Abstract
The calcium transport ATPase and the copper transport ATPase are members of the P-ATPase family and retain an analogous catalytic mechanism for ATP utilization, including intermediate phosphoryl transfer to a conserved aspartyl residue, vectorial displacement of bound cation, and final hydrolytic cleavage of Pi. Both ATPases undergo protein conformational changes concomitant with catalytic events. Yet, the two ATPases are prototypes of different features with regard to transduction and signaling mechanisms. The calcium ATPase resides stably on membranes delimiting cellular compartments, acquires free Ca2+ with high affinity on one side of the membrane, and releases the bound Ca2+ on the other side of the membrane to yield a high free Ca2+ gradient. These features are a basic requirement for cellular Ca2+ signaling mechanisms. On the other hand, the copper ATPase acquires copper through exchange with donor proteins, and undergoes intracellular trafficking to deliver copper to acceptor proteins. In addition to the cation transport site and the conserved aspartate undergoing catalytic phosphorylation, the copper ATPase has copper binding regulatory sites on a unique N-terminal protein extension, and has also serine residues undergoing kinase assisted phosphorylation. These additional features are involved in the mechanism of copper ATPase intracellular trafficking which is required to deliver copper to plasma membranes for extrusion, and to the trans-Golgi network for incorporation into metalloproteins. Isoform specific glyocosylation contributes to stabilization of ATP7A copper ATPase in plasma membranes.
Keywords: Transport ATPases, Ca2+ ATPase, Cu2+ ATPase, Ca2+ signaling, Copper trafficking, Energy transduction
Introduction
Transport ATPases of the P-type family are members of the acid dehalogenases superfamily (Burroughs et al. 2006), and are membrane bound enzymes whose catalytic cycle includes ATP utilization by formation of a phosphorylated enzyme intermediate, vectorial displacement of bound cation, and final hydrolytic cleavage of Pi from the phosphoenzyme intermediate. Transport ATPases (Inesi and Nakamoto 2008) are essential components of numerous mechanisms pertinent to signaling, homeostasis and energy metabolism. They are also relevant to diseases of cardiac muscle, cancer, osteoporosis, retinal degeneration, immune deficiency, cystic fibrosis, diabetes, gastric ulcer, hearing loss, skin alterations, and copper-related disorders. Compounds of pharmacological and therapeutic interest, and their interaction with transport ATPases, such as ouabain, thapsigargin and gastric H+ pump inhibitors, have been recognized. Furthermore, an important role of transport ATPases in the establishment of drug resistance has become apparent.
Within the wide family of ion-motive P-type ATPases, it is interesting to compare the calcium and the copper transport ATPases, as they are prototypes of enzymes that retain basic structural and catalytic features, but at the same time display prominently diverse features. In fact, the plasma (Brini and Carafoli 2009) and intracellular Ca2+ ATPases (de Meis and Vianna 1979; Inesi 1985; Møller et al. 2010) reside on membranes delimiting distinct cellular compartments and, in this rather stable position, collect free Ca2+ from one side of the membrane to release it on the other side, yielding a three to four orders of magnitude free Ca2+ gradient. On the other hand, copper is acquired by the copper ATPase (Lutsenko et al. 2007) through exchange with donor proteins, and is then delivered to acceptor proteins by vectorial displacement, without formation of high free copper gradients. In addition, the copper ATPase undergoes intracellular trafficking to reach rather distant locations for copper delivery and extrusion.
The calcium ATPase
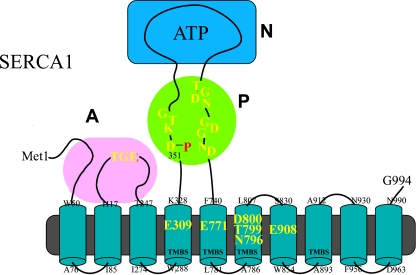
Calcium ATPases include transporters located on plasma membranes (PMCA: Brini and Carafoli 2009), on the Golgi/secretory pathway (Ca2+/Mn2+-SPCA: Ton and Rao 2004; Van Baelen et al. 2004), and on Sarco-Endoplasmic Reticulum membranes (SERCA). The latter is discussed here due to its detailed characterization. SERCA enzyme isoforms are encoded by three highly conserved genes (SERCA1, 2, and 3) localized on different chromosomes, and undergo alternative splicing following transcription (Periasamy and Kalyanasundaram 2007). The SERCA1 isoform (MacLennan et al. 1985) has been studied in detail, and the sequence of reactions and conformational changes comprising its catalytic and transport cycle is well known. Its amino acid sequence comprises (Fig. 1) ten transmembrane mostly helical segments, where the fourth, fifth, sixth and eighth segments contribute residues to a Transmembrane Binding Site (TMBS) for two Ca2+ required for enzyme activation and undergoing active transport. The SERCA1 headpiece includes an N domain with the ATP binding site, a P domain with the aspartyl residue undergoing phosphorylation as a catalytic intermediate, and an A domain containing the conserved TGE motif involved in catalytic assistance of the final hydrolytic reaction.
Fig. 1.
Two-dimensional folding model of the SERCA1 sequence. The diagram shows ten transmembrane segments including the calcium binding sites (TMBS) involved in enzyme activation and transport. Yellow residues contribute side chain oxygen atoms for calcium binding. The extramembranous region comprises: a nucleotide binding domain (N); the P domain, with several residues (in red) conserved in P-type ATPases, including D351 that undergoes phosphorylation to form the catalytic phosphoenzyme intermediate (EP); and the A domain with the TGE conserved sequence involved in catalytic assistance of EP hydrolytic cleavage
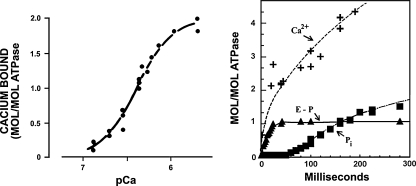
From the functional stand point, the equilibrium binding isotherm (Inesi et al. 1980) shown in Fig. 2 demonstrates that each molecule of SERCA1 protein binds two Ca2+ with high affinity 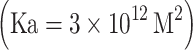 and high cooperativity (Hill coefficient = 1 .82). Mutational analysis has demonstrated that E309, E771, N796, T799, D800 and E908 in the transmembrane region (Fig. 1) are specifically required for Ca2+ dependent catalytic functions (Clarke et al. 1989; Andersen and Vilsen 1992), and are directly involved in Ca2+ binding (Strock et al. 1998) as elegantly shown by crystallography (Toyoshima et al. 2000).
and high cooperativity (Hill coefficient = 1 .82). Mutational analysis has demonstrated that E309, E771, N796, T799, D800 and E908 in the transmembrane region (Fig. 1) are specifically required for Ca2+ dependent catalytic functions (Clarke et al. 1989; Andersen and Vilsen 1992), and are directly involved in Ca2+ binding (Strock et al. 1998) as elegantly shown by crystallography (Toyoshima et al. 2000).
Fig. 2.
Functional characterization of SERCA1. The left panel shows an equilibrium binding isotherm obtained by incubation of SERCA with different concentrations of CaCl2 (EGTA-Ca buffer) in the absence of ATP. The right panel shows a pre-steady state experiment initiated by addition of ATP to SERCA saturated with Ca2+. Rapid formation of phosphorylated intermediate is accompanied by occlusion of bound calcium. Hydrolytic cleavage of Pi occurs following a lag period and continues with steady state velocity. ATP utilization and Ca2+ transport occur with stoichiometric ratio of 1:2. Adapted from Inesi et al. (1980)
The high specificity of the SERCA activating and transport sites (TMBS) for Ca2+ is revealed by high binding affinity and selectivity for Ca2+ in the presence of much higher concentrations of Mg2+ which is required in conjunction with ATP for phosphoryl transfer at the catalytic site within the P domain. It is of interest that Mn2+ appears able to substitute to some extent for both Ca2+ and Mg2+ (Chiesi and Inesi 1981).
Addition of ATP (Fig. 2) to the calcium activated ATPase (E1.Ca2) is rapidly followed by formation of the phosphorylated intermediate (E1-P . Ca2) and occlusion of the two bound Ca2+ that become unavailable for exchange on the surface of the membrane (Inesi et al. 1980). Isomerization of the phosphoenzyme (E2-P . Ca2) is then followed by vectorial dissociation of the two bound Ca2+. Final hydrolytic cleavage of Pi completes the cycle, yielding a turnover of 2 Ca2+ pumped per one ATP utilized by the ATPase. At neutral pH, Ca2+ binding and dissociation occur in concomitance with H+ exchange, as occupancy of pertinent acidic residues favors the E2 conformation, and H+ dissociation favors the E1 state. It is apparent that a pK change, in parallel with phosphoenzyme isomerization, contributes to reduction in affinity and dissociation of bound Ca2+ (Tadini-Buoninsegni et al. 2006). The sequential reactions comprising the catalytic and transport cycle are shown in the following scheme:
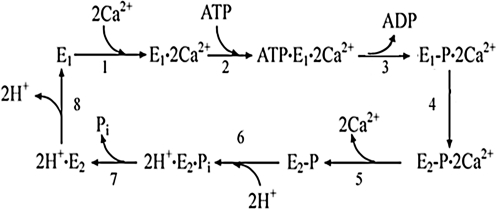
The equilibrium constants of the partial reactions listed in the scheme yield an overall free energy requirement that matches the standard free energy derived from hydrolytic cleavage of ATP γ-phosphate (Inesi et al. 1990). It is of interest that the equilibrium constants for enzyme phosphorylation by ATP (reaction 2), as well as the final hydrolytic cleavage of Pi (reaction 6) are nearly 1, indicating that the free energy of ATP is conserved by the enzyme and used to change the Ca2+ binding characteristics through conformational work. This means that the chemical potential of ATP does not manifest itself in the phosphoryl transfer (ATP . E . Ca2 ←→ ADP . E-P . Ca2) or hydrolytic cleavage (H2E-P ←→ H2E . Pi) reactions, but rather in the drastic reduction of the enzyme affinity for Ca2+, as the enzyme transitions from E . Ca2. to E2-P . Ca2 . If we consider that the free energy required for one ATPase cycle is used to change the equilibrium constant of reaction 1 to the equilibrium constant of reaction 4, under standard conditions,
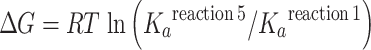 |
where K a reaction 1 is the association constants of the enzyme for Ca2+ in the ground state (H2E + 2Ca2+out ←→ E . Ca2 + 2H+out) and and K a reaction 5 is the association constant of the enzyme for Ca2+ following the phosphoenzyme transition (H2E-P + 2Ca2+in ←→ E-P . Ca2 + 2H+in). The corresponding values, at 25°C and pH 7.0, are 3 × 1012 M2 and 3 × 105 M2, yielding a free energy requirement of ∼9 Kcal per cycle which is used to change K a reaction 1 to K a reaction 5 . This value approximates the expected yield of free energy by cleavage of ATP γ-phosphate under standard conditions. Free energy changes in the presence of various concentrations of substrates and products were subjected to analysis and explained elsewhere (Inesi et al. 1990). It is of interest that the entire cycle can be reversed by phosphorylating the enzyme with Pi in the absence of Ca2+, and then adding high (mM) Ca2+ and ADP to form ATP (Masuda and de Meis 1973). Therefore, chemical and binding energy can be interconverted in either direction of the cycle, by means of protein conformational work.
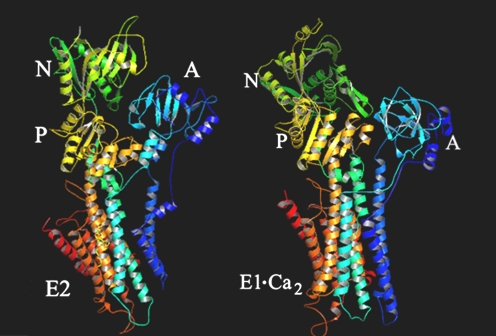
Functional characterization of the SERCA cycle indicates that the basic coupling mechanism of catalysis and transport consists of mutual destabilization of Ca2+ and phosphorylation sites. Considering that the Ca2+ binding and phosphorylation site are separated by a relatively large distance (∼50 Å: Figs. 1 and 3), it is apparent that interconversion of cation-binding and phosphorylation potentials requires a long-range intramolecular linkage, rendered possible by protein conformational changes. To start with, catalytic activation by Ca2+ binding produces a prominent conformational change, as apparent by comparing (Fig. 3) the three-dimensional structure of SERCA1 in the absence of Ca2+ (commonly referred to as E2) and in the presence of bound Ca2+ (commonly referred to E1 . Ca 2). In fact, calcium binding to the TMBS affects the A, N and P domain positions in the headpiece, as well as transmembrane segments. Further conformational changes occur in parallel with subsequent sequential reactions, including rotation and bending of entire domains as well as changes in orientation of side chains involved in catalysis and binding. This explains the linkage of phosphoenzyme isomerization to vectorial displacement of bound Ca2+ (Toyoshima and Inesi 2004).
Fig. 3.
Crystallographic models of SERCA1 in the E2 (left panel) and E1.Ca2 (right panel) states. The E2 state, stabilized with thapsigargin, was derived from Protein Data Bank code 1iw0, and the E1.Ca 2 state was derived from Protein Data Bank code 1su4
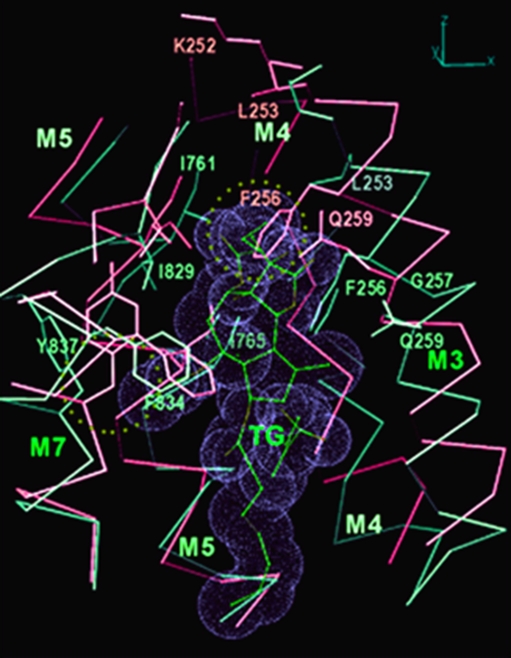
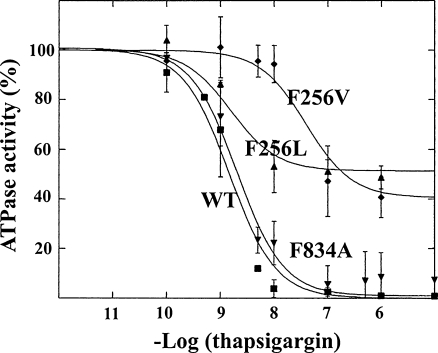
A cogent demonstration of the interdependence of Ca2+ binding, protein conformational change and enzyme activation is provided by thapsigargin (TG), a very high affinity and specific inhibitor of Ca2+ binding and SERCA activation (Sagara and Inesi 1991). It is shown in Fig. 4 that the presence of TG within the SERCA transmembrane region produces steric interference (Xu et al. 2004) with displacement of the Phe256 side chain, which is normally associated with Ca2+ binding. Transition from E2 to E1 . Ca 2 is thereby prevented, with consequent stabilization of the E2 conformation, and loss of catalytic activity. It is noteworthy that single mutations of Phe256 interfere with the inhibitory effect of TG (Fig. 5).
Fig. 4.
Binding of thapsigargin (TG) to SERCA interferes with the E2 to E1.Ca2 transition. TG is shown with van der Waals surface represented by dotted spheres. Transmembrane portions of M3, M4, M5, and M7 helices are in violet (E1·2Ca2+) and in light green (E2(TG)) and superimposed. Note how steric collisions (dotted circle) with TG would occur if the F256 side chain would transition from the E2 state to the E1·2Ca2+ state in the presence of TG. Derived from Xu et al. (2004)
Fig. 5.
Interference of Phe256 mutations with the inhibitory effect of TG on SERCA1 ATPase activity. Site directerd mutations were obtained by expression of mutated cDNA delivered by viral vectors to COS-1 cells. Experiments on ATPase activity were conducted with the microsomal fraction of the COS-1 cells sustaining heterologous expression. Derived from Xu et al. (2004)
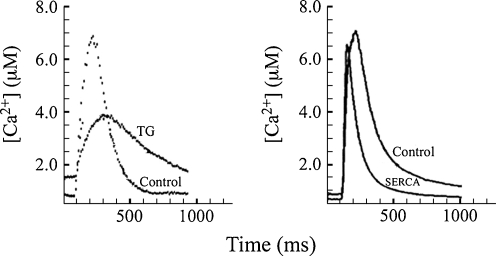
SERCA is a prominent intracellular protein of muscle, where it sustains ATP dependent Ca2+ transport across the sarcoplasmic reticulum membrane which, in concomitance with passive release through gated channels, allows variations of cytosolic Ca2+ concentrations and control of Ca2+ signaling functions (Clapham 2007). A clear example of the SERCA role in Ca2+ signaling is observed in cardiac myocytes (Fig. 6) where specific SERCA inhibition with TG produces a prominent reduction of Ca2+ released upon electrical stimulation, as well as slowing of the decay phase of the Ca2+ signal (Prasad and Inesi 2009). This indicates that Ca2+ pumping by SERCA is required to fill the Ca2+ stores to provide sufficient Ca2+ for subsequent for release to activate contraction, and is also required for removal of cytosolic Ca2+ to allow decay of the Ca2+ signal and relaxation of contraction. It is also shown in Fig. 6 that heterologous expression of additional SERCA protein by gene transfer, does not increase the amount of Ca2+ released, but increases the decay rate of the signal (Cavagna et al. 2000). This demonstrates that endogenous SERCA is sufficient to fill the Ca2+ stores for release of maximal Ca2+ upon electric stimuli, but the level of endogenous SERCA is rate limiting for the Ca2+ signal decay, which can be accelerated by expression of additional SERCA. Regulation of SERCA2 activity and level plays important roles in cardiac physiology and pathology (Bers 2008; Prasad and Inesi 2010). It is of interest that the SERCA affinity for Ca2+ and its catalytic turnover can be modulated through phosphorylation of the small partner protein phospholamban, in connection with adrenergic mechanisms in cardiac muscle (MacLennan and Kranias 2003). Generally, as mentioned above, the transduction features of Ca2+ transport ATPases are basic components of Ca2+ signaling mechanisms in numerous and diverse cell systems.
Fig. 6.
Effects of thapsigargin (TG) and heterologous SERCA expression (SERCA) on cytosolic Ca2+ transients of cardiac myocytes subjected to electrical field stimulation. Myocytes treated with TG (10 nM) or overexpressing exogenous SERCA following gene transfer by adenovirus vectors, are compared with control myocytes. Note the reduction in peak height and decay rate following TG treatment, and the inreased decay rate following SERA overexpression. Adapted from Prasad and Inesi (2009) and Cavagna et al. (2000)
The copper ATPase
The mammalian copper ATPases ATP7A (Dierick et al. 1995; Tümer et al. 1995) and ATP7B (Petrukhin et al. 1994) encode enzyme isoforms that sustain active transport of copper by utilization of ATP, and are involved in copper transfer from enterocytes to blood, copper export from the liver, copper delivery to the secretory pathways for incorporation in metalloprotein, and general copper homeostasis as reviewed in detail by Lutsenko et al. (2007). Genetic defects of ATP7A and ATP7B yield the human Menkes (Vulpe et al. 1993) and Wilson (Gitlin 2003; Harada 2002) diseases, respectively.
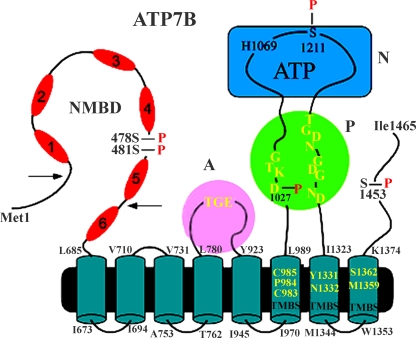
Structural and functional features of the copper ATPase proteins have been reviewed (Mercer et al. 2003; Tsivkovskii et al. 2004; Voskoboinik et al. 2001a), indicating that ATP7A and ATP7B possess a similar architecture, with considerable sequence homology but functionally significant differences. It is shown in Fig. 7 that the ATP7B isoform comprises eight transmembrane segments (as compared to ten in SERCA), including a copper binding site (TMBS) involved in catalytic activation and transport, and a headpiece including the N, P and A domains with conserved catalytic motifs analogous to SERCA1. A specific feature of mammalian copper ATPases is an amino-terminal extension (NMBD) that includes six copper binding sites in addition to the TMBS (Lutsenko et al. 2007). This N-terminal extension is involved in interactions with partner proteins and sustains copper-induced and functionally relevant conformational effects (Banci et al. 2009a).
Fig. 7.
Two-dimensional folding model of the ATP7B sequence. The diagram shows eight transmembrane segments including a copper binding site (TMBS). Yellow residues at the TMBS are likely involved in copper binding. The extramembranous region comprises: a nucleotide binding domain (N) with the H1069 residue whose mutation is frequently found in the Wilson disease; the P domain, with several residues (in yellow) conserved in P-type ATPases, where D1027 undergoes phosphorylation to form the catalytic phosphoenzyme intermediate (EP); the A domain with the TGE (yellow) conserved sequence involved in catalytic assistance of EP hydrolytic cleavage; the N-metal binding domain (NMBD) with six copper binding sites; and a C-terminus chain. Serines undergoing kinase assisted phosphorylation (Ser478, Ser481, Ser1121 and Ser1453) reside within flexible loops of the protein
Copper binding is attributed to characteristic CPX or XPC sequences that are present also in other P1B-type ATPases involved in transport of other metals such as cadmium, zinc, lead, cobalt and silver. On the other hand, other amino acid side chains are likely to participate in transient metal coordination during transport, thereby determining metal selectivity (Argüello 2003). Although the crystal structure of the mammalian copper ATPases is not available as yet, solution structures of specifc domains have been obtained by spectroscopic methods (Banci et al. 2009b; Dmitriev et al. 2006). Furthermore, crystal structures of microbial copper ATPases headpiece domains have been obtained (Sazinsky et al. 2006a, b; Tsuda and Toyoshima 2009). Most importantly, the occurrence of conformational changes concomitant with sequential reactions of the catalytic cycle has been demonstrated by characterization of proteolytic digestion patterns under pertinent conditions (Hatori et al. 2009). This indicates that, in analogy to the calcium ATPase, copper ATPases undergo functionally related conformational changes.
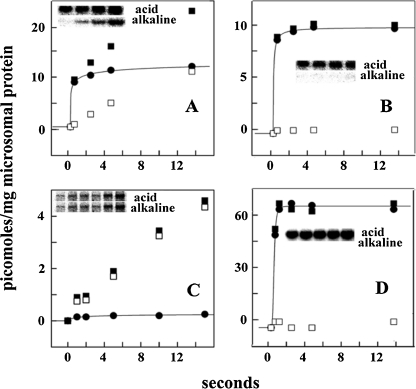
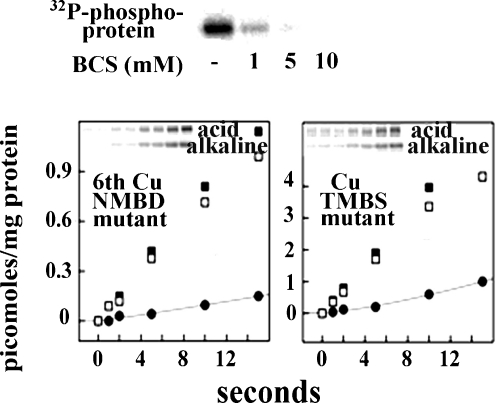
The native abundance of copper ATPases is quite low and, for this reason, biochemical experimentation requires recombinant protein obtained by heterologous expression in insect or mammalian (Barnes et al. 2005; Tsivkovskii et al. 2002; Voskoboinik et al. 2001b; Pilankatta et al. 2009) cells. It has been found that the copper ATPase undergoes phosphorylation upon addition of ATP (Barnes et al. 2005; Tsivkovskii et al. 2002; Voskoboinik et al. 2001b; Hung et al. 2007; Pilankatta et al. 2011). It is very important, in this regard, to distinguish formation of the ATPase catalytic intermediate from independent kinase assisted phosphorylation. In fact, phosphorylation of ATP7B protein (Fig. 8) obtained by heterologous expression in COS-1 cells includes a rapid and alkali labile component obtained within a few seconds, and a much slower alkali resistant phosphorylation (see below). Only the fast component is due to formation of phosphorylated intermediate (analogous to other P ATPases), and is not observed (Fig. 9) following mutation of the conserved aspartate (D1027) at the catalytic site, or following mutation within the transmembrane copper binding site (TMBS). Under optimal conditions, the phosphoenzyme intermediate undergoes decay by hydrolytic cleavage within the second time scale, as expected upon completion of the catalytic site.
Fig. 8.
Phosphorylation of WT ATP7B (a, b), ATP7B D1027N mutant (c) and WT SERCA1 (d), in the absence (a, c and d) and in the presence (b) of PKD inhibitor. Microsomes obtained from COS-1 cells sustaining heterologous expression of WT ATP7B (a, b), ATP7B D1027N mutant (c), or WT SERCA1 (d) were incubated with 50 μM [γ-32P]ATP at 30°C, in the absence (a, c and d) or in the presence (b) of 20 μM CID755673 (PKD inhibitor). Electrophoresis in acid buffer or alkaline buffer was then performed to distinguish total [32P]phosphoprotein (■) from alkali resistant [32P]phosphoprotein (□). The difference is considered alkali labile [32P]phosphoprotein (aspartate: ●) and attributed to formation of phosphorylated enzyme intermediate. The stoichiometry of phosphoprotein refers to total microsomal protein. Derived from Pilankatta et al. (2011)
Fig. 9.
Copper dependence of ATP7B phosphorylation. and effects of site directed mutations. Top: Total phosphorylation (aspartate and serines) is inhibited by copper chelation with BCS. Lower quadrants: total phosphorylation (aspartate and serines) is prevented by C575A/C578A mutation at the 6th NMBD copper site (6thCuNMBD mutant), while only aspartate phosphorylation is prevented by C983A & C985A mutation of the TMBS (Cu TMBS mutant). Note the different stoichiometric scale on the vertical axis of the two panels. Derived from Pilankatta et al. (2011)
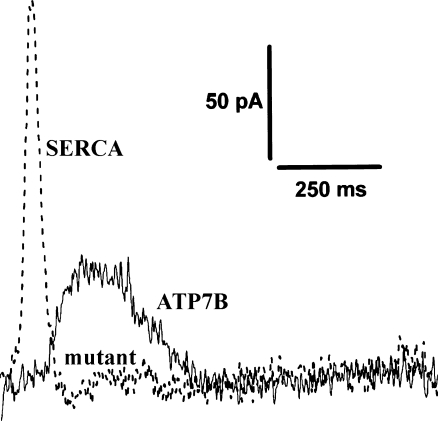
Steady state copper transport has been observed with ATP7A expressed in cultured Chinese hamster ovary cells (Voskoboinik et al. 1998) and with reconstituted preparations of ATP7A expressed in insect cells (Hung et al. 2007), but the reported rates are much lower than expected from ATPase activity. In fact, measurements of copper transport are rendered difficult by unfavorable characteristics of the radioactive copper isotope, as well as by occurrence of non-specific high affinity copper binding. These difficulties make it impossible to obtain direct measurements of copper movements within the short time scale of a single catalytic cycle, as done in detail for the Ca2+ ATPase (Fig. 2). Nevertheless, indirect demonstration of copper movements was obtained by measurements of charge transfer upon addition of ATP to ATP7B protein adsorbed on a solid supported membrane. It is shown in Fig. 10 that, within the pre-steady state following addition of ATP, a current transient is in fact obtained, due to movement of positive charge towards the electrode. The current is not observed in the presence of copper chelators or following mutation of copper binding sites in the protein (Tadini-Buoninsegni et al. 2010). It is of interest that the decay time constant of the copper signal is 140 +/− 4 milliseconds, which is significantly slower than that obtained with the Ca2+ ATPase, i.e. 25 +/− 0.3 milliseconds under the same conditions (Fig. 10). This indicates that the catalytic and transport kinetics sustained by the copper ATPase are approximately one order of magnitude slower that those of the Ca2+ ATPase, even though the general catalytic mechanism retains similar features.
Fig. 10.
Current transients obtained following rapid addition of ATP to recombinant WT copper ATPase (in the presence of 5 μM CuCl2, solid line) or Ca2+-ATPase (in the presence of 10 μM free Ca2+, dotted line). Charge movements were measured with microsomes containing recombinant Cu+-ATPase (ATP7B) or Ca2+ ATPase (SERCA1) adsorbed on a solid supported membrane anchored to a gold electrode. No currents are obtained with an inactive ATP7B mutant. Net charge across the activated protein was compensated for by a flow of electrons along the external circuit to keep the applied voltage ΔV constant across the whole metal/solution interphase, and the resulting current transient was monitored under potentiostatic conditions as a function of time. This technique detects pre-steady state current transients within the first transport cycle, and is not sensitive to stationary currents following the first cycle. (Derived from Tadini-Buoninsegni et al. 2010)
In addition to utilization of ATP for formation of phosphorylated intermediate, phosphorylation of serine residues has an influence on trafficking of ATP7A expressed in both polarized and non-polarized cells (Veldhuis et al. 2009). Furthermore, it was recently reported (Pilankatta et al. 2011) that using ATP7B protein expressed in COS-1 cells, a prominent alkali stable phosphorylation is obtained following addition of ATP. This alkali stable phosphorylation is formed at slow rates, in addition to rapid formation of alkali labile phosphorylated enzyme intermediate, and is specifically and totally prevented by protein kinase D inhibition (Fig. 8). An important feature of Protein kinase D is its association with the trans-Golgi network, where it favors fission of transport carriers specifically destined to the cell surface (Valverde et al. 1994; Liljedahl et al. 2001). It should be noted that alkali resistant phosphorylation is not observed at all with the Ca2+ ATPase (skeletal muscle SERCA1) under the same conditions (Fig. 8). However, serine phosphorylation sustained by Ca2+/calmodulin-dependent protein kinase has been reported for SERCA2, with consequent activation of Ca2+ pumping ATPase in cardiac and slow twitch muscle, but not in fast twitch skeletal muscle (Hawkins et al. 1994).
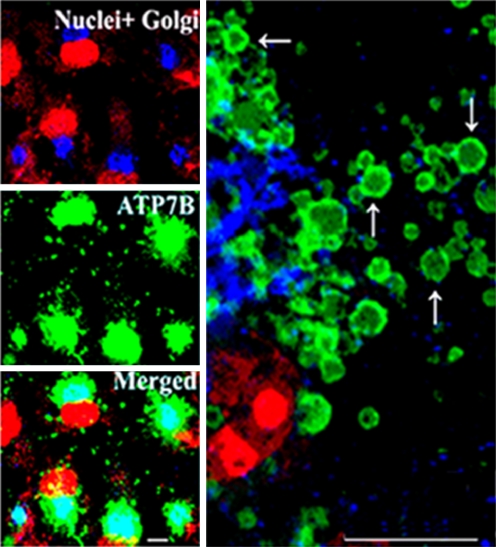
Alkali resistant phosphorylation of ATP7B involves serine residues at least in part identified as S478, S481, S1121 and S1453 (Fig. 7), requires copper and, most importantly, is required for ATP7B trafficking. Copper regulated movement from the Golgi apparatus to vesicles approaching the plasma membrane was first observed with ATP7A (Petris et al. 1996, 2002; La Fontaine et al. 1998), consistent with the notion that copper-induced trafficking of the copper ATPase is a key mechanism for copper homeostasis (Mercer et al. 2003). Compartmentalization and trafficking has also been observed with ATP7B (Guo et al. 2005; Schaefer et al. 1999). In fact, copper induced displacement from the trans-Golgi network to cytosolic trafficking vesicles can be shown clearly with ATP7B expressed in COS-1 cells (Fig. 11) and hepatocytes (Tsivkovskii et al. 2002). Most importantly, trafficking is not observed following mutations within the sixth copper binding site of the NMBD, or mutation of serines involved in phosphorylation assisted by protein kinase D (Pilankatta et al. 2011). It is therefore apparent that a concerted mechanism, based on copper occupancy of NMBD sites and kinase assisted phosphorylation of serines, is permissive of ATP7B trafficking. It is possible that the negative charge acquired by serines through phosphorylation, is involved in electrostatic interactions (such as arginine residues) with consequent effects on conformation and binding to partner proteins. The serine residues undergoing phosphorylation reside at rather distant locations within the TP7B sequence (Pilankatta et al. 2009), including the N terminal extension, the N domain and the C terminal. This suggests a global conformational response. In fact, a critical role of the C-terminus has been recently demonstrated (Braiterman et al. 2011). It is also important to recognize that a copper and phosphorylation dependent conformation is required to avoid degradation by proteasome mediated quality control, and to allow high levels of expressed ATP7B protein (Pilankatta et al. 2011).
Fig. 11.
Heterologous expression, distribution and trafficking of WT ATP7B in COS-1 cells. COS-1 cells were exposed overnight to 200 μM CuCl2 1 day after infection with adenovirus vector for delivery of WT ATP7B cDNA. The cells were stained with antibodies specific for the the Golgi marker protein Giantin (left upper panel), and the ATP7B c-myc tag (left middle panel) 24 h following infection. Secondary antibodies yield green color for the ATP7B c-myc tag, and blue color for Golgi. Red color indicates nuclei stained with propidium iodide. The lower left panel shows merging of the stains, demonstrating colocalization of ATP7B with the Golgi marker, in a distinct and polarized location relative to the nuclei. The right panel shows an enlarged view of cytosolic vesicles containing ATP7B (green), budding from the Golgi region (blue) and trafficking throughout the cytoplasm. Bars: 15 μm. Derived from Pilankatta et al. (2011)
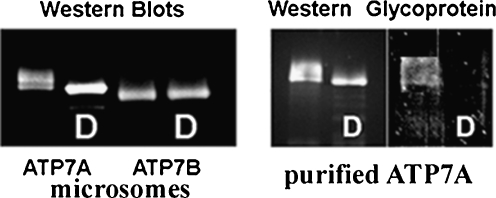
The differential effects of TMBS and NMBD copper occupancy are of special interest. Mutations within the transmembrane copper binding site (TMBS) produces specific inhibition of catalytic phosphorylation (Asp1027) in analogy to the effect of the calcium TMBS mutations in the Ca2+ ATPase. In fact, it was recently proposed that soluble copper chaperones transfer copper directly to transmembrane transport sites (González-Guerrero and Argüello 2008). Furthermore, mutation of the TMBS copper site does not interfere with kinase assisted phosphorylation of serines. On the other hand, mutation of the sixth NMBD copper site produces inhibition of both catalytic (aspartate) and kinase assisted (serines) phosphorylation (Fig. 9). It should be pointed out that a functional, global conformation of ATP7B requires the entire NMBD segment, since deletion of the segment including the first five copper NMBD sites results in extensive degradation of the expressed protein. Levels approximating those obtained with wild type constructs can be only obtained by specific inhibition of proteasome mediated degradation but, in this case, inactive enzyme is produced (Pilankatta et al. 2011). It is possible that, in various tissues, different requirements may be involved in conformational mechanisms to allow interactions with different partner proteins, thereby accounting for specific trafficking patterns and functions of copper ATPases. It was reported in this regard that in polarized hepatic cells, copper response and apical targeting of ATP7B is only dependent on a short segment of the NH2-extension rather than the entire NMBD (Guo et al. 2005). It was proposed that the copper-binding sites at the N-terminal domain of ATP7A are sensors of low concentrations of copper but are not essential for the overall catalytic activity (Voskoboinik et al. 2001c). Finally, specific sequence features of copper ATPases may determine diverse behaviors. For instance, the ATP7A isoform udergoes post-translational glycosylation, while the ATP7B does not ((Liu et al. 2010) and Fig. 12). This feature confers plasma membrane stabilization to the ATP7A isoform (Fig. 13).
Fig. 12.
Electrophoretic analysis of ATP7A or ATP7B protein derived from COS-1 cells sustaining heterologous expression of ATP7A or ATP7B and purifiedATP7A protein, and demonstration of ATP7A glycosylation. COS-1 cells were infected with viral vectors delivering cDND encoding WT ATP7A or ATP7B with a myc tag. Microsomal or purified protein stain was stained with Coomassie Blue R-250; glyocoprotein stain was obtained with Prop Q Emerald 300, and Western blots were obtained with 9E10 monoclonal antibodies against the c-myc tag in the expressed protein. D denotes samples that were subjected to deglycosylation before electrophoresis. Derived from Liu et al. (2010)
Fig. 13.
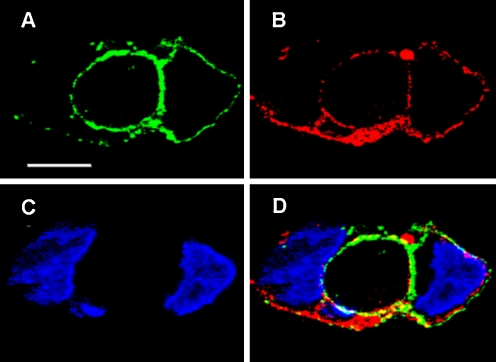
Heterologous expression and association of ATP7A protein with the plasma membrane. COS-1 cells were infected with viral vectors delivering cDND encoding WT ATP7A with a c-myc tag. 200 μM CuCl2 was added following the infection. After 24 h, the cells were fixed with paraformaldehyde and permeabilized with Triton X-100. Immunostaining of ATP7A with anti-myc tag antibody is shown in green (a), and plasma membrane immunostaining with the anti-PanCadherin antibody is shown in red (b). Nuclear staining with DAPI is shown in blue (c). The overlay in panel C demonstrates colocalization of ATP7A and anti-Pan Cadherin antibodies in the plasma membrane. The bar is 10 μm. Derived from Liu et al. (2010)
Conclusions
Calcium and copper transport ATPases retain an analogous catalytic mechanism for utilization of ATP, but present a wide range of differences in the mechanisms of transduction and signaling. The Ca2+ ATPase is well characterized from the structural, biochemical, thermodynamic and kinetic points of view. It sustains active transport of free ion across biological membranes, providing Ca2+ concentration gradients for subsequent release of signaling Ca2+. Its conformational states are dictated by occupancy of calcium, nucleotide and phosphorylation sites specifically involved in catalysis and active transport. On the other hand, functionally competent conformation of the copper ATPase requires proper folding of a rather long NH2-terminal extension which is not present in the calcium ATPase. Copper occupancy of the NH2-terminal extension (NMBD) allows kinase assisted phosphorylation of serine residues and cytosolic trafficking. Occupancy of the copper TMBS and of the ATP site on the N domain then triggers the catalytic cycle. Copper is mostly acquired from, and delivered to partner proteins.
From a conceptual point of view, transport ATPases have been viewed as chemiosmotic pumps where ATP utilization operates, in reverse direction, the mechanism originally conceived for ATP synthesis in mitochondria (Mitchell 1961). We show here that the Ca2+ ATPase does in fact yield a chemiosmotic gradient by active transport of free Ca2+. On the contrary, the copperATPase delivers copper by active transfer from donors to acceptor proteins. Due to difficulties in measuring copper binding affinity constants, we cannot predict the extent of copper ion gradients formed by the pump. In fact, due to high binding affinity, copper ion concentrations are expected to remain low. However, we can conclude that in both calcium and copper ATPases the ATP chemical energy is utilized to produce specific protein conformational changes that affect binding affinity and orientation of specific ligands. Therefore, the common mechanistic feature of transport ATPases is protein conformational work, yielding in some cases an ionic gradient, and in other cases vectorial displacement of bound ligands as in the copper ATPase and, even more evidently, in drug transport ATPases (Ernst et al. 2010).
Acknowledgment
The author’s research is supported by National Institutes of Health Grant NHBLI RO301-69830.
Competing interests
The author declares that he has no competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- NMBD
N-methyl-terminus domain
- SERCA
Sarco-endoplasmic reticulum Ca2+ ATPase
- TMBS
Transmembrane binding site
References
- Andersen JP, Vilsen B. Functional consequences of alterations to Glu309, Glu771, and Asp800 in the Ca2+ ATPase of sarcoplasmic reticulum. J Biol Chem. 1992;267:19383–19387. [PubMed] [Google Scholar]
- Argüello JM. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. J Membr Biol. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- Banci L, Bertini I, Cantini F, Massagni C, Migliardi M. An NMR study of the interaction of the N-terminal cytoplasmic tail of the Wilson disease protein with copper(I)-HAH1. J Biol Chem. 2009;284:9354–9360. doi: 10.1074/jbc.M805981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci L, Bertini I, Cantini F, Migliardi M, Natile G, Nushi F, Rosato A. Solution structures of the actuator domain of ATP7A and ATP7B, the Menkes and Wilson disease proteins. Biochemistry. 2009;48:7849–7855. doi: 10.1021/bi901003k. [DOI] [PubMed] [Google Scholar]
- Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S. The copper-transporting ATPases, Menkes and Wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem. 2005;280:9640–9645. doi: 10.1074/jbc.M413840200. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Braiterman L, Nyasae L, Leves F, Hubbard AL (2011) Critical roles for the C-Terminus of the Cu-ATPase, ATP7B, in protein stability, trans-Golgi network retention, copper sensing and retrograde trafficking. Am J Physiol Gastrointest Liver Physiol, Mar 31. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- Burroughs AM, Allen KN, Dunaway-Mariano D, Aravind L. Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J Mol Biol. 2006;361:1003–1034. doi: 10.1016/j.jmb.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Cavagna M, O’Donnell JM, Sumbilla C, Inesi G, Klein MG. Exogenous Ca2+-ATPase isoform effects on Ca2+ transients of embryonic chicken and neonatal rat cardiac myocytes. J Physiol. 2000;528:53–63. doi: 10.1111/j.1469-7793.2000.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi M, Inesi G. Mg2+ and Mn2+ modulation of Ca2+ transport and ATPase activity in sarcoplasmic reticulum vesicles. Arch Biochem Biophys. 1981;208:586–592. doi: 10.1016/0003-9861(81)90547-6. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Clarke DM, Loo TW, Inesi G, MacLennan DH. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+ ATPase. Nature. 1989;339:476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- de Meis L, Vianna AL. Energy interconversion by the Ca2+ dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Ambrosini L, Spencer J, Glover TW, Mercer JF. Molecular structure of the Menkes disease gene (ATP7A) Genomics. 1995;28:462–469. doi: 10.1006/geno.1995.1175. [DOI] [PubMed] [Google Scholar]
- Dmitriev O, Tsivkovskii R, Abildgaard F, Morgan CT, Markley JL, Lutsenko S. Solution structure of the N-domain of Wilson disease protein: distinct nucleotide-binding environment and effects of disease mutations. Proc Natl Acad Sci USA. 2006;103:5302–5307. doi: 10.1073/pnas.0507416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst R, Kueppers P, Stindt J, Kuchler K, Schmitt L. Multidrug efflux pumps: substrate selection in ATP-binding cassette multidrug efflux pumps–first come, first served? FEBS J. 2010;277:540–549. doi: 10.1111/j.1742-4658.2009.07485.x. [DOI] [PubMed] [Google Scholar]
- Gitlin JD. Wilson disease. Gastroenterolog. 2003;125:1868–7187. doi: 10.1053/j.gastro.2003.05.010. [DOI] [PubMed] [Google Scholar]
- González-Guerrero M, Argüello JM. Mechanism of Cu+−transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc Natl Acad Sci USA. 2008;105:5992–5997. doi: 10.1073/pnas.0711446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nyasae L, Braiterman LT, Hubbard AL. NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol. 2005;289:G904–G916. doi: 10.1152/ajpcell.00010.2005. [DOI] [PubMed] [Google Scholar]
- Harada M. Wilson disease. Med Electron Microsc. 2002;35:61–66. doi: 10.1007/s007950200007. [DOI] [PubMed] [Google Scholar]
- Hatori Y, Lewis D, Toyoshima C, Inesi G. Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants. Biochemistry. 2009;48:4871–4880. doi: 10.1021/bi900338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C, Xu, Narayanan N. Sarcoplasmic reticulum calcium pump in cardiac and slow twitch skeletal muscle but not fast twitch skeletal muscle undergoes phosphorylation by endogenous and exogenous Ca2+/calmodulin-dependent protein kinase. Characterization of optimal conditions for calcium pump phosphorylation. J Biol Chem. 1994;269:31198–31206. [PubMed] [Google Scholar]
- Hung YH, Layton MJ, Voskoboinik I, Mercer JF, Camakaris J. Purification and membrane reconstitution of catalytically active Menkes copper-transporting P-type ATPase (MNK; ATP7A) Biochem J. 2007;401:569–579. doi: 10.1042/BJ20060924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G. Mechanism of calcium transport. Annu Rev Physiol. 1985;47:573–601. doi: 10.1146/annurev.ph.47.030185.003041. [DOI] [PubMed] [Google Scholar]
- Inesi G, Nakamoto RK. Special issue on transport ATPases. Arch Biochem Biophys. 2008;476:1–2. doi: 10.1016/j.abb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, Kurzmack M, Coan C, Lewis DE. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980;255:3025–3031. [PubMed] [Google Scholar]
- Inesi G, Sumbilla C, Kirtley ME. Relationships of molecular structure and function in Ca2+ transport ATPase. Physiol Rev. 1990;70:749–760. doi: 10.1152/physrev.1990.70.3.749. [DOI] [PubMed] [Google Scholar]
- La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Parton RG, Camakaris J, Mercer JF. Functional analysis and intracellular localization of the human menkes protein (MNK) stably expressed from a cDNA construct in Chinese hamster ovary cells (CHO-K1) Hum Mol Genet. 1998;7:1293–1300. doi: 10.1093/hmg/7.8.1293. [DOI] [PubMed] [Google Scholar]
- Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/S0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pilankatta R, Hatori Y, Lewis D, Inesi G. Comparative features of copper ATPases ATP7A and ATP7B heterologously expressed in COS-1 cells. Biochemistry. 2010;49:10006–10012. doi: 10.1021/bi101423j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Brandl CJ, Korczak B, Green NM. Amino-acid sequence of a Ca2+ and Mg2+ dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985;316:696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Masuda H, de Meis L. Phosphorylation of the sarcoplasmic reticulum membrane by orthophosphate. Inhibition by calcium ions. Biochemistry. 1973;12:4581–4585. doi: 10.1021/bi00747a006. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Barnes N, Stevenson J, Strausak D, Llanos RM. Copper-induced trafficking of the cU-ATPases: a key mechanism for copper homeostasis. Biometals. 2003;16:175–184. doi: 10.1023/A:1020719016675. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Møller JV, Olesen C, Winther AM, Nissen P. The sarcoplasmic Ca2+−ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys. 2010;43:501–566. doi: 10.1017/S003358351000017X. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, Strausak D, Camakaris J, Mercer JF. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem. 2002;277:46736–46742. doi: 10.1074/jbc.M208864200. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet. 1994;3:1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- Pilankatta R, Lewis D, Adams CM, Inesi G. High yield heterologous expression of wild-type and mutant Cu+−ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper-dependent phosphorylation. J Biol Chem. 2009;284:21307–21316. doi: 10.1074/jbc.M109.023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilankatta R, Lewis D, Inesi G. Involvement of protein kinase D in expression and trafficking of ATP7B (Copper ATPase) J Biol Chem. 2011;286:7389–7396. doi: 10.1074/jbc.M110.171454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AM, Inesi G. Effects of thapsigargin and phenylephrine on calcineurin and protein kinase C signaling functions in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C992–C1002. doi: 10.1152/ajpcell.00594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AM, Inesi G. Downregulation of Ca2+ signalling proteins in cardiac hypertrophy. Minerva Cardioangiol. 2010;58:193–204. [PubMed] [Google Scholar]
- Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- Sazinsky MH, Mandal AK, Argüello JM, Rosenzweig AC. Structure of the ATP binding domain from the Archaeoglobus fulgidus Cu+ ATP ase. J Biol Chem. 2006;281:11161–11166. doi: 10.1074/jbc.M510708200. [DOI] [PubMed] [Google Scholar]
- Sazinsky MH, Agarwal S, Argüello JM, Rosenzweig AC. Structure of the actuator domain from the Archaeoglobus fulgidus Cu+ ATPase. Biochemistry. 2006;45:9949–9955. doi: 10.1021/bi0610045. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- Strock C, Cavagna M, Peiffer WE, Sumbilla C, Lewis D, Inesi G. Direct demonstration of Ca2+ binding defects in sarco-endoplasmic reticulum Ca2+ ATPase mutants overexpressed in COS-1 cells transfected with adenovirus vectors. J Biol Chem. 1998;273:15104–15109. doi: 10.1074/jbc.273.24.15104. [DOI] [PubMed] [Google Scholar]
- Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Guidelli R, Inesi G. Pre-steady state electrogenic events of Ca2+/H+ exchange and transport by the Ca2+ ATPase. J Biol Chem. 2006;281:37720–37727. doi: 10.1074/jbc.M606040200. [DOI] [PubMed] [Google Scholar]
- Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Pilankatta R, Lewis D, Inesi G. ATP dependent charge movement in ATP7B Cu+−ATPase is demonstrated by pre-steady state electrical measurements. FEBS Lett. 2010;584:4619–4622. doi: 10.1016/j.febslet.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton VK, Rao R. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am J Physiol Cell Physiol. 2004;287:C580–C589. doi: 10.1152/ajpcell.00135.2004. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Inesi G. Structural basis of ion pumping by Ca2+−ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. doi: 10.1146/annurev.biochem.73.011303.073700. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Nakasako M, Nomura H, Ogawa H. Structure determination of the calcium pump of sarcoplasmic reticulum. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- Tsivkovskii R, Eisses JF, Kaplan JH, Lutsenko S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson’s disease protein) expressed in insect cells. J Biol Chem. 2002;277:976–983. doi: 10.1074/jbc.M109368200. [DOI] [PubMed] [Google Scholar]
- Tsivkovskii R, Purnat T, Lutsenko S. Copper transporting ATPases: key regulators of intracellular copper concentration. In: Futai, Kaplan, Wada, editors. Handbook of ATPases. Weinheim: Weiley-VCH Verlag; 2004. pp. 99–158. [Google Scholar]
- Tsuda T, Toyoshima C. Nucleotide recognition by CopA, a Cu+−transporting P-type ATPase. EMBO J. 2009;28:1782–1791. doi: 10.1038/emboj.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer Z, Vural B, Tønnesen T, Chelly J, Monaco AP, Horn N. Characterization of the exon structure of the Menkes disease gene using vectorette PCR. Genomics. 1995;26:437–442. doi: 10.1016/0888-7543(95)80160-N. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Veldhuis NA, Valova VA, Gaeth AP, Palstra N, Hannan KM, Michell BJ, Kelly LE, Jennings I, Kemp BE, Pearson RB, Robinson PJ, Camakaris J. Phosphorylation regulates copper-responsive trafficking of the Menkes copper transporting P-type ATPase. Int J Biochem Cell Biol. 2009;41:2403–2412. doi: 10.1016/j.biocel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Brooks H, Smith S, Shen P, Camakaris J. ATP-dependent copper transport by the Menkes protein in membrane vesicles isolated from cultured Chinese hamster ovary cells. FEBS Lett. 1998;435:178–182. doi: 10.1016/S0014-5793(98)01059-X. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Greenough M, La Fontaine S, Mercer JF, Camakaris J. Functional studies on the Wilson copper P-type ATPase and toxic milk mouse mutant. Biochem Biophys Res Commun. 2001;281:966–970. doi: 10.1006/bbrc.2001.4445. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Mar J, Strausak D, Camakaris J. The regulation of catalytic activity of the menkes copper-translocating P-type ATPase. Role of high affinity copper-binding sites. J Biol Chem. 2001;276:28620–28627. doi: 10.1074/jbc.M103532200. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Jasmine Mar J, Daniel Strausak D, Camakaris J. The regulation of catalytic activity of the Menkes copper-translocating P-type ATPase the regulation of catalytic activity of the Menkes copper-translocating P-type ATPase: Role of high affinity copper-binding sites. J Biol Chem. 2001;276:28620–28627. doi: 10.1074/jbc.M103532200. [DOI] [PubMed] [Google Scholar]
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Xu C, Ma H, Inesi G, Al-Shawi MK, Toyoshima C. Specific structural requirements for the inhibitory effect of thapsigargin on the Ca2+ ATPase (SERCA) J Biol Chem. 2004;279:17973–17979. doi: 10.1074/jbc.M313263200. [DOI] [PubMed] [Google Scholar]