Abstract
Chondrocytes forming articular cartilage are embedded in a vast amount of extracellular matrix having physical stiffness and elasticity, properties that support the mechanical load from bones and enable the flexible movement of synovial joints. Unlike chondrocytes that conduct the growth of long bones by forming the growth plate, articular chondrocytes show suppressed cell proliferation, unless these cells are exposed to pathological conditions such as mechanical overload. In the present study, we found that one of the members of the CCN family, CCN3, was significantly expressed in chondrocytes isolated from the epiphyseal head in developing rat synovial joints. Evaluation of the effect of recombinant CCN3 on those chondrocytes revealed that CCN3 promoted proteoglycan synthesis, whereas this factor repressed the proliferation of the same cells. These results suggest a critical role for CCN3 in the regulation of the biological properties of articular chondrocytes.
Keywords: CCN family, CCN3, Articular cartilage, Chondrocytes, NOV
Introduction
In vertebrates, cartilage has a pivotal role in both the development and maintenance of the musculoskeletal system. Cartilage can be roughly classified into 2 types, transient and permanent, based on the fate of the tissue. The most typical transient cartilage is the growth-plate cartilage, which leads to the genesis and growth of long bones, such as femurs and ribs. In this type of cartilage, chondrocytes undergo a series of proliferation and differentiation processes and finally are removed after hypertrophy, in parallel with the replacement of the cartilage with bone tissue, which process is called endochondral ossification. In contrast, chondrocytes involved in permanent cartilage remain present and quiescent in the tissue throughout life, even after the completion of growth and development. A typical permanent cartilage is the articular cartilage, which contains chondrocytes that do not enter the endochondral ossification pathway and remain at the joint surface, probably to maintain joint integrity (Karaplis 2002). Therefore, articular cartilage cells are characterized by restricted cell proliferation and the potential to supply extracellular matrix molecules, particularly cartilaginous proteoglycans. However, the molecular background to support such properties of chondrocytes that form and maintain articular cartilage has not been fully addressed.
The CCN family is a novel family of matricelluar proteins with characteristic structure and multiple functions (Brigstock 2003; Perbal 2004; Perbal and Takigawa 2005; Chen and Lau 2009). These proteins are composed of 4 evolutionally conserved modules: insulin-like growth factor-like (IGFBP), von Willebrand factor type C repeat (VWC), thrombospondin type 1 repeat (TSP1), and C-terminal (CT) modules, except for CCN5, which lacks the CT module (Perbal and Takigawa 2005; Leask and Abraham 2006). CCN family members have been implicated in key steps of cartilage metabolism. In fact, all of the members are produced in cartilage anlagen for long bones during mouse development (Kawaki et al. 2008b). Especially, CCN2/connective tissue growth factor (CTGF) is known to play an important role as a signal conductor in the orchestration of growth and differentiation in both growth-plate and articular cartilage (Nakanishi et al. 2000; Nishida et al. 2002; Kubota and Takigawa 2007 and 2011). However, the role of the other CCN family members in the chondrocytes forming articular cartilage remains unclear, and no factors that maintain the quiescence of these chondrocytes have been identified. Recently, it was reported that targeted disruption of CCN3 causes joint deformity (Heath et al. 2008). Therefore, in this study, we evaluated the effect of CCN3 (Joliot et al. 1992; McCallum and Irvine 2009), which has been reported to counteract CCN2 (Kawaki et al. 2008b; Riser et al. 2009), on the chondrocytes isolated from the epiphysis of developing rats.
Materials and methods
Isolation and culture of chondrocytes
Chondrocytes were obtained from the distal third of the femoral distal epiphysis from 5-day-old Sprague Dawley rats. The tissue was minced into small fragments, and digested at 37°C with 2 rounds of 0.15% (w/v) collagenase (Wako Pure Chemicals, Japan) treatment in serum-free α-MEM (ICN Biomedicals, Inc., USA) for 30 min each time. After enzymatic digestion, the cells were filtered through a 100-μm nylon mesh (BD Falcon, Franklin Lakes, NJ, USA) and then cultured with α-MEM supplemented with 10% fetal bovine serum (Cancera International, Rexclale, ON, Canada) at 37°C in a humidified incubator with a 5% CO2 atmosphere. Unless stated otherwise, the medium was replaced every 3 days. The cells were frozen for storage, and all experiments were carried out with cells within 2 passages after restarting the culture from the frozen stock.
RNA extraction and reverse transcription
The total RNAs of the cells were isolated by using an RNeasy Mini Kit (QIAGEN, Hilden, Germany), following the directions of the manufacturer. One microgram of total RNA was used for the reverse transcription reaction conducted at 42°C for 30 min with avian myeloblastosis virus (AMV) reverse transcriptase supplied in an RNA PCR Kit (Takara, Ohtsu, Japan).
Quantitative real-time PCR
Quantitative real-time PCR was performed as described previously (Yanagita et al. 2007; Kawaki et al. 2008a). Amplification was carried out with the cDNA templates obtained from the reverse transcription reaction by using the specific primers listed in Table 1 and an SYBR® Green Real Time PCR Master Mix (Toyobo, Osaka, Japan). Specific amplification was confirmed by melting curve analysis and by agarose gel electrophoresis of the endpoint samples.
Table 1.
Oligodeoxynucleotide primers used in real-time PCR analysis
| Gene name | Strand | Nucleotide sequence |
|---|---|---|
| gapdh | sense | 5’- ATCTTGGGCTACACTGAGGA -3’ |
| antisense | 5’- CAGGAAATGAGCTTGACAAAGT -3’ | |
| tenascin-C | sense | 5’- ACCAACTGTGCCCTGTCCTA -3’ |
| antisense | 5’- ATTTCGGAAGTTGCTGGGTC -3’ | |
| ccn3 | sense | 5’-TGAAGTCTCTGACTCCAGCATT -3’ |
| antisense | 5’-TGGCTTTCAGGGATTTCTTG -3’ |
[35S]-sulfate incorporation assay
Cells seeded in 48-well plates were grown to confluence. Twenty-four hours after the first medium replacement upon confluence, the cells were incubated with or without recombinant CCN3 (rCCN3), which was produced and purified as described previously (Lazar et al. 2007). Five hours later, [35S] sulfate was added to all culture wells to a final concentration of 370 kBq/mL and the cultures were continued for a further 17 h. The medium of each well was collected, and the glycosaminoglycans therein was precipitated by 1% cetylpyridinium chloride (CPC) and was trapped in a glass fiber filter. The filter was transferred to a scintillation tube containing Clear-Sol (Nacalai Tesque, Kyoto, Japan) scintillation cocktail, and the radioactivity was counted by a scintillation counter. The cell layer remaining in the wells was treated with actinase-E for 21 h at 55°C, and the cell lysate was analyzed in the same way as the medium.
[3H] thymidine incorporation assay
Cells seeded in 48-well plates were grown to sub-confluence. Twenty-four hours after medium replacement, the cells were treated or not with rCCN3. Twenty-four hours later, all wells received [3H] thymidine at a final concentration of 350 kBq/mL; and incubation was then continued for 4 h. Next, the medium was removed; and the cells were exposed to 5% trichloroacetic acid (TCA) (w/v) for 10 min. Thereafter, the TCA was replaced with an ethanol-ether mixture (3:1), in which the cells were kept for 2 min. The cells were then dissolved with 0.3 M NaOH for 1 h at 37°C. After the neutralization with HCl, the cell lysate was transferred to a scintillation tube containing Clear-Sol scintillation cocktail; and the radioactivity was counted in a scintillation counter.
Statistical analysis
Statistical analysis was performed, where required, by using Student's paired t-test.
Results
Expression of CCN3 and tenascin C (TNC), a marker for articular chondrocytes, in the epiphyseal chondrocytes
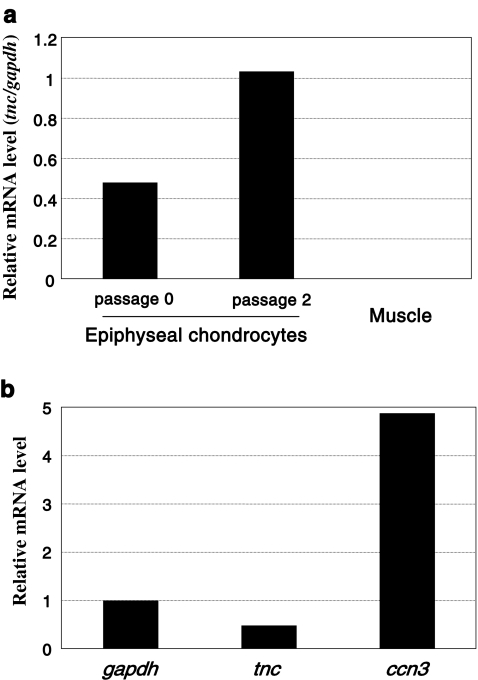
Prior to functional evaluation of CCN3, we initially examined the expression of CCN3 in chondrocytes from developing articular cartilage. Chondrocytes were isolated from the joint heads of 5-day-old rats, and total RNA was extracted after a few passages of the cells in culture. Subsequently, the mRNA levels of CCN3 and tenascin C, which is a typical marker of articular chondrocytes (Nishida et al. 2002), were evaluated by quantitative real-time PCR analysis. Tenascin C mRNA was detected, indicating the presence of chondrocytes with the articular chondrocytic phenotype in the cell population, which would also contain chondrocytes that would form the secondary ossification center. Of note, the expression of tnc was retained a high level, even after 2 passages in cell culture in vitro, suggesting the utility of these cells as a model of articular chondrocytes (Fig. 1a). As also suggested by a previous report (Kawaki et al. 2008b), strong expression of CCN3 was distinctly confirmed, suggesting a significant role for CCN3 in articular cartilage (Fig. 1b).
Fig. 1.
Gene expression analysis of the chondrocytes isolated from developing rat epiphyseal heads. a Expression of the tenascin C gene (tnc), a typical marker of articular chondrocytes. The tnc mRNA levels in the chondrocytes in primary cell cultures and in those after 2 rounds of passages were quantified by real-time RT-PCR. RNA extracted from rat muscle tissue was used as a negative control. Data are presented as relative values against those of the glyceraldehyde 3-dehydrogenase gene (gapdh). b Strong expression of ccn3 in primary cultures of chondrocytes prepared from the epiphyseal heads. Messenger RNA expression levels of ccn3 and tnc genes were standardized against that expression level of the gapdh gene. Note that ccn3 mRNA showed even remarkably higher levels of expression than the housekeeping (gapdh) or articular chondrocyte marker (tenascin C: tnc) gene
Repression of DNA synthesis in the epiphyseal chondrocytes by rCCN3
Next, we estimated the effect of rCCN3 on the proliferation of the chondrocytes from the epiphysis by evaluating the uptake of radiolabeled thymidine. According to previous reports, CCN3 represses cell proliferation in a variety of cells, including chondrocytes isolated from mouse rib cartilage (Bleau et al. 2007; Fukunaga-Kalabis et al 2008; Kawaki et al. 2008b; Shimoyama et al. 2010). Consistent with these past findings, rCCN3 significantly repressed the uptake of radiolabeled thymidine by the chondrocytes from rat joint heads (Table 2). Our present results not only support the general idea that CCN3 acts as a universal anti-proliferative agent, but also indicate its significant role in maintaining the quiescence of articular chondrocytes.
Table 2.
Anti-proliferative effect of rCCN3 on chondrocytes in developing joint heads
| Treatment | [3 H]-Thymidine uptake | |
|---|---|---|
| cpm/well: (average±SD) | % of control | |
| control | 33,077 ± 1,552 | 100.0 ± 4.7 |
| rCCN3 (100 ng/mL) | 30,927 ± 1,368* | 93.5 ± 4.1* |
* p = 0.011
Promotion of sulfated proteoglycan synthesis in epiphyseal chondrocytes by rCCN3
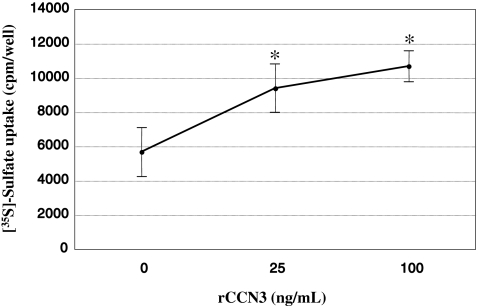
Although CCN3 was described to promote the differentiation of the cells of particular origins (Sin 2011), our previous study showed that sulfated proteoglycan synthesis (Kawaki et al. 2008b), which is a typical phenotypic marker of differentiated chondrocytes, in mouse costal chondrocytes is rather repressed by rCCN3. In order to examine whether CCN3 exerts a similar, or different, effect on the chondrocytes from the joint head, we comparatively evaluated the uptake of radiolabeled sulfate by those cells in the absence or presence of rCCN3 at different doses. Unexpectedly, sulfate uptake, representing sulfated proteoglycan synthesis, was remarkably enhanced in a dose-dependent manner (Fig. 2). These results suggest a function of CCN3 in the articular cartilage distinct from that in chondrocytes following the route of endochondral ossification.
Fig. 2.
Dose-dependent enhancement of sulfate uptake into glycosaminoglycans by chondrocytes from developing rat epiphyseal cartilage by rCCN3. Radiolabeled sulfate incorporated by the cells during a 17-h incubation period after the addition of rCCN3 at the indicated concentration was measured. These data are presented as the mean values and SD of the data obtained from 8 independent cell cultures. *p < 0.01 versus control (0)
Discussion
In the present study, we evaluated the effect of CCN3 that was actually produced by the cells constituting developing joint heads. Consequently, CCN3 was found to repress the proliferation of those cells, whereas this factor promoted the sulfated proteoglycan synthesis by the same cells. One of our previous studies involved similar analysis of chondrocytes from mouse costal cartilage, which is a typical temporary cartilage that supports the growth of rib cage through the endochondral ossification process. In that previous study, the effect of CCN3 was comparatively analyzed with that of CCN2 on the chondrocytes following the osteogenic pathway. In the case of these costal cells, CCN3 exerted effects entirely opposite to those of CCN2. Namely, CCN3 inhibited the synthesis of both DNA and proteoglycans, which is known to be promoted by CCN2 (Kawaki et al. 2008b). The similarity and discrepancy between the data from epiphyseal and costal cartilage present a few critical issues as to the role of CCN3 in cartilage biology.
First, cell proliferation was inhibited in both types of chondrocytes. These findings are fully in agreement with those reported for a variety of cells of different origins (Bleau et al. 2007; Fukunaga-Kalabis et al 2008; Kawaki et al. 2008b; Shimoyama et al. 2010). Therefore, this anti-mitotic effect is indicated to be a quite specific and general function of CCN3. In contrast, CCN3 promoted sulfated proteoglycan synthesis in the chondrocytes from developing joint heads, whereas it repressed that of chondrocytes fated to hypertrophy and ossification. Generally, CCN3 has been shown to promote cellular differentiation. In fact, recent study has revealed that CCN3 promotes neurite outgrowth (Sin 2011). In this context, our present result with epiphyseal chondrocytes seems to be feasible. Interestingly, the inhibitory effect of CCN3 on proteoglycan synthesis was seen only in CCN2-producing wild-type costal chondrocytes, whereas no such effects were evident in corresponding CCN2-null chondrocytes (Kawaki et al. 2008b). The apparent repressive effect of CCN3 on the proteoglycan synthesis is supposedly due to its antagonistic effect on CCN2, which can be displayed only in the presence of CCN2. It should be also noted that costal chondrocytes proceeding to mineralization through hypertrophic differentiation possess a molecular background different from that of epiphyseal chondrocytes constructing permanent cartilage. Since the functionality of any CCN family member highly depends on the co-factors present in the microenvironment, it is not surprising for CCN3 to exert apparently opposite effects in different tissues, as actually reported (Lin et al. 2003; Perbal and Takigawa 2005; Ohgawara et al. 2011).
Unlike growth-plate chondrocytes, the proliferation of chondrocytes to form articular cartilage needs to be highly restricted to construct a structure of the cartilage with quiescent and isolated chondrocytes embedded in a vast amount of cartilaginous ECM. To yield such a large amount of ECM, the chondrocytes have to be quite active in producing ECM molecules, including proteoglycans. As found in this present study, these functional properties of CCN3 of repressing the proliferation and enhancing the ECM production of chondrocytes from joint heads are thus quite appropriate for the formation and maintenance of articular cartilage. Investigations to clarify in detail the role of CCN3 in the formation of articular cartilage is currently underway.
Acknowledgments
This work was supported by the programs Grants-in-Aid for Scientific Research (S) [to M.T.] and (C) [to S.K.] from Japan Society for the Promotion of Science, and by a research grant from Terumo Life Science Foundation [to S.K.]. D.J. is supported by a scholarship provided by the Japanese Ministry of Education, Culture, Sports, Science, and Technology. Work performed in B. Perbal's laboratory was funded by French Ministry of Education: EA1556; and by European PROTHETS (Prognosis and Therapeutic Targets of Ewing Family of Tumors, FP6 Contract 503036).
Contributor Information
Satoshi Kubota, Phone: +81-86-2356646, FAX: +81-86-2356649, Email: kubota1@md.okayama-u.ac.jp.
Masaharu Takigawa, Phone: +81-86-2356646, FAX: +81-86-2356649, Email: takigawa@md.okayama-u.ac.jp.
References
- Bleau AM, Planque N, Lazar N, Zambelli D, Ori A, Quan T, Fisher G, Scotlandi K, Perbal B. Antiproliferative activity of CCN3: involvement of the C-terminal module and post-translational regulation. J Cell Biochem. 2007;101:1475–1491. doi: 10.1002/jcb.21262. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Telson SM, Liu ZJ, Balint K, Juhasz I, Elder DE, Perbal B, Herlyn M. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene. 2008;27:2552–60. doi: 10.1038/sj.onc.1210896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis AC. Embryonic development of bone and the molecular regulation of intramembranous and endochondral bone formation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego: Academic; 2002. pp. 33–58. [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Yamada T, Matsumura T, Mandai T, Yao M, Maeda T, Lyons KM, Takigawa M. Functional requirement of CCN2 for intramembranous bone formation in embryonic mice. Biochem Biophys Res Commun. 2008;366:450–456. doi: 10.1016/j.bbrc.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M (2011). The role of CCN2 in cartilage and bone development. J Cell Commun Signal in press [DOI] [PMC free article] [PubMed]
- Lazar N, Manara C, Navarro S, Bleau AM, Llombart-Bosch A, Scotlandi K, Planque N, Perbal B. Domain-specific CCN3 antibodies as unique tools for structural and functional studies. J Cell Commun Signal. 2007;1:91–102. doi: 10.1007/s12079-007-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- McCallum L, Irvine AE. CCN3 - A key regulator of the hematopoietic compartment. Blood Rev. 2009;23:79–85. doi: 10.1016/j.blre.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M. Effect of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/en.141.1.264. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol. 2002;192:55–63. doi: 10.1002/jcp.10113. [DOI] [PubMed] [Google Scholar]
- Ohgawara T, Kubota S, Kawaki H, Kurio N, Abd El Kadar T, Hoshijima M, Janune D, Shimo T, Perbal B, Sasaki A, Takigawa, M (2011). Association of the metastatic phenotype with CCN family members among breast and oral cancer cells. J Cell Commun Signal, in press. [DOI] [PMC free article] [PubMed]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal B, Takigawa M. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174:1725–34. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T, Hiraoka S, Takemoto M, Koshizaka M, Tokuyama H, Tokuyama T, Watanabe A, Fujimoto M, Kawamura H, Sato S, Tsurutani Y, Saito Y, Perbal B, Koseki H, Yokote K. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol. 2010;30:675–82. doi: 10.1161/ATVBAHA.110.203356. [DOI] [PubMed] [Google Scholar]
- Sin WC. CCN3 promotes neurite outgrowth. J Cell Commun Signal. 2011;5:18. doi: 10.1007/s12079-020-00556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, Tanaka S, Takigawa M. Expression and physiological role of CCN4/Wnt-induced secreted protein 1 mRNA splicing variants in chondrocytes. FEBS J. 2007;274:1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]