant damage in the articular cartilage underneath the scaffold. The discrepancy between the in vitro and in vivo models was likely influenced by the abundant tissue generated within the scaffold and the unexpected tissue that regenerated within the site of the partial meniscectomy. We are currently augmenting our suite of tests so that we can pre-clinically evaluate the functional performance at time zero and as a function of time after implantation.
Keywords: meniscus, scaffold, knee, cartilage
Introduction
The clinical management of young active patients with osteoarthritis presents a challenge in terms of providing long-term pain relief while allowing for a return to high-level activities. Although total joint arthroplasty is considered a safe and effective procedure [10, 11, 15], the risk of subsequent revisions in young active patients and the associated reduction in longevity with each revision [29] advocates delaying the age for a primary total joint replacement. One such paradigm is to repair injured musculoskeletal soft tissue early in the course of the problem in an attempt to prevent or delay the progression of osteoarthritis from the index injury. The meniscus, for example, functions to provide knee joint stability, load bearing, and chondroprotection by distributing loads over a broad area of articular cartilage [19, 21, 22]. Once torn, or damaged, the chondroprotective ability of the tissue is disrupted and the cascade towards osteoarthritis initiated [26]. A goal in the surgical management of meniscal tears or defects is to recreate a mechanically functional tissue in an attempt to halt disease progression. Use of scaffolds to facilitate tissue ingrowth into meniscal defects is one such option that is under investigation.
Although not yet used clinically, cell-seeded scaffolds designed to be cultured in a laboratory setting prior to implantation have been developed for the treatment of meniscal defects [4, 18, 33]. The quest to engineer such implants has used significant funding from federal sources, venture capitalists, and private foundations, the magnitude of which is difficult to estimate, but likely exceeds hundreds of millions of dollars. Resources have thus far been largely directed to the sciences of polymer chemistry, cell biology, and cell–matrix interactions. Few resources, if any, have been used to standardize methods to assess the mechanical properties and functional performance of the candidate materials. Currently, information about the mechanical performance of materials intended for meniscal replacement such as their ability to provide knee joint stability and chondroprotection without damaging the articular cartilage is rarely reported in the scientific literature. As a result, despite 20 years of tissue engineering innovation, only one scaffold for the treatment of meniscal defects has translated into clinical use in the US—Menaflex™ (Regen Biologics, NJ, USA), yet there have been no published reports which quantify the ability of the scaffold to mechanically function in the knee joint. Given that the scaffold is intended to function in the highly loaded environment of the knee, which is exposed to three to five times body weight during activities of daily living [14, 27] and subjected to millions of loading cycles each year, this lack of information negatively impacts regulatory decisions as to the safety and efficacy of an implant and suggests the urgent need to standardize tests aimed at evaluating functional performance.
The closest we have come to developing pre-clinical tests that simulate functional mechanical loading is in the use of animal models; however, controversy persists as to the optimal animal model [3]. Small animal models have been used to provide an assessment of the biological response to new materials implanted into the knee joint [2, 16, 31]; however, the implants are not subjected to the high loads which would occur in the human knee. Large animal models, such as sheep [18, 25], offer the opportunity to subject the candidate materials to immediate and substantial loads, but the models are costly and time consuming to conduct. Moreover, in the event of a negative result, it is often difficult to identify the features of an implant or scaffold that caused the unfavorable result and therefore should be modified for the next design iteration. For example, every synthetic meniscal implant studied thus far in animal models has led to some degree of degeneration of articular cartilage, including fibrillation and depletion of matrix [17, 35], cartilage softening, or the formation of osteophytes [26]. None of these studies have provided definite information about the cause of failure to feed back into the design process, leading to the abandonment rather than refinement of new technologies. A platform is needed in which the functional performance of meniscal scaffolds (whether cell-seeded or non-cell based) can be assessed prior to large-scale animal models and clinical trials. Such a platform should allow for the systematic evaluation and optimization of implant design features and screen out those constructs that are unlikely to mechanically function in vivo.
The goal of this study was to consolidate a suite of in vitro [6, 12] and in vivo test modules [24] into a pre-clinical test platform to assess the functional performance of scaffolds intended to replace a damaged meniscus. Our objective was to assess the ability of a scaffold to carry load and the degree to which the scaffold would detrimentally abrade against articular cartilage. Finally, we wished to compare these in vitro evaluations to an in vivo model which used MRI imaging to assess the structure and composition of the articular cartilage in the tibial plateau 12 months after implantation of the scaffold.
Methods
Three test modules were developed to assess the functional performance of a porous degradable polyurethane scaffold (Actifit™; Orteq Sports Medicine Ltd., London, UK) intended as a temporary scaffold for a partial meniscal defect. The first module tested the ability of the scaffold to carry load in an in vitro model designed to measure the change in normal contact stress magnitude and contact area on the tibial plateau of cadaveric knees after scaffold implantation. This model was designed to give us confidence that the scaffold could carry load under physiological loading conditions [6]. The second module assessed the frictional coefficient of the scaffold against cartilage. This was performed in an in vitro model designed to measure the frictional characteristics of the scaffold against cartilage, immediately after implantation. This model was designed to test the likelihood that the scaffold would destructively abrade against articular cartilage in vivo [12]. The third module consisted of an assessment of functional performance in vivo. An in vivo model was designed to measure the structure and composition of articular cartilage across the tibial plateau 12 months after scaffold implantation. Our goal was to understand if the two in vitro test models predicted the in vivo response of the knee joint to the scaffold [24].
The ability of the scaffold to carry load was tested on a load controlled Instron-Stanmore KCI Knee Joint Simulator (University College London, Middlesex, UK) that was adapted to accept cadaveric ovine knees (in vivo animal model of choice). The simulator was chosen because of the ability to program and dynamically control the axial force, anterior and posterior force, and rotational moment (internal/external torque), as a function of flexion angle towards simulating activities of gait and stair climbing. By combining this technology with a Tekscan piezoelectric sensor (4,010 N; Tekscan, South Boston, MA, USA) located underneath the meniscus and attached to the tibial plateau, a measurement of the normal contact stresses during gait was enabled. Six ovine knees were subjected to loads and flexion–extension profiles corresponding to that experienced in the ovine knee joint during gait [30]. The effect of injury (in this case a partial meniscectomy) and repair (in this case via scaffold implantation) on the joint contact stresses transmitted to the tibial plateau were assessed [6].
To study the frictional coefficient of the scaffold against cartilage, an apparatus was designed to measure the coefficient of friction of two materials which articulate against each other over a range of velocities to simulate a range of boundary conditions. In brief, the apparatus consists of a biaxial load cell to simultaneously measure frictional shear forces and compressive axial loads, while a variable-speed oscillating table is programmed to control the relative speed of the two materials. The test apparatus was used to measure the frictional coefficients of two groups of materials, which included calf cartilage discs articulated against a candidate scaffold and calf cartilage disks articulated against polished stainless steel, to simulate conditions of a hemi-arthroplasty [12]. Equine synovial fluid (ESF) from the stifle joints of three skeletally mature horses (ages 3–5 years) was used as lubricant.
Functional performance in vivo was studied in 12 skeletally mature Columbia × Rambouillet ewes subjected to unilateral partial excision (50% the AP length of the native tissue to within 1 mm of the capsule) of the lateral meniscus. In four animals, the defect was left unfilled. A precontoured biodegradable aliphatic polyurethane scaffold with a porosity of 80% and pores ranging in size from 150 μm to 355 μm (Actifit™; Orteq) was used to fill the defect site in the remaining eight animals and attached to the meniscus using three horizontal mattress 3-0 Ethibond sutures [24]. All animals were euthanized at 12 months, at which point the knees were assessed by magnetic resonance (MR) imaging using a previously validated cartilage-sensitive fast spin echo sequence to assess cartilage morphology [17], as well as quantitative T2 mapping to assess the collagen orientation and T1rho to assess proteoglycan distribution [34].
Joint contact stress data were analyzed using repeated measures ANOVA with post hoc contrasts; the type I error rate (alpha) was set at 0.05. Mann–Whitney tests were used to assess the coefficient of friction data, and differences in T1 and T2rho values were assessed using Student’s t tests, with an alpha of 0.05.
Results
Our in vitro tests demonstrated that a partial meniscectomy extending to 50% of the length of the native meniscus in the sheep knee led to a significant increase in joint contact stress close to the mid-line of the joint. The increase in contact stress as measured in vitro led to cartilage degeneration in vivo in the form of fibrillation, proteoglycan loss, and collagen breakdown in that zone. The relatively high coefficient of friction between the scaffold and articular cartilage as measured experimentally did not result in articular cartilage damage in vivo; this was likely influenced by the fact that abundant tissue was generated within the scaffold and unexpected tissue also regenerated within the site of the partial meniscectomy at 12 months. Our results suggest the need to include temporal effects in our in vitro models.
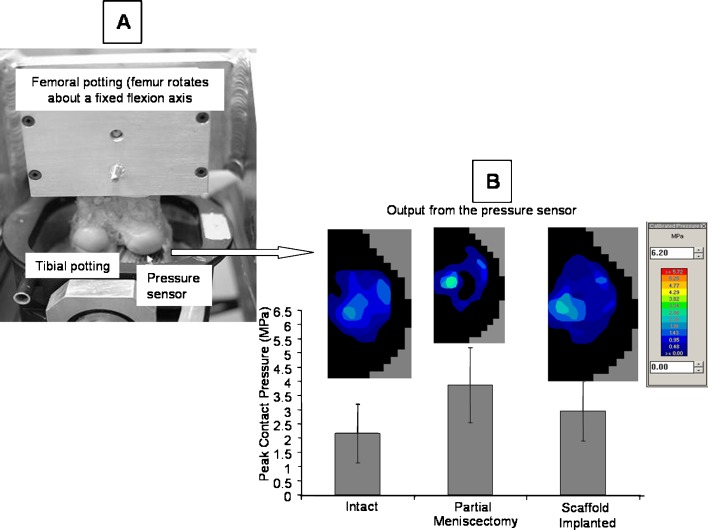
The tibial plateau mounted pressure sensor (Fig. 1a) indicted that while the intact knee manifested a wide area of contact across the tibial plateau, no contact was detectable under the site of the lateral partial meniscectomy. Thus, the contact area was decreased and joint contact stress significantly increased adjacent to the defect site increase by 1.9 MPa (p = 0.01). Scaffold implantation facilitated a more even distribution of contact across the tibial plateau, with a contact stress that was lower (p = 0.01) than that of the partially meniscectomized condition (Fig. 1b). These data suggest that even at time zero, without cell ingress or tissue ingrowth, the scaffold might function to carry and distribute loads across the tibial plateau much in the way of the native tissue.
Fig. 1.
a The experimental apparatus used to measure the distribution of load across the tibial plateau of sheep knees is demonstrated in this photograph. b Representative images of the contact stress distribution across the tibial plateau for each condition are illustrated. The effect of meniscal manipulation on the peak joint contact stresses across the tibial plateau is shown
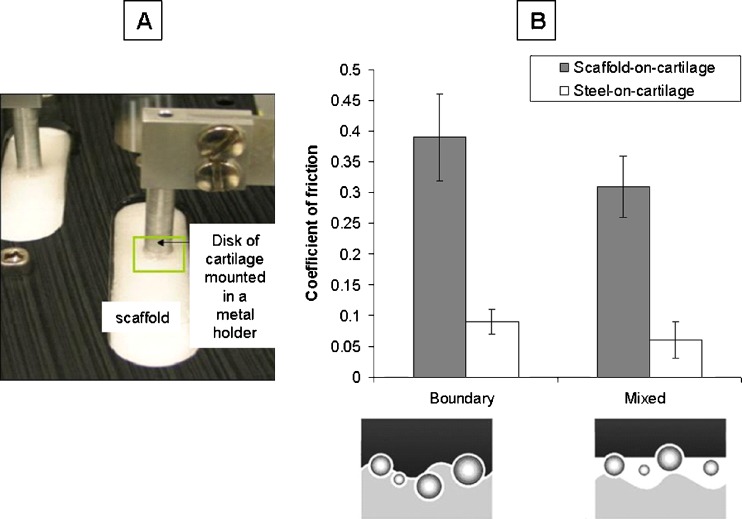
The coefficient of friction was higher (3–5-fold; p = 0.01) for the scaffold against cartilage (Fig. 2a) than for stainless steel against cartilage at all applied strains and entraining speeds (Fig. 2b). This data suggests that at time zero the scaffold might detrimentally abrade against the cartilage.
Fig. 2.
a The experimental apparatus used to measure the coefficient of friction between articular cartilage and the scaffold is shown in this photograph. b This graph displays the coefficient of friction values as measured for boundary and mixed mode lubrication where boundary lubrication is achieved at slow speeds and mixed mode lubrication is achieved at higher speeds
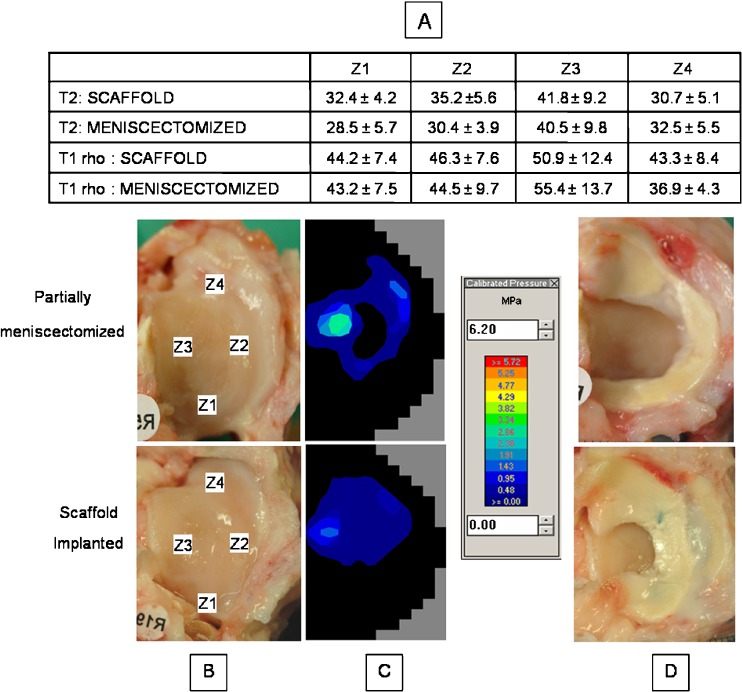
MR imaging revealed damage to the extracellular matrix of articular cartilage over the central weight-bearing area of the lateral tibial plateau in both groups. Disruption of the normally high-ordered collagen (increased T2 values) and diminished proteoglycan content (increased T1rho values) were seen (Fig. 3a). This area (zone 3) corresponded to the region close to the mid-line of the joint (Fig. 3b) in which an increase in joint contact stress was seen in the experimental model at time zero (Fig. 3c). No significant differences were found in the T2 or T1rho values for any zone between the scaffold implanted and non-scaffold implanted groups (p > 0.1).
Fig. 3.
Representative images of knees from both groups are shown illustrating the a distribution of T2 and T1rho values across the four zones for the tibial plateaus of each group. b Gross photographs of representative tibial plateaus are included and the zones used for MRI analysis are labeled. c The representative contact stress distributions for each condition are depicted. d These photographs show the gross appearance of knees with the menisci intact
Discussion
The ability of the native meniscus to distribute loads across the cartilage of the knee joint is its defining functional characteristic. A requirement of any material intended to replace or repair the meniscus is that it should restore the load-carrying ability to the damaged site and prevent damage to the cartilage with which it articulates. However, there are no pre-clinical test models with which to evaluate the functional properties of candidate materials for meniscal repair or replacement. The goal of this study was to consolidate a suite of in vitro and in vivo test modules into a pre-clinical test platform to assess the functional performance of scaffolds intended to replace a damaged meniscus. The functional parameters assessed were the ability of a scaffold to carry load without detrimentally abrading against articular cartilage. The tests were applied to evaluate a porous polyurethane degradable scaffold (Actifit™; Orteq) intended for implantation into a partial meniscal defect. Our in vitro tests demonstrated that a partial meniscectomy extending to 50% of the length of the native meniscus in the sheep knee led to a significant increase in joint contact stress close to the mid-line of the joint. The increase in contact stress as measured in vitro led to cartilage degeneration in vivo in the form of fibrillation, proteoglycan loss, and collagen breakdown in that zone. The experimental in vitro finding that implantation of the scaffold significantly decreased the contact stresses on the tibial plateau relative to the partially meniscectomized knee suggested that, in vivo, less damage would be seen in the scaffold implanted knees. This was not born out in vivo, which demonstrated no significant difference in the condition of cartilage in that zone between the two groups. Furthermore, the high coefficient of friction between the scaffold and articular cartilage as measured experimentally did not result in articular cartilage damage. This was likely influenced by the fact that abundant tissue was generated within the scaffold and unexpected tissue also regenerated within the site of the partial meniscectomy at 12 months (Fig. 3d).
Our models had their limitations. We specifically chose the ovine model for this study, realizing that controversy surrounds the optimal animal model for the in vivo evaluation of meniscal scaffolds [3, 7]. The sheep model was chosen because it has been previously used to evaluate meniscal repair and regeneration technology [8, 9]. The mechanical properties of its meniscus are similar to that of the human meniscus and the contact stresses transmitted to the tibial plateau are within the range of that of human knees [6]. However, the tissue ingrowth that occurred in the empty meniscal defect group raises concern about the suitability of using a partial meniscectomy as a control in the ovine model.
Our experimental in vitro model to assess meniscal load carrying ability is unique in that it allows for the application of multi-directional physiological loads, a significant improvement over the static and quasi-static loads under which knee joint loading is typically assessed [1, 2, 5, 20]. The input loading profile was designed to match ovine knee gait loads thereby allowing for a direct link between the in vitro and in vivo results. By measuring the peak contact stress and contact area on the tibial plateau, our intent was to understand if implantation of a candidate material could restore the stress distribution (hence load-carrying ability) of the meniscus to that of the native tissue and thereby avoid articular cartilage degeneration. The experimental in vitro finding that implantation of the scaffold significantly decreased the contact stresses on the tibial plateau relative to the partially meniscectomized knee suggested that, in vivo, less damage would be seen in the scaffold implanted knees. This was not born out in the in vivo model, which demonstrated no significant difference in the condition of cartilage in that zone between the two groups. Furthermore, the in vitro measure of the frictional characteristics between the scaffold and cartilage suggested that cartilage damage may occur in vivo. Again, this was not the case. There was little or no damage in the cartilage immediately beneath the scaffold. The discrepancy between the outcome measured of the in vitro and in vivo models was caused by the robust matrix generation within the scaffold and within the site of the partial meniscectomy (which was unexpected) over time. To truly use our tests as effective pre-clinical evaluation tools, the temporal changes in the knee, scaffold degradation, cell migration, and matrix generation should ideally be included. To address this need, we have started to measure the load-carrying ability and frictional characteristics of scaffold implanted knees post-sacrifice. For example, by quantifying the frictional characteristics of the scaffold after 3 months of in vivo implantation, we found that the frictional characteristics of the scaffold approach that of the native tissue [12], further emphasizing the need to include temporal effects in any pre-clinical model.
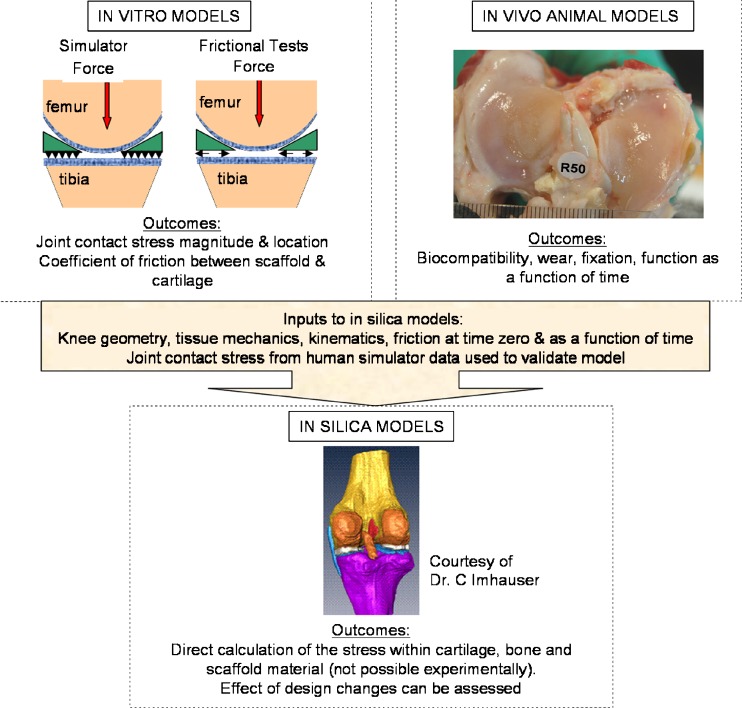
Ultimately, our goal is to use this pre-clinical platform as a scaffold design tool. To this end, the knee joint simulator has recently been further modified to allow for the effect of meniscal manipulation on knee joint contact stress distribution across human knees. This modification will ultimately allow for outputs from the in vitro and in vivo models to be combined and used for the creation of an in silica finite element model (FEM), which can more readily be used to understand the effect of scaffold implantation on the functional performance of the joint (Fig. 4). The in silica model of choice, a computational FEM, allows the user to define inputs—geometry, material properties, applied loads, constraints—and to understand what effect those inputs have on outputs—in the form of contact stress in the joint, and stress distribution within the cartilage and underlying bone, which cannot be measured experimentally. This concept is not new; FEMs have been used to understand the effects of native tissue structural and material properties on the functional performance of the knee joint [13, 32], but often simplify the complex mechanics of the soft tissue structures and do not mimic physiological activities, thereby limiting the clinical relevance of the data generated. Our goal is to experimentally validate a physiological model, which incorporates the viscoelastic biphasic behavior of the soft tissues and employs physiological loading conditions. Given the complexity of such models, and the wide range of possible input parameters (scaffold modulus, anisotropy, geometry, fixation, activity, and percent knee flexion), the diversity of modeling conditions needed to map the entire input–output space is computationally prohibitive. Statistically based predictors can be used to increase the computational efficiency of this process by identifying those variables that have the greatest impact on the model outputs. These statistical methods can be used to target the computational runs required to map the design space, thereby saving both resources and time, and have been used recently to understand the factors that affect the performance of hip resurfacing [23].
Fig. 4.
This figure provides a pre-clinical test platform for the functional evaluation of meniscal scaffolds. The platform consists of in vitro experimental tests and in vivo animal models upon which validated in silica computational models are built
In summary, by combining in vitro measures of the load-distribution and frictional characteristics of candidate materials for meniscal repair and replacement and combining it with an in vivo assessment of cartilage “health”, we have demonstrated an ability to assess the functional performance of candidate materials for meniscal replacement. It is our intent to augment this suite of tests with models that can assess the temporal changes that occur in the scaffold in vivo, but ultimately with the help of statistically driven computational modeling we will systematically evaluate and optimize implants and scaffolds intended for meniscal replacement/repair. With our unique approach, we hope to facilitate the translation of novel technologies into clinical use to provide functional solutions for the estimated 850,000 patients for whom meniscal surgery is carried out annually [28].
Acknowledgments
This study was funded by Orteq Sports Medicine Ltd., London, UK. The research was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, NIH.
Footnotes
This study was funded by Orteq Sports Medicine Ltd., London, UK. The project was supported in part by Award Number RO1AR057343 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAMS or NIH. This research was conducted in a facility constructed with support from Research Facilities Improvement Program CO6-RR12538-01 from the National Center for Research Resources, NIH.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Agneskirchner JD, Hurschler C, Stukenborg-Colsman C, Imhoff AB, Lobenhoffer P. Effect of high tibial flexion osteotomy on cartilage pressure and joint kinematics: a biomechanical study in human cadaveric knees. Arch Orthop Trauma Surg. 2004;124:575–84. doi: 10.1007/s00402-004-0728-8. [DOI] [PubMed] [Google Scholar]
- 2.Angele P, Johnstone B, Kujat R, Zellner J, Nerlich M, Goldberg V, Yoo J. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85(2):445–55. doi: 10.1002/jbm.a.31480. [DOI] [PubMed] [Google Scholar]
- 3.Arnoczky SP, Cook JL, Carter T, Turner S. Translational Models for Studying Meniscal Repair and Replacement: What they can and cannot tell us.Tissue Eng Part B Rev. 2009 Aug 21. (Epub ahead of print) [DOI] [PubMed]
- 4.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 5.Becker R, Wirz D, Wolf C, Göpfert B, Nebelung W, Friederich N. Measurement of meniscofemoral contact pressure after repair of bucket-handle tears with biodegradable implants. Arch Orthop Trauma Surg. 2005;125(4):254–60. doi: 10.1007/s00402-004-0739-5. [DOI] [PubMed] [Google Scholar]
- 6.Brophy RH, Cottrell J, Rodeo SA, Wright TM, Warren RF, Maher SA. Implantation of a synthetic meniscal scaffold improves joint contact mechanics in a partial meniscectomy cadaver model. J Biomed Mater Res A. 2010;92(3):1154–61. doi: 10.1002/jbm.a.32384. [DOI] [PubMed] [Google Scholar]
- 7.Burger C, Mueller M, Wlodarczyk P, Goost H, Tolba RH, Rangger C, Kabir K, Weber O. The sheep as a knee osteoarthritis model: early cartilage changes after meniscus injury and repair. Lab Anim. 2007;41(4):420–31. doi: 10.1258/002367707782314265. [DOI] [PubMed] [Google Scholar]
- 8.Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD.Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res. 2009 Feb 25 (Epub ahead of print) [DOI] [PubMed]
- 9.Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, Ambrosio L, Tognana E, Kon E, Salter D, Nehrer S. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthritis Cartilage. 2006;14(10):1056–65. doi: 10.1016/j.joca.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67–70. doi: 10.1016/j.arth.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Duffy GP, Trousdale RT, Stuart MJ. Total knee arthroplasty in patients 55 years old or younger. 10- to 17-year results. Clin Orthop Relat Res. 1998;356:22–7. doi: 10.1097/00003086-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Gleghorn JP, Doty SB, Warren RF, Wright TM, Maher SA, Bonassar LJ. Analysis of frictional behavior and changes in morphology resulting from cartilage articulation with porous polyurethane foams. J Orthop Res. 2010;28(10):1292–1299. doi: 10.1002/jor.21136. [DOI] [PubMed] [Google Scholar]
- 13.Haut Donahue TL, Hull ML, Rashid MM, Jacobs CR. How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. J. Biomech. 2003;36(1):19–34. doi: 10.1016/S0021-9290(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 14.Heinlein B, Kutzner I, Graichen F, Bender A, Rohlmann A, Halder AM, Beier A, Bergmann G. ESB Clinical Biomechanics Award 2008: complete data of total knee replacement loading for level walking and stair climbing measured in vivo with a follow-up of 6–10 months. Clin Biomech (Bristol, Avon) 2009;24(4):315–26. doi: 10.1016/j.clinbiomech.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann Aaron A., Heithoff Scott M., Camargo Marcelo. Cementless Total Knee Arthroplasty in Patients 50 Years or Younger. Clinical Orthopaedics and Related Research. 2002;404:102–107. doi: 10.1097/00003086-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kang SW, Son SM, Lee JS, Lee ES, Lee KY, Park SG, Park JH, Kim BS. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J Biomed Mater Res A. 2006;78(3):659–71. doi: 10.1002/jbm.a.30579. [DOI] [PubMed] [Google Scholar]
- 17.Kelly BT, Robertson W, Potter HG, Deng XH, Turner AS, Lyman S, Warren RF, Rodeo SA. Hydrogel meniscal replacement in the sheep knee: preliminary evaluation of chondroprotective effects. Am J Sports Med. 2007;35(1):43–52. doi: 10.1177/0363546506292848. [DOI] [PubMed] [Google Scholar]
- 18.Kon E, Chiari C, Marcacci M, Delcogliano M, Salter DM, Martin I, Ambrosio L, Fini M, Tschon M, Tognana E, Plasenzotti R, Nehrer S. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Eng Part A. 2008;14(6):1067–80. doi: 10.1089/ten.tea.2007.0193. [DOI] [PubMed] [Google Scholar]
- 19.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint. Clin Orthop Relat Res. 1980;149:283–90. [PubMed] [Google Scholar]
- 20.Lee SJ, Aadalen KJ, Malaviya P, et al. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006;34:1334–44. doi: 10.1177/0363546506286786. [DOI] [PubMed] [Google Scholar]
- 21.Levy IM, Torzilli PA, Gould JD, Warren RF. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg Am. 1989;71(3):401–6. doi: 10.2106/00004623-198971030-00014. [DOI] [PubMed] [Google Scholar]
- 22.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior–posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883–8. doi: 10.2106/00004623-198264060-00011. [DOI] [PubMed] [Google Scholar]
- 23.Long JP, Santner TJ, Bartel DL. Hip resurfacing increases bone strains associated with short-term femoral neck fracture. J Orthop Res. 2009;27(10):1319–25. doi: 10.1002/jor.20884. [DOI] [PubMed] [Google Scholar]
- 24.Maher Suzanne A., Rodeo Scott A., Doty Stephen B., Brophy Robert, Potter Hollis, Foo Li-Foong, Rosenblatt Lauren, Deng Xiang-Hua, Turner Anthony S., Wright Timothy M., Warren Russell F. Evaluation of a Porous Polyurethane Scaffold in a Partial Meniscal Defect Ovine Model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(11):1510–1519. doi: 10.1016/j.arthro.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Martinek V, Ueblacker P, Bräun K, Nitschke S, Mannhardt R, Specht K, Gansbacher B, Imhoff AB. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg. 2006;126(4):228–34. doi: 10.1007/s00402-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 26.Messner K, Gillquist J. Prosthetic replacement of the rabbit medial meniscus. J Biomed Mater Res. 1993;27:1165–73. doi: 10.1002/jbm.820270907. [DOI] [PubMed] [Google Scholar]
- 27.Mündermann A, Dyrby CO, D’Lima DD, Colwell CW, Jr, Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. J Orthop Res. 2008;26(9):1167–72. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 28.Rodkey WG. Basic biology of the meniscus and response to injury. Instr Course Lect. 2000;49:189–93. [PubMed] [Google Scholar]
- 29.Sheng PY, Konttinen L, Lehto M, Ogino D, Jämsen E, Nevalainen J, Pajamäki J, Halonen P, Konttinen YT. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish arthroplasty registry. J Bone Joint Surg Am. 2006;88(7):1425–30. doi: 10.2106/JBJS.E.00737. [DOI] [PubMed] [Google Scholar]
- 30.Tapper JE, Fukushima S, Azuma H, Thornton GM, Ronsky JL, Shrive NG, Frank CB. Dynamic in vivo kinematics of the intact ovine stifle joint. J Orthop Res. 2006;24(4):782–92. doi: 10.1002/jor.20051. [DOI] [PubMed] [Google Scholar]
- 31.Testa Pezzin AP, Cardoso TP, do Carmo Alberto Rincón M, de Carvalho Zavaglia CA, de Rezende Duek EA. Bioreabsorbable polymer scaffold as temporary meniscal prosthesis. Artif Organs. 2003;27(5):428–31. doi: 10.1046/j.1525-1594.2003.07251.x. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri A, Nayeb-Hashemi H, Singh A, Tafti BA. Influence of meniscectomy and meniscus replacement on the stress distribution in human knee joint. Ann Biomed Eng. 2008;36(8):1335–44. doi: 10.1007/s10439-008-9515-y. [DOI] [PubMed] [Google Scholar]
- 33.Weinand C, Peretti GM, Adams SB, Jr, Bonassar LJ, Randolph MA, Gill TJ. An allogenic cell-based implant for meniscal lesions. Am J Sports Med. 2006;34(11):1779–89. doi: 10.1177/0363546506290666. [DOI] [PubMed] [Google Scholar]
- 34.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan by T1 rho imaging. JOR. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood DJ, Minns RJ, Strover A. Replacement of the rabbit medial meniscus with a polyester–carbon fibre bioprosthesis. Biomaterials. 1990;11:13–16. doi: 10.1016/0142-9612(90)90045-R. [DOI] [PubMed] [Google Scholar]