Abstract
The ability of TGFβ1 to act as a potent pro-fibrotic mediator is well established, potently inducing the expression of fibrogenic genes including type I collagen (COL1A2) and CCN2. Previously we have shown elevated expression of the TGFβ accessory receptor, endoglin on Systemic Sclerosis (SSc) dermal fibroblasts. Here we sought to assess the cell surface expression of the TGFβ receptor complex on SSc dermal fibroblasts (SDF), and investigate their role in maintaining the elevated expression of CCN2. SDF exhibited elevated expression of the TGFβ accessory receptors betaglycan/TGFβRIII and endoglin, but not type I or type II receptors. To determine the effect of altered receptor repertoire on TGFβ responses, we investigated the effect of exogenous TGFβ on expression of two pro-fibrotic genes. SDF exhibited higher basal expression of COL1A2 and CCN2 compared to healthy controls. TGFβ induced a marked increase in the expression of these genes in normal dermal fibroblasts, whereas SDF exhibited only a modest increase. We next sought to determine if higher basal expression in SDF was a result of autocrine expression of TGFβ. Surprisingly basal expression was not affected by a pan-neutralizing TGFβ antibody. To explore if altered accessory receptor expression alone could account for these changes, we determined their effects on CCN2 promoter activity. Endoglin inhibited CCN2 promoter activity in response to TGFβ. TGFβRIII alone or in combination with endoglin was sufficient to enhance basal CCN2 promoter activity. Thus TGFβ accessory receptors may play a significant role in the altered expression of fibrogenic genes in SDF.
Keywords: CCN2, TGFβRIII, Endoglin, TGFβ
Introduction
Systemic sclerosis (SSc) is a complex fibrotic disease of unknown etiology characterised by the elevated deposition of extracellular matrix (ECM) proteins, such as collagen type I. The excessive expression of connective tissue growth factor (CTGF/CCN2), a member of the CCN family of genes is now widely considered a hallmark of fibrotic pathologies, including SSc. Early studies demonstrated explant cultured fibroblasts from SSc patients maintained their apparent profibrotic phenotype (Leroy 1972), however the precise mechanisms which contribute to this remains unclear.
Transforming Growth Factor beta (TGFβ) has been proposed as a central mediator in fibrotic pathologies, inducing many of the hall mark genes associated with fibrosis (Leask and Abraham 2004). Indeed TGFβ is perhaps the most potent inducer of CCN2 thus far identified (Holmes et al. 2001). TGFβ isoforms 1–3 exert their biological effects through a heteromeric complex containing the transmembrane Ser/Thr kinases TGFβRI (Activin receptor-like kinase/ALK) and TGFβRII receptors (Ten and Arthur 2007). The resultant receptor complex activates members of the SMAD transcription factor family, in addition to members of the Mitogen Activated Protein kinase signalling pathway, including JNK and ERK (Leask and Abraham 2004; Ten and Arthur 2007). In addition to TGFβ type I and II receptors, auxiliary membrane-associated proteins, endoglin (CD105) and TGFβRIII (betaglycan), have been shown to regulate ligand receptor interaction and subsequently effect downstream signalling (Perez-Gomez et al. 2010; Bilandzic and Stenvers 2011).
Recent studies suggest alterations in normal repertoire of TGFβ receptor expression may promote the development of a pro-fibrotic environment (Denton et al. 2003; Liu et al. 2002; Hermida et al. 2009). Previously we and others have demonstrated endoglin to be elevated on SSc dermal fibroblasts, and expression to significantly alter cellular responses to TGFβ (Morris et al. 2011; Leask et al. 2002). In addition recent studies have demonstrated TGFβRIII may play a role in the development of organ fibrosis (Liu et al. 2002; Hermida et al. 2009). Here we sought to profile the expression of the TGFβ receptor cell surface expression on SSc dermal fibroblasts, and explore the impact on collagen type I and CCN2 expression.
Materials and methods
Cell culture Primary dermal fibroblasts were grown from biopsies from SSc and healthy controls by explant culture as described previously (Leask et al. 2002) and were used between passages 2 and 5. Dermal and NIH-3T3 fibroblasts were maintained in DMEM with 10% fetal bovine serum (Invitrogen).
Flow cytometry Flow cytometry was performed as previously described (Leask et al. 2002). Betaglycan, ALK1 and ALK5 cell surface expression were assessed by flow cytometry. The reagents used were FITC-labelled anti-TGFβRIII, anti-ALK1 or anti-ALK5 (R&D Systems) or with isotype-matched control IgGs. Labelling was detected with FITC-conjugated secondary antibodies (Invitrogen) and labelled cells were analyzed by flow cytometry using FACSCalibur (Becton Dickinson, Mountain View, CA). Isotype matched irrelevant primary monoclonal antibody was used as a control for nonspecific binding, and the average fluorescence intensities were determined by subtracting background values from experimental values.
Western blot analysis Cells were cultured until 80% confluence in DMEM 10% FBS. Cells were cultured in DMEM, 1% FBS for an additional 24 h in the presence or absence of 1 ng/ml TGFβ1 (R&D systems). Experiments involving the pan-neutralizing TGFβ antibody, 1D11 (R&D Systems) were performed in the presence of 10 μg/ml 1D11 or IgG control (R&D systems) Cell layers were harvested using RIPA buffer and proteins were quantified (Bradford, Bio-Rad). Equal amounts of protein (25 μg) were subjected to SDS/PAGE using 4%–12% polyacrylamide gels (Invitrogen). Gels were blotted onto nitrocellulose, and proteins were detected using anti-CCN2, anti-type I collagen (Biodesign), anti-Actin (Sigma Aldrich), antibodies followed by an appropriate HRP-conjugated secondary antibody (Jackson Immunoresearch). Proteins were detected using an enhanced chemiluminescence kit (Amersham). Pan-TGFβ neutralizing and IgG control antibodies were used at concentrations specified.
Quantitative PCR Fibroblasts were serum-starved for 18 h and treated with 1 ng/ml TGFβ1 (R&D systems). Total RNA and cDNA was prepared as previously described, and expression of target genes determined (Xu et al. 2004) Duplicate samples were run, transcripts were measured in picograms, and expression values were standardized to values obtained with control 28 S RNA primers. Primers (Invitrogen) were as follows: COL1A2, 5′-ATAGGTTTCCCAGGGCAGC3-′ (F) and 5′-CCACGCTCTCCTTCAATCC-3′ (R); CCN2, 5′-CTCGCGGCTTACCGACTG-3′ (F) and 5′-GCACTTGAACTCCACCGG-3′ (R); and 28 S, 5′-TTGAAAATCCGGGGGAGAG-3′ (F) and 5′-ACATTGTTCCAACATGCCAG-3′ (R).
Transfection and reporter gene assays The 5 kb upstream promoter region of CCN2 was cloned into pGL3 (Promega). Promoter/reporter constructs were transfected into fibroblasts using FuGENE6 transfection reagent (Roche Applied Science) according to the manufacturer’s instructions. Transfection efficiency was normalized by co-transfected with pTK-rluc (Promega). Following transfection, cells were incubated in DMEM with 2% fetal bovine serum for 18 h. Media were changed, and cells were incubated for an additional 24 h in the presence or absence of 1 ng/ml TGFβ1 (R&D systems). Reporter gene activity was measured by luminometry (Turner Designs). Values given are mean ± S.E.M of duplicate assays from three individual experiments.
Statistical analysis
The data show the mean ± standard deviation of at least three independent experiments (n = 3), unless otherwise indicated. Statistical significance was determined using Student’s t test. Statistical analysis was performed using GraphPad Prism software.
Results
Previously we had shown SSc dermal fibroblasts (SDF) exhibit an elevated expression in the accessory receptor, endoglin (CD105). We sought to profile the expression of the associated TGFβ signalling receptors ALK1, ALK5 and TGFβRIII (Betaglycan) by fluorescence-activated cell sorting analysis on these same cells. Three healthy subjects and three SSc patients were used for these analyses. SDF exhibited significantly higher cell surface expression of the accessory receptor TGFβRIII (Table 1). By contrast cell surface levels of TGFβ type I (ALK1 and ALK5) or type II (TGFβRII) receptors were not significantly elevated compared control dermal fibroblasts (NDF).
Table 1.
Flow cytometry analysis of cell surface expression of receptor members of the TGFβ family
| Control (AFI) | SSc (AFI) | |
|---|---|---|
| ALK1 | 10.3 (0.5) | 9.9 (0.2) |
| ALK5 | 10.3 (1.4) | 12.5 (0.9) |
| TGFβRII | 7.3 (0.8) | 7.5 (1.6) |
| TGFβRIII | 79.3 (2.5) | 115 (2.8)a |
| Endoglin | 41 (6) | 58 (1.4)a |
Dermal fibroblasts from three healthy control subjects and three patients with diffuse cutaneous SSc were assessed for expression of ALK1, ALK5, TGFβRII, TGFβRIII and endoglin by FACSCalibur, and the average fluorescence intensities (AFIs) determined by subtracting the background values from the experimental values. Values given are expressed as the mean AFI ± SEMa, statistically significant difference in expression values (p < 0.05) relative to controls
TGFβ accessory receptors have previously been implicated in modulating cellular responses of TGFβs. To evaluate the impact of enhanced cell surface expression of TGFβRIII and endoglin we initially assessed the effects of exogenous TGFβ on the expression of two fibrogenic genes, collagen type I and CCN2 in SDF. Consistent with previous studies, basal gene transcript and protein levels of collagen type I and CCN2 were significantly elevated in SDF compared to healthy controls (Fig. 1). Addition of exogenous stimulation with TGFβ1 induced a significant fold increase in gene transcription and protein expression of collagen type I and CCN2 in NDF, SDF exhibited a blunted response to TGFβ1 with only a modest increase in these genes (Fig. 1).
Fig. 1.
SSc dermal fibroblasts have reduced responsiveness to exogenous TGFβ1. Control (n = 3) and SSc (n = 3) dermal fibroblasts were assessed for the expression of COL1A2 and CCN2 in the presence or absence of TGF-β1 (1 ng/ml) after 4 h by Q-PCR (Upper panel). Protein expression of collagen type I and CCN2 was confirmed in the cell monolayers of these cells after 24 h in the presence or absence of TGFβ1 (1 ng/ml) by Western blot (Lower Panel). Protein levels were controlled for by levels of actin expression
We postulated that the apparent blunted response to exogenous responses to TGFβ1, and enhanced basal expression exhibited by SDF, as determined by collagen type I and CCN2 expression, may be due to autocrine expression of TGFβ1 by SDF. To assess this the effects of the pan-neutralizing TGFβ antibody, 1D11 (Fig. 2) on SDF collagen type I and CCN2 expression was determined by Western blot (Fig. 2). Maximal induction of CCN2 and collagen type I in NDF was previously shown to peak at 2 ng/ml, and remains maximal at 10 ng/ml (data not shown). Incubation of NDF with 10 μg/ml of 1D11 inhibited induction of collagen type I and CCN2 by exogenous TGFβ (10 ng/ml). Incubation of SDF with 1D11 failed to inhibit the enhanced expression of either collagen type I or CCN2. No significant effect of an isotype matched IgG control was noted.
Fig. 2.
Effect of neutralizing TGFβ antibody on collagen type I and CCN2 expression. The ability of a pan-neutralizing TGFβ antibody to inhibit TGF-β1 induced expression of collagen type I and CCN2 was assessed by Western blot. Expression of collagen type I and CCN2 in the presence or absence of TGFβ1 (10 ng/ml) and/or a 10 μg/ml of pan-neutralizing TGFβ antibody (1D11) or IgG control, was assessed in control dermal fibroblasts (N) by Western blot (Top panel; representative experiment of n = 2). The effects of 1D11 (10 μg/ml) or IgG control antibodies on basal expression of collagen type I and CCN2 in 2 control (N) and 4 SSc (S) dermal fibroblasts was assessed after 24 h by Western blot (Lower Panel). Protein levels were controlled for by levels of actin expression
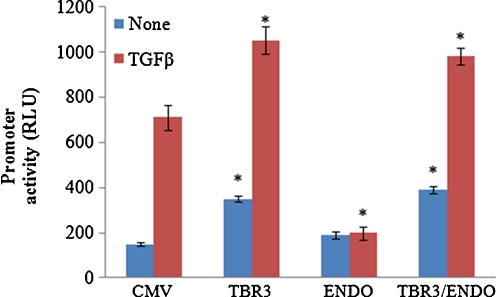
To investigate the impact of enhanced expression of the TGFβ accessory receptors, we tested the ability of an expression vector encoding either TGFβ RIII or endoglin to modulate the TGFβ induction of a target promoter, CCN2 in transfected mouse NIH3T3 (Fig. 3). We found that relative to co-transfection of empty expression vector, co-transfection of an TGFβRIII CCN2 promoter/luciferase reporter construct enhanced both basal and TGFβ induced activity. Consistent with previous studies over expression of endoglin suppressed CCN2 promoter/luciferase reporter construct induction by TGFβ. Whereas over-expression of TGFβIII and endoglin led to significant increase in basal and TGFβ induced activity (Fig. 3).
Fig. 3.
TGFR3 enhances basal and TGF-β induced CCN2 promoter activity. Fibroblasts were transiently transfected with a luciferase reporter construct containing a 5 kb region of the CCN2 promoter is the presence of 0.5 ug/well of empty expression vector (CMV) or expression vectors encoding endoglin (ENDO) or TGFβRIII (TBR3) and the effect on CCN2 promoter reporter activity (RLU) in the presence or absence of TGFβ1 (1 ng/ml) was assessed after 24 h. Results shown are for three individual experiments with duplicate replicates per experiment. Values given are expressed as RLU mean ± SEM.*, statistically significant difference in expression values (p < 0.05) relative to controls
Discussion
Our results provide additional insight into the repertoire of TGF-β receptors expressed on the cell surface of SSc fibroblasts, and their putative functions. Here we demonstrate SDF exhibit an enhanced cell surface expression of the TGF-β1 accessory receptor TGFβRIII (betaglycan), but not type I (ALK1 and ALK5) or type II TGF-β receptors. Consistent with this we and others had previously reported increased expression of a second TGF-β accessory receptor, endoglin (Leask et al. 2002; Morris et al. 2011). Indeed recent studies from Trojanowska group have demonstrated an important pro-fibrotic role for endoglin in SDF, acting via endoglin/ALK1/Smad1 pathway to promote pro-fibrotic genes including ET-1 (Morris et al. 2011; Pannu et al. 2007). To determine cumulative effects of these changes in cell surface receptor levels, we assessed the response of SDF to exogenous TGF-β1. Consistent with previous studies by ours and other groups, SDF exhibited elevated basal expression of collagen type I and CCN2 compared health controls (Leask et al. 2002; Morris et al. 2011). Whilst stimulation with TGF-β1 led to a significant increase in collagen type I and CCN2 expression in control fibroblast, SDF responses was less pronounced. To ascertain if this blunted response to TGF-β1 was the result of autocrine expression of TGFβ by SDFs, we sought to determine the impact of the neutralizing antibody TGFβ1, 1D11 on CCN2 and collagen type I expression. Whilst 1D11 markedly attenuated the effects of exogenous TGFβ1 on control dermal fibroblasts, neutralization of TGF-β failed to impact on the heighted basal expression of collagen type I and CCN2 by SDF. Suggesting the elevated expression of the genes was ligand independent. Previously we had shown endoglin to repress ligand independent and dependent activation of matrix associated genes. We thus sought to determine the effect of TGFβRIII on CCN2 promoter/reporter activity. Consistent with previous observation endoglin markedly repressed TGFβ inducted CCN2 promoter activity (Leask et al. 2002). In contrast, over-expression of TGFβRIII alone or in conjunction with endoglin led to a significant increase in basal expression of the CCN2 promoter. Collectively this data suggests TGFβ accessory receptors may play a significant role in regulating CCN2 activity independently of ligand. Altered expression of members of the TGFβ superfamily of receptors has been implicated in a number of human pathologies, including pulmonary arterial hypertention (Burton et al. 2011). Determining if this apparent imbalance in the TGFβ receptor repertoire is a systemic feature of the disease and contributes other underlying pathologies by altering the normal cellular response to members of the TGFβ superfamily remain key questions for the future.
References
- Bilandzic M, Stenvers KL (2011) Betaglycan: a multifunctional accessory. Mol Cell Endocrinol [DOI] [PubMed]
- Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood. 2011;117:333–341. doi: 10.1182/blood-2010-05-285973. [DOI] [PubMed] [Google Scholar]
- Denton CP, Zheng B, Evans LA, Shi-Wen X, Ong VH, Fisher I, Lazaridis K, Abraham DJ, Black CM, Crombrugghe B. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFbeta) receptor leads to paradoxical activation of TGFbeta signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278:25109–25119. doi: 10.1074/jbc.M300636200. [DOI] [PubMed] [Google Scholar]
- Hermida N, Lopez B, Gonzalez A, Dotor J, Lasarte JJ, Sarobe P, Borras-Cuesta F, Diez J. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res. 2009;81:601–609. doi: 10.1093/cvr/cvn315. [DOI] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ, Finlay DR, Holmes A, Pennington D, Shi-Wen X, Chen Y, Venstrom K, Dou X, Ponticos M, Black C, Bernabeu C, Jackman JK, Findell PR, Connolly MK. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857–1865. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- Leroy EC. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972;135:1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Suga M, Maclean AA, St George JA, Souza DW, Keshavjee S. Soluble transforming growth factor-beta type III receptor gene transfection inhibits fibrous airway obliteration in a rat model of Bronchiolitis obliterans. Am J Respir Crit Care Med. 2002;165:419–423. doi: 10.1164/ajrccm.165.3.2102108. [DOI] [PubMed] [Google Scholar]
- Morris E, Chrobak I, Bujor A, Hant F, Mummery C, Ten DP, Trojanowska M (2011) Endoglin promotes TGF-beta/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol [DOI] [PMC free article] [PubMed]
- Pannu J, Nakerakanti S, Smith E, Ten DP, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez E, Del CG, Juan FS, Lopez-Novoa JM, Bernabeu C, Quintanilla M. The role of the TGF-beta coreceptor endoglin in cancer. ScientificWorldJournal. 2010;10:2367–2384. doi: 10.1100/tsw.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten DP, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]