Abstract
Mice lacking the pro-adhesive matricellular protein connective tissue growth factor (CTGF/CCN2) display an embryonic lethal phenotype due to defects in bone and cartilage. However, the specific role of CCN2 in skin development is unknown. Here, we generated mice deleted for CCN2 in the entire body (using a cre/lox system in which CCN2 is deleted in the entire body due to the presence of a constitutively expressed cre recombinase). We found that CCN2 was not required for the development of skin as defined by skin thickness measurements, trichrome staining and immunostaining with anti-CD31 (to detect endothelial cells) and anti-α−SMA (to detect smooth muscle cells and pericytes) antibodies. Thus, although recently we have shown that CCN2 is required for fibrogenesis in postnatal mice, CCN2 is not required for skin development during embryogenesis.
Keywords: CTGF, CCN2, Skin
Introduction
Skin consists of epithelia overlying supporting connective tissue, which consists principally of fibroblasts and extracellular matrix (ECM). One of the prototypical members of the CCN family of proteins, CTGF (Connective tissue growth factor/CCN2), a matricellular protein, promotes adhesive signaling through integrins and heparan sulfate-containing proteoglycan (HSPG)s (Leask and Abraham 2006; Perbal 2004; Brigstock 2009; Lau and Lam 1999). The vivo of CCN2, however, is poorly understood.
Studies using whole body CCN2 knockout mice have revealed that loss of CCN2 results in perinatal lethality, associated with defects in ribcage ossification (Ivkovic et al. 2003). CCN2 is an effective surrogate marker of fibrosis (Leask et al. 2009). Recently, we found, using conditional CCN2 knockout mice in which CCN2 is deleted in adult fibroblasts, that CCN2 was required for bleomycin-induced skin fibrosis (Liu et al. 2011). Yet, the role of CCN2 in skin development is unknown. In this report, we use animals deficient in CCN2 to probe the role of CCN2 in skin development. Our data provide new insights into the role that CCN2 plays in skin biology.
Methods
Generation of whole body CCN2 knockout mice
As described previously, mice harboring a floxed CCN2 allele were made under contract by genOway (Lyon, France) on a fee-for-service basis (Liu et al. 2011). These were crossed by genOway with mice expressing cre recombinase under the control of the cytomegalovirus promoter/enhancer (Jackson Labs). Mice were then backcrossed into a C57/BL6J background. To generate Cre/CCN2 heterozygote mice, mice hemizygous for Cre and heterozygous for the loxP-CCN2 allele were crossed with mice possessing a homozygous loxP-CCN2 allele (genOway Laboratories). The resultant mice were crossed each other second cross obtained Cre/CCN2 homozygote (KO) as well as CCN2 homozygous mice lacking cre (WT). Animals used for experiments were genotyped by polymerase chain reaction. CCN2 and cre alleles were identified as described previously (Liu et al. 2011; Zheng et al. 2002). To screen for the CCN2 alleles, the primers 5′-AATACCAATGCACTTGCCTGGATGG-3′ and 5′-GAAACAGCAATTACTACAACGGGAGTGG-3′ were used. The sizes for the wild type, flox and knockout bands are: 878 bp, 1003 bp and 587 bp respectively. KO mice displayed perinatal lethality (Ivkovic et al. 2003); skin from these animals and age-matched WT mice were taken and processed for further analysis. All animal experiments were approved by the appropriate board at the University of Western Ontario.
Assessment of skin thickness and ECM synthesis
Formalin fixed and paraffin embedded skin was sectioned (0.5 μm) using a microtome (Leica) and placed onto Superfrost Plus slides (Fisher Scientific). Sections were de-waxed in xylene and then rehydrated by successive immersion in descending concentrations of alcohol. Sections were subsequently stained with Hemotoxylin and Eosin (H&E; Fisher Scientific) according to the manufacturer’s recommendation; skin thickness was measured using a software program (Northern Eclipse, Empix, and Mississauga, ON, Canada). Additional sections were subjected to Masson’s trichrome stain, in which collagen appears as a blue stain. Semi-quantitative analysis was employed to assess the amount of collagen was assessed by three blinded observers: 0 signifies: No collagen; 1 signifies: little collagen; 2 signifies: Moderate amount of collagen; 3 signifies: Excessive amount of collagen.
Immunohistochemistry
Sections were created as described above and subjected to immunofluorescence staining. Tissue sections were incubated with 5% donkey serum for 1 h, washed three times with phosphate-buffered saline (PBS) and incubated with primary antibodies for 1 h at room temperature under humidified conditions. Primary antibodies used were: mouse anti-alpha-smooth muscle actin (α-SMA, 1:100 dilution, Sigma) and rabbit anti-CD31 (Millipore, 1:500 dilution). After primary antibody incubation, sections were then washed with PBS and incubated with appropriate fluorescent secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) for 1 h at room temperature. Sections were washed, mounted using 4′,6-diamidino-2-phenylindole (DAPI) and then photographed using a Zeiss fluorescence microscope and Northern Eclipse software (Empix, Missassagua, ON, Canada). The number of blood vessels per unit area was calculated with the aid of Northern Eclipse software (Empix, Missassagua, ON, Canada).
Results
Generation of mutant mice bearing a whole body CCN2 knockout
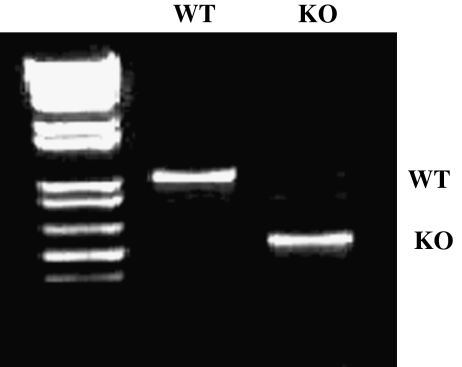
To assess the effect of loss of CCN2 on skin development, we crossed mice harboring a CCN2 allele bordered by loxP sites with mice containing a cre recombinase gene expressed under the control of a cytomegalovirus promoter/enhancer (CMV) (Liu et al. 2011). Littermate mice homozygous for the loxP-CCN2 that were hemizygous for CMV-cre (KO) were generated as well as mice homozygous for loxP-CCN2 but lacking CMV-cre (WT). Deletion of CCN2 was verified using polymerase chain reaction analysis of tail DNA (Fig. 1). Mice deleted for CCN2 showed perinatal lethality, as previously described (Ivkovic et al. 2003). Further analysis of these mice will be reported elsewhere; but by this stage of development CCN2 was absent from skin as revealed by immunohistochemistry (not shown).
Fig. 1.
Generation of whole body CCN2 knockout. As described in methods, mice deleted (KO) or not (WT) for the CCN2 gene in the entire body were generated. Deletion of CCN2 was tested by PCR genotyping of tail DNA. Mice containing the CCN2 gene (WT) or not (KO) are shown. Please note that KO mice showed perinatal lethality, as expected (Ivkovic et al. 2003). The sizes for the wild type, flox and knockout bands are: 878 bp, 1003 bp and 587 bp respectively
Whole body CCN2 knockout mice show normal skin development
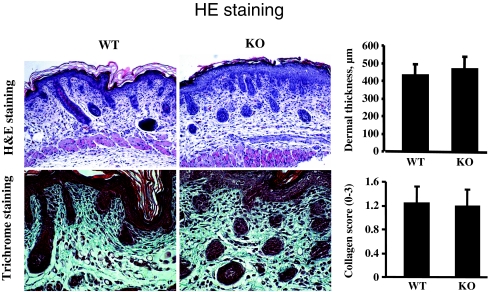
Skin of mice deleted for CCN2 (KO) and age-matched control mice (WT) were then fixed, sectioned and subjected to histological examination using hematoxylin and eosin (H and E) and trichrome staining (to examine ECM deposition). WT and KO mice were indistinguishable in regards to skin appearance, thickness and ECM deposition (Fig. 2).
Fig. 2.
CCN2 is not required for skin development. H and E and trichrome analysis. Mice deleted (KO) or not (WT) for the CCN2 gene were generated. a H and E stain b Trichrome stain. For all assays, 6 mice were analyzed. Representative sections are shown. Note that no difference in skin thickness or collagen score (0–3) were observed between WT and KO skin
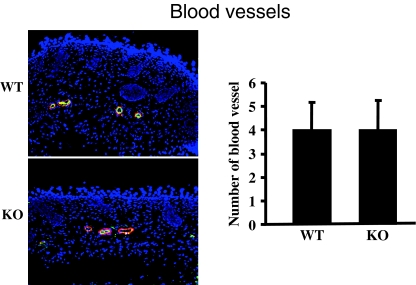
To assess whether CCN2 was required for formation of the vasculature, sections were stained with anti-CD31 (to detect endothelial cells) and anti-α−SMA (to detect smooth muscle cells and pericytes) antibodies. WT and KO mice were indistinguishable in regards to formation of the vasculature (Fig. 3). Collectively, our results indicated that the loss of CCN2 does not impair skin development in embryogenesis.
Fig. 3.
CCN2 is not required for skin development. CD31 and α−SMA staining. Mice deleted (KO) or not (WT) for the CCN2 gene in the entire body. Immunohistochemical analysis of skin derived was performed with anti-CD31 (green, to detect endothelial cells) and anti-α−SMA (red, to detect pericytes and smooth muscle cells) antibodies. For all assays, cells from 6 mice were analyzed. Number of blood vessels per unit area is shown. Representative sections are shown. Note that loss of CCN2 does not affect the appearance of blood vessels in skin
Discussion
Although CCN2 is required for bleomycin-induced skin fibrosis in adult mice (Liu et al., epub), the in vivo function of CCN2 in skin development and homeostasis is unknown. To assess the possible contribution of CCN2 to skin development in vivo, we use conditional knockout technology. We used mice homozygous for a CCN2 gene flanked by loxP sites and hemizygous for a transgene encoding a cre recombinase driven by a CMV promoter (Liu et al. 2011). Confirming previous data, CCN2-knockout mice showed perinatal lethality (Ivkovic et al. 2003). However, CCN2-knockout mice were indistinguishable from their wild-type counterparts in terms of skin development as revealed by H and E and trichrome analysis. Similarly, the vasculature of CCN2-knockout mice appeared normal. Thus, CCN2 is not required for skin development during embryogenesis, in spite of the fact that CCN2 is expressed by embryonic fibroblasts cultured from E13.5 mice (Shi-wen et al. 2006), in vivo CCN2 is largely present in the developing vasculature, hair follicles and epithelia (Jones et al. 2007). These observations are consistent with the notion that, when fibroblasts are concerned, CCN2 expression is intimately linked with the presence of fibrogenic stimuli such as TGFβ (Leask et al. 2009). Although CCN2 may be required for postnatal skin homeostasis, it is nonetheless interesting to note that CCN2 did not appear to be required for baseline ECM production in post-natal skin, at least ~1 month post-deletion of CCN2 in dermal fibroblasts (Liu et al. 2011).
Combined with previous data showing that CCN2 was essential for bleomycin-induced fibrogenesis in vivo, that an antibody directed against CCN2 results in reduced bleomycin-induced lung fibrosis (Ponticos et al. 2009) and that a CCN2 siRNA reduces collagen synthesis in scleroderma fibroblasts (Xiao et al. 2006), these data suggest that CCN2 may represent a highly specific target for antifibrotic drug intervention (Brigstock 2009).
Acknowledgements
Funding from the Canadian Foundation for Innovation and the Canadian Institutes of Health Research is gratefully recognized.
References
- Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): from pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal. 2009;3:5–18. doi: 10.1007/s12079-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Gray MR, Oliveira BE, Koch M, Castellot JJ., Jr CCN5 expression in mammals: I. Embryonic and fetal tissues of mouse and human. J Cell Commun Signal. 2007;1:127–43. doi: 10.1007/s12079-007-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shi-Wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis. Arthritis Rheum. 2011;63:239–46. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–55. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006;281:10715–26. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- Xiao R, Liu FY, Luo JY, Yang XJ, Wen HQ, et al. Effect of small interfering RNA on the expression of connective tissue growth factor and type I and III collagen in skin fibroblasts of patients with systemic sclerosis. Br J Dermatol. 2006;155:1145–53. doi: 10.1111/j.1365-2133.2006.07438.x. [DOI] [PubMed] [Google Scholar]
- Zheng B, Zhang Z, Black CM, Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160:1609–17. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]