Abstract
Several studies have shown physiological functions of interleukin (IL)-32, a novel cytokine. However, the role of IL-32 in cancer development has not been reported. In this study, we showed that IL-32γ inhibited tumor growth in IL-32γ-overexpressing transgenic mice inoculated with melanoma as well as colon tumor growth in xenograft nude mice inoculated with IL-32γ-transfected colon cancer cells (SW620). The inhibitory effect of IL-32γ on tumor growth was associated with the inhibition of constitutive activated nuclear transcription factor-κB (NF-κB) and of signal transducer and activator of transcription 3 (STAT3). The expression of antiapoptotic, cell proliferation and tumor-promoting genes (bcl-2, X-chromosome inhibitor of apoptosis protein (IAP), cellular IAP and cellular FADD-like IL-1β-converting enzyme-inhibitory protein, cyclin D), cyclin-dependent kinase 4, cycolooxygenase-2 and inducible nitric oxide synthase was decreased, whereas the expression of apoptotic target genes (caspase-3 and -9, bax) increased. In tumor, spleen and blood, the number of cytotoxic CD8+ T cells and CD57+ natural killer cells and the levels of IL-10 increased, but that of tumor necrosis factor-α (TNF-α), IL-1β and IL-6 decreased. We also found that forced overexpression of IL-32γ inhibited colon cancer cell (SW620 and HCT116) growth accompanied with the inhibition of activated NF-κB and STAT3 in vitro. In addition, when IL-32γ was knocked down by small interfering RNA (siRNA) or neutralized with an anti-IL-32γ antibody, IL-32γ-induced colon cancer cell growth inhibition, the IL-32γ-induced decrease of TNF-α, IL-1 and IL-6 production, and the increase of IL-10 production were abolished. However, siRNA of NF-κB and STAT3 augmented IL-32γ-induced colon cancer cell growth inhibition. These findings indicate significant pathophysiological roles of IL-32γ in cancer development.
Keywords: IL-32, cytokine, colon cancer, NF-κB, STAT3
Introduction
Interleukin-32 (IL-32) was cloned as a gene induced by IL-18 and was formerly known as natural killer (NK) cell transcript 4 (Dahl et al., 1992). IL-32 is produced by mitogen-activated lymphocytes, interferon-γ-activated epithelial cells and IL-12-activated NK cells (Kim et al., 2005). IL-32 has six splice variants, IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ɛ and IL-32ζ, but the functional differences between these isoforms remain unknown (Nold-Petry et al., 2009). IL-32 modulates the generation of tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-10 and two C–X–C chemokine family members involved in inflammatory and/or autoimmune diseases (Dinarello and Kim, 2006; Kang et al., 2009). Although there are several limitations in the studies describing IL-32 pathophysiological functions, significant roles of IL-32 have been reported in the development of several diseases, such as arthritis, psoriasis, ulcerative colitis and Crohn's disease, as well as chronic obstructive pulmonary disease (Dinarello and Kim, 2006; Calabrese et al., 2008). However, the cognate receptor for IL-32 has not yet been identified despite numerous investigations. The lack of an identified receptor as well as the presence of multiple IL-32 isoforms is very uncommon among cytokines (Kim et al., 2005; Nold-Petry et al., 2009). It is also unusual that soluble IL-32 is barely detectable in culture medium and that IL-32 is localized in both the cytosol and the nucleus (Dinarello and Kim, 2006; Kang et al., 2009). Thus, there may be non-canonical and/or cell-associated functions of IL-32 besides its canonical role as a soluble factor.
Cytokines are involved in inflammatory and/or immune disease-associated cancer development (Lin and Karin, 2007). Cytokines that are released in response to infection can affect tumor development in different ways. IL-8, which induces motility in non-hematopoietic tumor cells, directly modulates endothelial cell proliferation and migration, thus promoting angiogenesis (Fujimoto et al., 2002). Tumor histology revealed the collapse of large blood vessels and disorganized vessel formation in mice that were inoculated with IL-8-transfected cancer cells (Araki et al., 2007). IL-6 increases antiapoptotic activity and, consequently, tumorigenic potency in basal cell carcinoma (Jee et al., 2001). However, Hisada et al. (2004) have reported that IL-27 potently induces tumor-specific antitumor activity. IL-2-stimulated lymphocytes have proven to be effective in destroying tumors (Groscurth et al., 1990). IL-10 inhibits tumor growth and metastasis in an animal model (Huang et al., 1996; Kundu and Fulton, 1997), and it inhibits tumor metastasis through an NK cell-dependent tumor killing mechanism (Zheng et al., 1996). IL-10-deficient transgenic mice are more susceptible to inflammation-associated adenocarcinoma (Westbrook et al., 2009). Moreover, a negative correlation between the expression of IL-10 and human colon cancer development has also been shown (Cacev et al., 2008). A few recent, limited studies showed higher expression or higher protein level of IL-32 in the human lung (Sorrentino and Di Carlo, 2009), pancreas cancer patient tissue (Nishida et al., 2009) and stomach patient tissue (Seo et al., 2008) than in normal tissue or serum. In cancer cell line studies, IL-32 causes apoptotic cell death in a human leukemia cell (Marcondes et al., 2008) and HeLa cell lines (Goda et al., 2006). IL-32 is also involved in the cisplatin-induced apoptotic cell death of HeLa cells (Zhang et al., 2009), but in pancreatic cancer cells, IL-32 stimulates cancer cell growth (Nishida et al., 2009). However, the exact roles and mechanisms of IL-32 in cancer development in vivo have not been reported.
The signal transducer and activator of transcription (STAT) family proteins, especially STAT3 and nuclear transcription factor-κB (NF-κB), have been proposed to be important in inflammatory and/or immune disease-associated carcinogenesis (Lin and Karin, 2007). NF-κB and STAT3 are markedly activated in inflammatory and immune cells by cytokines and chemokines, including IL-6, IL-12, IL-17 and IL-23, which could stimulate tumor cell growth ( Lu et al., 2004; Lin and Karin, 2007; Yu et al., 2009). Activated NF-κB and STAT3 stimulate pro-survival, proliferative, antiapoptotic and pro-angiogenic genes in cancer development (Bromberg et al., 1999; Lee et al., 2009). Therefore, changes of cytokine levels in immune cells as well as in tumor cells could affect NF-κB and STAT3 activity, thus playing critical roles in tumorigenesis. In this study, we investigated the role and mechanisms of IL-32γ in melanoma and colon cancer development using IL-32γ-transfected colon cancer cells, xenograft nude mice inoculated with IL-32γ-transfected colon cancer cells and IL-32γ-overexpressing transgenic mice inoculated with melanoma, and we investigated the involvement of STAT3 and NF-κB pathways in the action of IL-32γ during cancer development.
Results
Generation of IL-32γ transgenic mice, and expression of IL-32γ in the mice
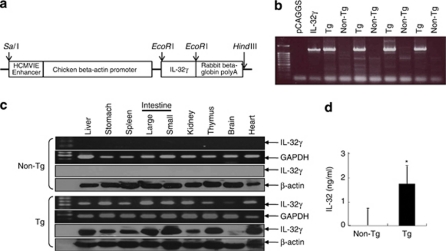
To investigate the role of IL-32γ in tumor growth in vivo, we generated transgenic mice overexpressing human IL-32γ (hIL-32γ). Before generating the IL-32γ transgenic mice, we confirmed that the IL-32γ cDNA was properly translated into the IL-32 protein using glutathione S-transferase-fused IL-32γ protein expression in Escherichia coli. The glutathione S-transferase-fused IL-32γ protein was detected by western blotting with an anti-IL-32-reactive monoclonal antibody, KU32-52, as described elsewhere (Kim et al., 2008). The IL-32γ cDNA was subcloned into the mammalian expression vector pCAGGS, and this construct was used for IL-32γ transgenic mice production (Figure 1a). The nature of the IL-32γ transgenic mice was confirmed by polymerase chain reaction (PCR) of mouse tail genomic DNA using allele-specific primers (Figure 1b). A 1.4-kb PCR product was amplified from the IL-32γ transgenic mice allele. Among the more than 20 resultant pups, three mice were found to be positive in the genotyping analysis. These three founder mice were each backcrossed into the C57BL6/J background for eight generations. The transgene was successfully transmitted to 50% of the pups from each littermate as evaluated by genotyping and western blotting. Reverse transcription (RT)–PCR and western blotting revealed that hIL-32γ was ubiquitously expressed in various tissues and highly expressed in the intestine, liver, thymus and heart, whereas there was no expression of hIL-32γ in the tissues of non-transgenic mice (Figure 1c). The male/female ratio was 50% for the IL-32γ transgenic and non-transgenic littermates. IL-32γ transgenic mice were viable, fertile and had no tissue or organ abnormalities. IL-32 level in the sera of IL-32γ transgenic mice (approximately 1.6 ng/ml) detected by enzyme-linked immunosorbent assay method was increased in the wild-type mice (Figure 1d).
Figure 1.
Generation of IL-32γ transgenic mice and detection of IL-32γ. (a) Scheme for IL-32γ transgenic generation. (b) PCR analysis was performed to analyze IL-32γ gene expression, as described in Materials and methods. (c) RT–PCR and western blotting analyses for IL-32γ in the spleen, thymus, liver, lung, kidney, colon and brain tissues of IL-32γ transgenic and non-transgenic mice. (d) Detection of IL-32 in the sera of transgenic mice or non-transgenic mice. The results are expressed as mean±s.d. of three mice. *P<0.05 compared with non-transgenic mice. A full colour version of this figure is available at the Oncogene journal online.
Inhibition of tumor growth in IL-32γ transgenic mice
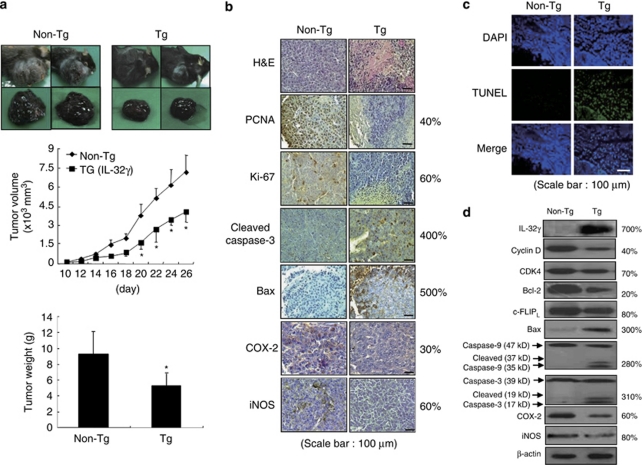
The antitumor effects of IL-32γ were investigated using transgenic mice (n=10) overexpressing IL-32γ. As IL-32γ was highly expressed in the intestine, and many cytokines play important roles in colon and melanoma cancer development, we were interested in studying the effect of IL-32γ on these cancers. Colon cancer cells could not be transplanted into the IL-32γ transgenic mice (C57BL6/J background) owing to the immune rejection response. B16 melanoma cells were inoculated subcutaneously into transgenic mice and non-transgenic mice (n=10). Tumor growth was monitored for 26 days as the inoculated melanoma cells grew rapidly. There was a significant difference in tumor growth between the transgenic mice and non-transgenic mice. The tumor volumes and weights of the transgenic mice were significantly smaller than in those of the non-transgenic mice determined on day 26 (volume: 4056.1±819.5 versus 7217.3±1243.3 mm3, P<0.05; tumor weight: 9.2±2.9 versus 4.8±0.9 g, P<0.05) (Figure 2a). The histological findings after hematoxylin and eosin staining indicated that the tumor tissues of the transgenic mice, but not those of the non-transgenic mice, contained large areas of necrosis. Immunohistochemically, high levels of cleaved caspase-3 and bax were found more frequently in the transgenic mice than in the non-transgenic mice. The expression levels of cell cycle regulatory proteins, cyclin D and cyclin-dependent kinase 4 (CDK4), proliferation makers, proliferating cell nuclear antigen and Ki-67, as well as inflammatory proteins, cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), were reduced in the transgenic mice (Figure 2b). TdT-mediated dUTP nick-end labeling (assay) (TUNEL) staining revealed a higher frequency of apoptotic cell death in the tumor tissues of the transgenic mice than in those of the non-transgenic mice (Figure 2c). Western blotting showed the expression of IL-32γ in the transgenic mice tissues, but not in the non-transgenic mice tissues. In the transgenic mice, the expression levels of cell cycle regulatory proteins, cyclin D and CDK 4, and antiapoptotic proteins, bcl-2 and cellular FADD-like IL-1β-converting enzyme-inhibitory protein (c-FLIPL), as well as inflammatory proteins, COX-2 and iNOS, were decreased, whereas the levels of the pro-apoptotic bax, and cleaved caspase-3 and -9 were increased (Figure 2d).
Figure 2.
Effect of IL-32γ on tumor growth in IL-32γ-overexpressing transgenic mice. (a) Tumor images, volumes and weights. The results are expressed as mean±s.d. *P<0.05 compared with the non-transgenic mice. (b) Tumor sections were analyzed by immunohistochemistry. (c) Apoptotic cells were examined by fluorescence microscopy after TUNEL staining. (d) Tumor extracts were analyzed by western blotting. Each image and band is representative of three independent experiments. The values on the right of panels b and d are average percentages of vector control over three independent experiments.
Inhibition of tumor growth in BALB/c athymic nude mice inoculated with IL-32γ-transfected colon cancer cells
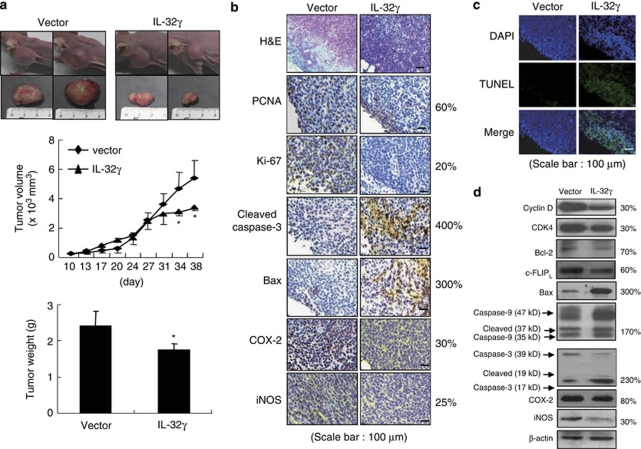
We investigated whether the inhibitory effects of IL-32γ on colon cancer cell growth could bring about tumor regression in vivo. Vector- or IL-32γ-transfected SW620 cells were inoculated subcutaneously into BALB/c athymic nude mice (n=10), and tumor growth and weight were monitored for 38 days. Tumor growth was gradually and time-dependently retarded in the mice inoculated with colon cancer cells expressing IL-32γ compared with the mice exposed to the empty vector. On day 38, when the final volumes were recorded, the tumor volume and tumor weight of the mice inoculated with the IL-32γ-expressing colon cancer cells were 48.7 and 75.3% of the corresponding values for the mice inoculated with the vector-expressing colon cancer cells, respectively (Figure 3a). Immunohistochemical analysis of tumor sections, stained with hematoxylin and eosin, and for proliferation antigens, proliferating cell nuclear antigen and Ki-67, revealed greater inhibitory effects of IL-32γ on tumor cell growth. We also confirmed that the expression levels of apoptotic cell death regulatory proteins, that is, cleaved caspase-3 and bax, were increased, but inflammatory proteins, iNOS and COX-2, were decreased in the mice tumor tissue that were inoculated with colon cancer cells expressing IL-32γ (Figure 3b). Apoptotic cell death was significantly induced in the mice tumor tissues that were inoculated with IL-32γ-expressing colon cancer cells (Figure 3c). Consistent with the immunohistochemical data, western blot analysis showed that IL-32γ increased the expression levels of cleaved caspase-3 and -9, and bax, whereas it decreased the expression levels of cyclin D, CDK4, bcl-2 and c-FLIPL, as well as iNOS and COX-2 (Figure 3d).
Figure 3.
Effect of IL-32γ on tumor growth in the SW620 xenograft in vivo model. (a) Tumor images, volumes and weights. The results are expressed as mean±s.d. *P<0.05 compared with the mice inoculated with vector-transfected colon cancer cells. (b) Tumor sections were analyzed by immunohistochemistry. (c) Apoptotic cells were examined by fluorescence microscopy after TUNEL staining. (d) Tumor extracts were analyzed by western blotting. Each image and band is representative of three independent experiments. The values on the right of panels b and d are average percentages of vector control over three independent experiments.
IL-32γ overexpression inhibits colon cancer cell growth through induction of apoptotic cell death in cultured cells
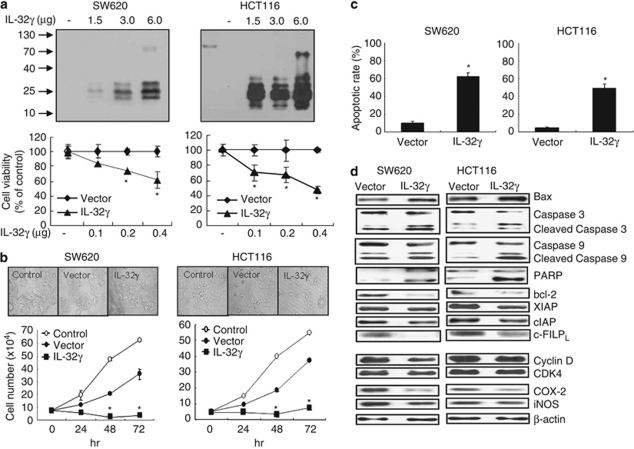
To further investigate whether the introduction of IL-32γ into cancer cells changes their growth in vitro, we carried out cell counting and analyses of morphological changes in colon cancer cells transfected with or without the IL-32γ gene. IL-32γ was marginally expressed in colon cancer cells without the introduction of IL-32γ determined with PCR analysis. The expression of IL-32γ was found to be increased depending on the amount of introduced IL-32γ cDNA, and correlated with the inhibition of colon cancer cell growth (Figure 4a). Morphological observations revealed that enforced expression of IL-32γ caused a gradual reduction in cell size and the rounding of individual colon cancer cells. The introduction of IL-32γ resulted in the inhibition of colon cancer cell growth in a time-dependent manner, as compared with the cells transfected with empty vector (Figure 4b). To confirm further the relationship between the introduction of IL-32γ and colon cancer cell growth inhibition, the anticancer drug paclitaxel was added to cancer cells, and the levels of growth inhibition were determined. In the presence of paclitaxel (5 n), the growth inhibition caused by IL-32γ was augmented (Supplementary Figure 1A). We also found that introduction of IL-32γ inhibited the prostate, liver and lung cancer cell growth (Supplementary Figure 1B). These results indicate that IL-32γ inhibits the growth of several cancer cells.
Figure 4.
Expression of IL-32γ, growth rates and apoptotic cell death in IL-32γ-transfected colon cancer cells. (a) Colon cancer cells (1 × 106) were transfected with various amounts of the IL-32γ plasmid (1.5–6.0 μg) for 24 h and harvested. IL-32γ expression was detected by western blotting using monoclonal antibody KU32-52. To determine the effects of different IL-32γ levels on colon cancer cell growth, the cells were inoculated into 24-well plates (5 × 104 cells per well) and transfected with the IL-32γ plasmid (0.1–0.4 μg per well) for 72 h. Cell growth was measured by direct counting after Trypan blue staining. (b) Colon cancer cells were inoculated into 24-well plates (5 × 104 cells per well) and transfected with the vector or IL-32γ plasmid (0.4 μg per well). At 24, 48 and 72 h post-transfection, the cells were harvested by trypsinization and counted after Trypan blue staining. Control: untransfected cells. (c) Colon cancer cells were transfected with the vector or the IL-32γ for 72 h. Apoptotic cells were examined under a fluorescence microscope after TUNEL staining. The total number of cells in a given area was determined by 4′,6-diamidino-2-phenylindole nuclear staining. The results are expressed as mean±s.d. of three experiments with each experiment performed in triplicate. *P<0.05 compared with the vector-transfected colon cancer cells. (d) Cells were transfected with the vector or the IL-32γ for 24 h. Cell extracts were analyzed by western blotting. Each band is representative of three independent experiments.
To determine whether apoptotic cell death contributed to the observed inhibitory effect of IL-32γ on colon cancer cell growth, we evaluated the changes in chromatin morphology of the IL-32γ-expressing colon cancer cells using TUNEL staining. The introduction of IL-32γ increased the number of apoptotic (4′,6-diamidino-2-phenylindole-stained TUNEL-positive) colon cancer cells. The frequencies of apoptotic cell death without and with the introduction of IL-32γ were 10 and 62% in the SW620 cells, and 4 and 49% in the HCT116 cells, respectively (Figure 4c). To elucidate the relationship between the induction of apoptotic cell death by IL-32γ and the expression of apoptotic cell death-related genes, the expression of apoptotic cell death regulatory proteins was investigated. In the IL-32γ-overexpressing colon cancer cells, the expression levels of pro-apoptotic proteins, that is, cleaved caspase-3, -9 and poly-(ADP-ribose) polymerase, were increased. However, the expression levels of the antiapoptotic proteins, bcl-2, inhibitor of apoptosis protein (IAP), X-chromosome IAP (XIAP), c-FLIP, and of cell proliferation markers, for example, cyclin D and CDK4, were decreased in the colon cancer cells by IL-32γ. Furthermore, IL-32γ reduced the expression of inflammatory marker proteins, COX-2 and iNOS (Figure 4d).
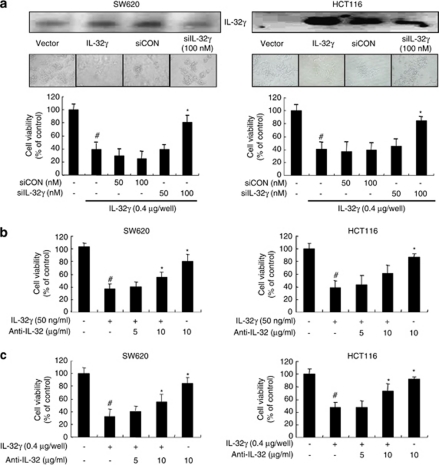
Silencing endogenous expression of IL-32γ abolishes IL-32γ-induced cell growth inhibition and apoptotic cell death
When the colon cancer cells were co-transfected with the IL-32γ gene and IL-32γ-specific small interfering RNA (siRNA), consistent with the reduced enforced expression of IL-32γ (Figure 5a), the IL-32γ transfection-induced inhibition of cell growth was significantly abolished in the colon cancer cells (Figure 5a). We also found that recombinant IL-32γ protein inhibited colon cancer cell growth (Figure 5b). However, IL-32 antibody abolished the inhibitory effect of IL-32γ on colon cancer cell growth in the IL-32γ-protein-treated (Figure 5b) or IL-32γ-transfected colon cancer cells (Figure 5c). Furthermore, the increase in apoptotic cell number induced by IL-32γ was diminished by transfection of the IL-32γ-specific siRNA (Supplementary Figure 1C) and by treatment of IL-32 antibody in SW620 colon cancer cells (Supplementary Figure 1D).
Figure 5.
Effect of silencing endogenous IL-32γ expression on colon cancer cell growth. (a) Colon cancer cells were inoculated into 24-well plates (5 × 104 cells per well) and co-transfected with the IL-32γ plasmid (0.4 μg per well) and the IL-32γ-specific siRNA (siIL-32; 50–100 n) or anti-IL-32 (5 or 10 μg/ml) for up to 72 h. The expression of IL-32γ was determined as described above. Thereafter, cell growth was measured by direct counting after Trypan blue staining. siCON: non-targeting control siRNA. (b) Colon cancer cells were treated with either recombinant IL-32γ alone or with its antibody and cultured for 72 h. (c) Colon cancer cells were inoculated into 24-well plates (5 × 104 cells per well) and transfected with the IL-32γ plasmid (0.4 μg per well) with/without anti-IL-32 (5 or 10 μg/ml) for up to 72 h. All results are expressed as mean±s.d. of three experiments with triplicate tests in each experiment. #P<0.05 compared with the colon cancer cells transfected with vector alone (a, c) or untreated control (b). *P<0.05 compared with the colon cancer cells transfected with IL-32γ alone (a, c) or with recombination IL-32γ protein (b). A full colour version of this figure is available at the Oncogene journal online.
IL-32γ decreases NF-κB activity in tumor tissues and in colon cancer cells
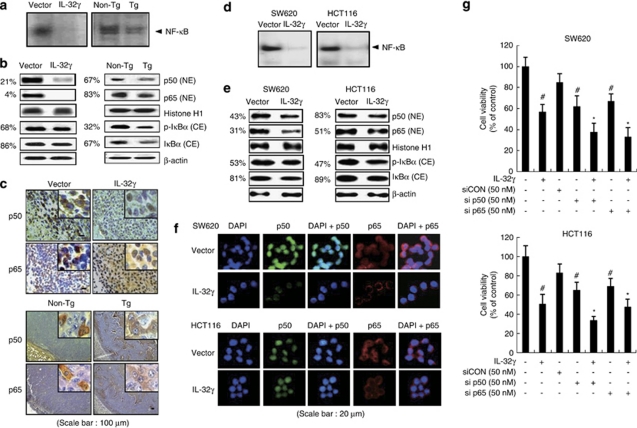
The activation of NF-κB plays a critical role in cancer cell survival. To determine whether IL-32γ inhibits the activation of NF-κB in colon cancer and melanoma cells as well as tumors, we determined the DNA-binding activity of NF-κB by electromobility shift assay, and translocation of p50 and p65 into the nucleus and IκBα protein degradation by western blot and immunohistochemical analysis. The DNA-binding activity of NF-κB, and translocation of p50 and p65 into the nucleus and IκBα protein degradation were significantly decreased in the tumor tissues of xenograft nude mice that were inoculated with IL-32γ-overexpressing colon cancer cells and transgenic mice that expressed IL-32γ (Figures 6a and b). Immunohistochemical analysis of p50 and p65 confirmed that the intensities of nuclear staining for p50 and p65 were decreased in the tumor tissues of nude and transgenic mice that expressed IL-32γ (Figure 6c). As NF-κB is highly activated in colon cancer cells (Ban et al., 2009), the DNA-binding activity of NF-κB was measured after the introduction of the vector or IL-32γ into the colon cancer cells. The introduction of IL-32γ inhibited the constitutive DNA-binding activity in the colon cancer cells (Figure 6d). Moreover, IL-32γ prevented the translocation of p50 and p65 into the nucleus through the inhibition of the phosphorylation of IκB (Figure 6e). Confocal microscopy confirmed that the translocation of p50 and p65 into the nucleus was decreased by IL-32γ (Figure 6f). We also showed that introduction of the other subtype of IL-32 also decreased constitutively activated NF-κB in the SW620 colon cancer cells transfected with IL-32α, -β and -γ (Supplementary Figure 2A), and IL32-γ transfection also inhibited constitutive or TNF-α-induced NF-κB in other cancer cells such as the prostate (PC3), liver (HepG2) and lung (NICH4610) (Supplementary Figure 2B). In addition, the treatment with recombinant IL-32γ protein inhibited the constitutive DNA-binding activity in the SW620 cells (Supplementary Figure 2C). The DNA-binding activity of NF-κB was confirmed by the competition and supershift assays (Supplementary Figure 2D). We also found that recombinant IL-32γ protein decreased TNF-α-induced NF-κB transcriptional activity (Supplementary Figure 2E). However, treatment with siRNA of IL-32γ abolished IL-32γ-induced p65 and p50 translocation (Supplementary Figure 3). To further investigate the effect of NF-κB inactivation on cancer cell growth, we analyzed the growth patterns of colon cancer cells, where p50 or p65 were knocked down by specific siRNAs. The knock down of p50 and p65 augmented the inhibitory effect of IL-32γ on colon cancer cell growth (Figure 6g). These data indicate that inhibition of NF-κB is implicated in IL-32γ-induced cancer cell growth inhibition.
Figure 6.
Effect of IL-32γ on NF-κB activation in tumor tissues and colon cancer cells. (a) The DNA-binding activity of NF-κB was determined by electromobility shift assay in the nuclear extracts of the xenograft mouse or IL-32γ transgenic mice tumor samples. (b, c) Expression of p50 and p65 in nuclear extracts and IκB phosphorylation in the cytosol of murine tumors, as determined by western blotting (b) and immunohistochemistry (c). Each image and band is representative of three independent experiments. (d) The DNA-binding activity of NF-κB was investigated using electromobility shift assay in nuclear extracts of colon cancer cells that were transfected by IL-32γ. (e, f) Expression of p50 and p65 in nuclear extracts and IκB phosphorylation in the cytosol, as determined by western blotting (e), and fluorescence microscopy (f). (g) Colon cancer cells were co-transfected with the IL-32γ and p50/p65 siRNA for up to 72 h. Cell growth was measured by direct counting of cells stained with Trypan blue. The results are expressed as mean±s.d. of three experiments with triplicate tests in each experiment. #P<0.05 compared with the colon cancer cells transfected with vector. *P<0.05 compared with the colon cancer cells transfected with IL-32γ alone. The values on the left of panels b and e are average percentages of vector control over three independent experiments.
IL-32γ decreases STAT3 activity in tumor tissues and in colon cancer cells
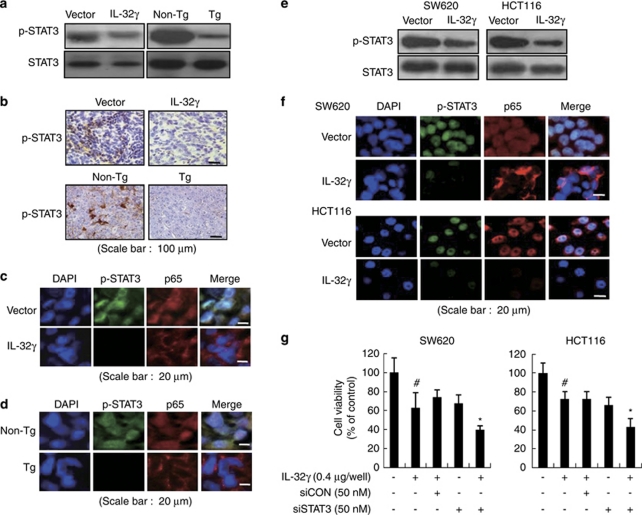
STAT3 is also implicated in inflammatory-associated carcinogenesis and in maintaining of the constitutive activation of NF-κB in cancer cells (Lee et al., 2009; Yu et al., 2009). Thus, we investigated whether IL-32γ inhibits STAT3 activation in colon and melanoma tumors as well as in colon cancer cells. Consistent with the inhibitory effect on NF-κB activation, STAT3 phosphorylation was significantly decreased in the tumor tissues of nude and transgenic mice that expressed IL-32γ (Figures 7a and b). Co-translocation of p65 and p-STAT3 into the nucleus was also decreased in the tumor tissues of nude and transgenic mice that expressed IL-32γ (Figures 7c and d). IL-32γ also inhibited the activated STAT3 activity (phosphorylation of STAT3) in the colon cancer cells (Figure 7e). Confocal microscopy confirmed that the translocation of p-STAT3 into the nucleus, and colocalization of p-STAT3 and p65 were decreased by IL-32γ in colon cancer cells (Figure 7f). To investigate further the inhibitory effect of IL-32γ on STAT3 activation on colon cancer cell growth, we analyzed the growth patterns of colon cancer cells, where STAT3 was knocked down by specific siRNAs. The knock down of STAT3 augmented the inhibitory effect of IL-32γ on colon cancer cell growth (Figure 7g). These data indicate that inhibition of STAT3 is implicated in IL-32γ-induced NF-κB inactivation and cancer cell growth inhibition.
Figure 7.
Effect of IL-32γ on STAT3 activation in tumor tissues and colon cancer cells. (a, b) Phosphorylation of STAT3 in whole extracts of murine tumors, as determined by western blotting (a) and immunohistochemistry (b). (c, d) Cellular localization p-STAT3 (green) and p65 (red) in tumor tissues of xenograft mice (c) and IL-32γ transgenic mice (d). Each image and band is representative of three independent experiments. (e) Colon cancer cells were transfected with the vector or the IL-32γ for 24 h. Whole-cell extracts were prepared and analyzed for phosphorylated STAT3 by western blotting. Each band is representative of three independent experiments. (f) Cellular localization of p-STAT3 (green) and p65 (red) was observed by confocal microscopy after immunofluorescence staining of colon cancer cells transfected with IL-32γ. (g) Colon cancer cells were co-transfected with the IL-32γ and STAT3 siRNA for up to 72 h. Cell growth was measured by direct counting of cells stained with Trypan blue. The results are expressed as mean±s.d. of three experiments with triplicate tests in each experiment. #P<0.05 compared with the colon cancer cells transfected with vector. *P<0.05 compared with the colon cancer cells transfected with IL-32γ alone.
IL-32γ induces the activation of CD8+ cytotoxic T cells and NK cells in the blood, tumor and immune organ tissues, and alters cytokine levels
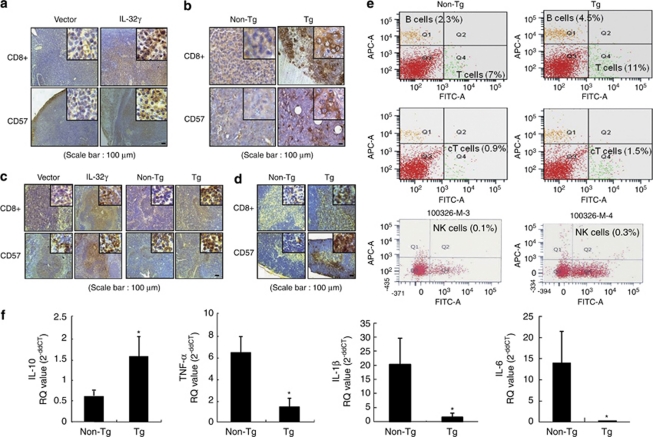
To investigate whether the inhibition of colon cancer growth by IL-32γ is related to tumor-specific immune responses, we analyzed the expression patterns of CD8+ (cytotoxic T cells) and CD57+ (NK cells) in tumor and immune tissues. Immunohistochemically, more CD8+ T and CD57+ cells were detected in the tumor sections of nude mice inoculated with colon cancer cells expressing IL-32 (Figure 8a). By staining the tumor sections from IL-32γ transgenic mice, we found that there were also a significant higher number of CD8+ T cells in the tumor, as well as an increase in the number of NK cells (Figure 8b). To investigate further the differences in the numbers of CD8+ T cells and NK cells between the control and IL-32γ-expressing cells in immunity-related organs, we analyzed the levels of CD8+ (cytotoxic T cells) and CD49+ reactive cell (NK cells) numbers in spleen and thymus tissues. With respect to the spleen, the numbers of CD8+ T cells were much higher in the IL-32γ transgenic mice than those in the non-transgenic mice, whereas there was a slight higher number in the xenograft nude mice inoculated with IL-32γ-expressing colon cancer cells (Figure 8c). Thymus sections of the IL-32γ transgenic mice also showed increased levels of CD8+ T cells and NK cells as compared with those of the non-transgenic mice (Figure 8d). Moreover, fluorescence-activated cell sorting (FACS) analysis also showed that the CD8+ T- and NK cell number as well as total B and T cells in the tumor tissues was higher in the blood of IL-32γ transgenic mice compared with those of the non-transgenic mice (Figure 8e). Similar increase of CD8+ T- and NK cell number was found in the blood and spleen even though the total number of T and B cells was not much different (Supplementary Table 1). These data suggest that IL-32γ induces antitumor immunity by promoting the activation of CD8+ T cells and NK cells in both tumor and immune organ tissues, which are potent cytotoxic effectors for tumors.
Figure 8.
Effect of IL-32γ on the infiltration of CD8+ T cells and NK cells into tumor tissues and immune organ tissues and on cytokine levels in tumor tissues. (a–d) Immunohistochemistry was used to determine the level of CD8+- and CD57-reactive cell number in tumor sections and immunity-related organs, as described in Materials and methods. Shown are the expression patterns of CD8+ and CD57 in the tumor sections of nude mouse xenografts (a) and IL-32γ transgenic mice (b), as well as in the spleens (c) and thymuses (d) of IL-32γ transgenic mice. The images shown are representative of three sections from each mouse (n=3). Each image and band is representative of three independent experiments. (e) Subpopulation of immune cells determined after FACS analysis, as described in detail in Materials and methods. The values in each area are the average subpopulation of immune cells (NK cells and CD8+ cells). Each image is representative of three independent experiments. (f) The levels of IL-10, TNF-α, IL-1β and IL-6 in the tumor tissues of transgenic mice (n=3) were measured using quantitative real-time PCR, as described in Materials and methods. *P<0.05 compared with non-transgenic mice tumor tissues.
To investigate whether IL-32γ alters other cytokine levels in tumor tissues and immune organ (spleen), and thus effect on the cancer cell growth, NF-κB and STAT3 signal and activation of immune cells in the tumor and/or immune organ, we analyzed cytokine levels in tumor tissues by quantitative RT–PCR. The levels of the tumor growth-prompting cytokines, TNF-α, IL-6 and IL-1β, were significantly decreased, whereas the level of IL-10, a tumor growth-inhibiting cytokine, was significantly elevated in the tumor tissues of IL-32γ transgenic mice than those in the non-transgenic mice (Figure 8c). Similar alteration of cytokine levels was found in the xenograft nude mice tumor tissues (Supplementary Figure 4A) as well as SW620 colon cancer cells expressing IL-32γ (Supplementary Figure 4B). However, treatment with siRNA of IL-32γ abolished IL-32γ transfection-induced alteration of cytokine levels (Supplementary Figure 4C).
Discussion
In this study, using IL-32γ, the most biological active form of the IL-32s (Choi et al., 2009), we found that tumor growth was inhibited in the xenograft BALB/C athymic nude mice inoculated with IL-32γ-overexpressing colon cancer cells and in the IL-32γ-overexpressing transgenic mice inoculated with B16 melanoma cells. The inhibitory effect of IL-32γ on tumor growth was associated with the inhibition of constitutively activated NF-κB and STAT3. This antitumor activity was also associated with decreased expression of antiapoptotic, cell proliferation and tumor-promoting genes (cleaved caspase-3 and -9, bax, cyclin D and CDK4, COX-2 and iNOS), but with increased expression of their target apoptotic genes (bcl-2, xIAP, cIAP and c-FLIPL). In tumor, blood and immune tissues, the number of cytotoxic CD8+ T cells and CD57+ NK cells and the levels of IL-10 increased, but that of TNF-α, IL-1β and IL-6 levels decreased. When IL-32γ was knocked down by siRNA IL-32, NF-κB or STAT3, or neutralized with an anti-IL-32 antibody, the IL-32γ-induced colon cancer cell growth inhibition; IL-32γ-induced decrease of TNF-α, IL-1 and IL-6 production; and increase of IL-10 production were abolished. These findings suggest that IL-32γ has an inhibitory influence on tumor development.
However, the fundamental mechanisms underlying these phenomena are not clear. Because of their abilities to induce the expression of a large array of inflammatory mediators and their roles as core transcription factors in diverse immune responses, NF-κB and STAT family signals have been recognized as major pathways responsible for cytokine-associated cancer development or antitumor immunity (Mantovani et al., 2008; Baud and Karin, 2009). When the colon cancer cells or the in vivo tumor tissues were overexpressed with IL-32γ, the expression of NF-κB target genes, especially inflammatory and tumor development-associated genes, such as iNOS and COX-2, and antiapoptosis genes, Bcl-2, cIAP, xIAP and c-FLIPL, and the DNA-binding activity of NF-κB, were significantly inhibited, but the expression of cell death NF-κB target genes, such as Bax and caspase-3 and -9, was enhanced. In addition, p50 and p65 translocation into the nucleus and phosphorylation of IκBα was also inhibited. The inhibitory effect on NF-κB activity was also found in conjunction with other subtypes of IL-32, such as α and β, and in other cancer cell lines, such as the prostate, liver and lung (data not shown). The colon cancer cell lines and human tumor samples, as well as the nuclei of stromal macrophages in sporadic adenomatous polyps, had increased levels of NF-κB (Hardwick et al., 2001; Lind et al., 2001), which is likely acting as a survival factor for colon and melanoma cancer cell growth. Constitutively activated NF-κB has been associated with several aspects of tumorigenesis, including tumor cell growth, antiapoptosis and tumor promotion in colon cancer (Karin et al., 2002). Thus, the inhibitory effects on NF-κB activity and on the expression of target proteins are critical in the IL-32γ-induced inhibition of tumor growth. Although it remains to be investigated, it is worth noting that NF-κB is activated or inactivated by many cytokines. It has been reported that IL-6 induces the activation of NF-κB in the intestinal epithelia, resulting in the induction of intercellular adhesion molecule 1 expression (Wang et al., 2003), increase of cell cycle progression and suppression of apoptotic cell death-leading, tumor promotion (Lin and Karin, 2007). In contrast, IL-10 blocks NF-κB activity, causing apoptotic cell death of cancer cells (Schottelius et al., 1999), and it inhibits the production of proinflammatory cytokines, such as TNF-α, IL-6, IL-1β and IL-12, which are closely associated with tumor development (Lin and Karin, 2007). We found that the levels of TNF-α, IL-1β and IL-6 were significantly reduced, but IL-10 expression increased in the tumor tissues of BALB/C athymic nude mice inoculated with colon cancers expressing IL-32γ and of IL-32γ-overexpressing transgenic mice inoculated with B16 melanoma cells. In addition, siRNA of IL-32γ or a neutralization with anti-IL-32 antibody abolished the constitutive activity of NF-κB (p50 and p65 translocation into the nucleus) accompanied with the abolishment of cell growth inhibition; decreased TNF-α, IL-1β and IL-6 production; increased IL-10 production; and the induction of apoptotic cell death in cultured colon cancer cells. Moreover, siRNA of p50 and p65 augmented IL-32γ-induced colon cancer cell growth inhibition. Very similar to this finding, it was also found that associated with attenuated tissue damage, IL-6 and TNF-α was lower, but IL-10 level was higher in IL-32γ-overexpressed transgenic mice compared with those levels of wild-type mice having dextran sodium sulfate-inflamed colitis (Choi et al., 2010). Therefore, it is possible that the inhibition of the NF-κB signal by IL-32γ could play important roles in the inhibitory effect of IL-32γ on colon cancer development and reduced production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), but an increase in the production of anti-inflammatory cytokine (IL-10) could be critically involved in this tumor killing effect of IL-32γ.
We also found that STAT3 activation was significantly reduced in the tumor tissues of xenograft BALB/C athymic nude mice inoculated with IL-32γ-overexpressing colon cancer cells and in those of the IL-32γ-overexpressing transgenic mice inoculated with B16 melanoma cells. Recently, the role of STAT3 and NF-κB and their relationship in inflammatory and cytokine-associated cancer development have been studied frequently (Lee et al., 2009; Yu et al., 2009). Activated nuclear STAT3 has been detected in many forms of cancer, including breast, colon, gastric, lung, head and neck, skin, prostate and others (Lee et al., 2009). Activation of STAT3 in the tumor contributes to the expression of pro-cancer inflammatory mediators and growth factors, leading to increased tumor growth. STAT3 also induces the expression of antiapoptotic genes in tumors (Lee et al., 2009). Conversely, conditional STAT3 ablation inhibited tumor development and progression (Chan et al., 2004; Jenkins et al., 2005). In addition to the inhibitory effect of IL-32γ on cancer cell growth and STAT3 activation, we found that knock down of STAT3 with siRNA further augmented the IL-32γ-induced inhibition of colon cancer cell growth. Therefore, it is also possible that inhibition of the STAT3 signal by IL-32γ could play important roles in the inhibitory effect of IL-32γ on cancer development too. Activated STAT3 can increase NF-κB activity in cancer, and persistent activation of NF-κB in tumors is dependent on STAT3 activation (Lee et al., 2009). In agreement with these findings, we also found that STAT3 and NF-κB were co-activated in colon cancer cells and tumor tissues, and the introduction of IL-32γ reduced the co-activation of STAT3 and NF-κB. Functional interaction between NF-κB and STAT3 can occur as well. Many genes whose products play important roles in tumor development are controlled by NF-κB and STAT3, either synergistically or individually (Bromberg et al., 1999; Bollrath and Greten 2009; Lee et al., 2009; Grivennikov and Karin, 2010). The expression of antiapoptotic genes, such Bcl-2, xIAP2 and cIAP2, which are prominent targets for NF-κB and STAT3, was increased by IL-32γ however, the expression of NF-κB-dependent tumor-promoting genes, such as c-FLIP, COX-2 and iNOS, was inhibited. The expression of STAT3 and NF-κB target genes controlling cell cycle and proliferation, such as cyclins D and B (Levy and Darnell, 2002; Jenkins et al., 2005; Naugler and Karin, 2008), also was decreased by treatment with IL-32γ, and it was accompanied with G0/G1-phase cell cycle arrest. Thus, the blocking ability of IL-32γ on STAT3 alone and/or coordination of the inhibitory effect on NF-κB could be significantly associated with the inhibition of tumor growth by IL-32γ. It remains to be determined how IL-32γ inhibited activated STAT3 and NF-κB, but it is also worth noting that several cytokines can modulate STAT3 and NF-κB activities to increase gene transcription. IL-6 activates STAT3, whereas IL-10 inhibits the activation of STAT3 during tumor growth inhibition (Yu et al., 2009). The acetylation and/or recruitment of p300 protein are critical in cytokine-induced activation of STAT3 and NF-κB, and this cytokine (IL-6) prolongs their retention in the nucleus (Choi et al., 2002; Hou et al., 2008; Ray et al., 2008; Ma et al., 2010). We found that acetylation of p65 and expression of p300 in the nucleus of colon cancer cells were inhibited by the introduction of IL-32γ and in the nucleus of tumor tissues overexpressing IL-32γ (data not shown). Thus, direct inhibition of acetylation and/or recruitment of p300 protein may partially contribute to the inhibitory effects of IL-32γ on the inactivation of STAT3 and NF-κB. Indirect inactivation via modulation of other cytokines in the tumor tissues (decreases of TNF-α, IL-6, and IL-1β and/or an increase of IL-10) may also play a role in the inhibitory effects of IL-32γ on the inactivation of STAT3 and NF-κB.
The interaction between tumor and immune cells is primarily responsible for overall tumor progression (or regression) and the spreading or induction of antitumor immune responses and tumor rejection (Shurin et al., 2006). Cytokines can act as inducers, causing the activation of T cells (cytotoxic CD8+ T cells) and NK cells in the tumor tissues, which results in the apoptotic cell death of tumor cells (Moretta et al., 2000). The expression of IL-2 and IL-10 at the site of tumors enhances tumor killing ability by inducing the generation of tumor-reactive cytotoxic T lymphocytes and by allowing the increased infiltration of activated T cells into the tumors (Lee et al., 1998). We found that IL-32γ-overexpressing transgenic mice and xenograft nude mice have higher levels of CD8+ T cells and CD57-positive NK cells in the tumor area, blood, spleen and thymus. Thus, activation of immune cells, such as cytotoxic T cells and NK cells, could be important in the inhibition of colon cancer development by IL-32γ. Several recent studies have reported that activation of STAT3 and NF-κB signals in tumors by cytokines, such as IL-6 and IL-12, impedes dendrite maturation and inhibits the generation of T cells, giving rise to immune tolerance against cancers (Lee et al., 1998; Moretta et al., 2000; Chan et al., 2004; Jenkins et al., 2005; Shurin et al., 2006). Thus, it is possible that inactivation of STAT3 and NF-κB by the overexpression of IL-32γ may also enhance immune responses to the tumor via an increase of traffic into the tumor area or maturation of immune cells, especially cytotoxic T cells and NK cells, thereby causing indirect inhibition of tumor growth. We are currently studying the roles and mechanisms of IL-32s on the immune responses against cancer development. The present data conclusively show that IL-32γ can act as a cytokine-inhibiting cancer cell growth and that the inhibition of NF-κB and STAT3 signaling and the reduced production of pro-inflammatory cytokines (but increased production of anti-inflammatory cytokines) play an important role in the inhibitory effect of IL-32γ on colon cancer and melanoma cell growth.
Materials and methods
Generation of IL-32γ transgenic mice
To generate transgenic mice that express hIL-32γ, concentrated hIL-32γ cDNA was prepared. The pCAGGS/hIL-32γ plasmid was prepared using the Qiagen MIDI-Prep Kit. To generate IL-32γ transgenic mice, a 705-base pair fragment of the hIL-32γ gene was subcloned into the EcoRI sites of the pCAGGs expression vector. The sequence of the IL-32γ insert was confirmed by automated sequencing. The IL-32γ gene-containing genomic fragment was released from the vector using SalI/HindIII digestion, and then separated from the pCAGGs vector using low-melting-point agarose and transverse alternating field-gel electrophoresis. The fragment was purified and microinjected at a concentration of 4 ng/μl into the embryos of BDF1 mice, as described previously (Wang et al., 2005; Kim et al., 2008). The experimental treatments were carried out according to the guidelines for animal experiments of the Faculty of Disease Animal Model Research Center, Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). IL-32γ insertion was confirmed by amplification of genomic DNA isolated from the transgenic mice tails using Super Taq PLUS Pre-mix (RexGeneBioTech, Seoul, Korea) and the following specific primer set: sense, 5′-GAAGGTCCTCTCTGATGACA-3′ and antisense, 5′-GAAGAGGGACAGCTATGACTG-3′ (nt 2245–2225). pCAGGS/IL-32γ was used as a positive control, and pCAGGS (4.8 kb) was used as a negative control. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Genomic DNA samples were extracted from transgenic mice tails and PCR analysis was performed for IL-32γ gene expression. The following conditions were used for the TaKaRa PCR Thermal Cycler: 94 °C for 10 min, followed by 35 cycles of 94 °C for 1 min, 63 °C for 1 min and 72 °C for 1.5 min, with a final step of 72 °C for 10 min. To analyze IL-32 gene expression in various tissues of transgenic mice, total RNAs were extracted and RT–PCR analysis was performed using primer sets as follows: sense, 5′-GAAGGTCCTCTCTGATGACA-3′ antisense, 5′-GGGGTTCAGAGCACTTCT-3′ (371 bp). GAPDH was used as an internal control as follows: sense, 5′-ACCACAGTCCATGCCATCAC-3′ antisense, 5′-TCCACCACCCTGTTGCTGTA-3′ (450 bp). IL-32γ protein expressed in the tissues of transgenic mice was detected by western blotting with an anti-IL-32 monoclonal antibody KU32-52 (Kim et al., 2008).
Antitumor activity of IL-32γ in IL-32γ transgenic mice
Male, 6- to 8-week-old IL-32γ transgenic and non-transgenic mice were maintained in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee (Chungbuk, Korea). B16 melanoma cells were injected subcutaneously (5 × 105 tumor cells in 0.1 ml phosphate-buffered saline per animal) into transgenic mice and non-transgenic mice. The weights and tumor volumes of the animals were monitored twice weekly. The tumor volumes were measured with Vernier calipers and calculated using the following formula: (A × B2)/2, where A is the larger and B is the smaller of the two dimensions. At the end of the experiment, the animals were killed and the tumors were separated from the surrounding muscles.
Detection of IL-32 in the sera of transgenic mouse
The level of IL-32 in the sera was detected by enzyme-linked immunosorbent assay methods as described elsewhere (Lee et al., 2010).
In vivo antitumor activity of IL-32γ in a xenograft animal model
Six-week-old male BALB/c athymic nude mice were purchased from Japan SLC (Hamamatsu, Japan). All experiments were approved and carried out according to the Guide for the Care and Use of Animals (Chungbuk National University Animal Care Committee). Human colon cancer SW620 cells that had been transfected with the vector or the IL-32γ plasmid (6 μg/1 × 106 cells) were injected subcutaneously (1 × 107 tumor cells in 0.1 ml PBS per animal) into the right-lower flanks of the carrier mice. The body weights and tumor volumes of the animals were monitored twice weekly. The formula described above was used to calculate tumor volume. At the end of the experiment, the animals were killed by cervical dislocation. The tumors were separated from the surrounding muscles and dermis, excised and weighed.
Immunohistochemistry
All specimens were fixed in formalin and embedded in paraffin for examination. Sections (4-μm thickness) were stained with hematoxylin and eosin and analyzed by immunohistochemistry as described elsewhere (Son et al., 2007) using primary mouse anti-human proliferating cell nuclear antigen, Ki-67, bax (1:200 dilution), CD3 (1:10) and CD57 (1:50) monoclonal antibodies or primary rabbit anti-human cleaved caspase-3 polyclonal antibody (1:100) and secondary biotinylated anti-mouse and anti-rabbit antibodies.
Detection of apoptosis
TUNEL assays were performed using the In situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany), as described elsewhere (Son et al., 2007). The total cell number in a given area was determined based on 4′,6-diamidino-2-phenylindole nuclear staining. The apoptotic index was calculated as the number of 4′,6-diamidino-2-phenylindole-stained, TUNEL-positive cells divided by the total number of cells counted × 100.
Reagents and cell culture
The HCT116 and SW620 colon cancer cell lines and B16 melanoma cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Colon cancer cells were grown at 37 °C in 5% CO2 humidified air in RPMI 1640 medium that contained 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. B16 melanoma cells were grown at same conditions in DMEM medium. RPMI 1640, DMEM, penicillin, streptomycin and FBS were purchased from Gibco Life Technologies (Grand Island, NY, USA). siRNA species for IL-32, p50 and p65, and a non-targeting control siRNA were purchased from Bioneer (Daejeon, Korea) and siRNA for STAT3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Transfection
Colon cancer cells (5 × 104 cells per well) were plated in 24-well plates and transiently transfected with 0.4 μg of the empty vector or the constitutively activated full-length IL-32γ plasmid per well, using a mixture of plasmid and the WelFect-EX PLUS reagent in OPTI-MEN, according to the manufacturer's specification (WelGENE, Seoul, Korea).
Cell viability
To determine viable cell numbers, the colon cancer cells were seeded onto 24-well plates (5 × 104 cells per well). The cells were trypsinized, pelleted by centrifugation for 5 min at 1500 r.p.m., resuspended in 10 ml of PBS and 0.1 ml of 0.2% Trypan blue was added to the tumor cell suspension in each solution (0.9 ml each). Subsequently, a drop of suspension was placed in a Neubauer chamber, and the living cancer cells were counted. Cells that showed signs of Trypan blue uptake were considered to be dead, whereas those that excluded Trypan blue were considered to be viable. Each assay was carried out in triplicate.
Western blotting
Western blot analysis was performed as described previously (Son et al., 2007). The membranes were immunoblotted with the following primary antibodies: mouse monoclonal antibodies directed against p65, p50 and c-FLIP (1:500 dilutions; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antibodies directed against bax, poly-(ADP-ribose) polymerase, COX-2, iNOS and CDK4 (1:500 dilutions; Santa Cruz Biotechnology) and against caspase-3, cleaved caspase-3, caspase-9, Bcl-2, XIAP, c-IAP-1 and Akt (1:1000 dilutions; Cell Signaling Technology, Beverly, MA, USA). The monoclonal anti-hIL-32 antibody KU32-52 was used as reported previously (Kim et al., 2008).
Gel electrophoretic mobility shift assay
A gel electromobility shift assay was performed according to the manufacturer's recommendations (Promega, Madison, WI, USA), as described elsewhere (Son et al., 2007).
Fluorescence microscopy
The fixed cells and tissues were exposed to following primary antibodies: p50, p65 and p-STAT3 (1:100 dilutions in blocking serum; Santa Cruz Biotechnology) at room temperature for 1 h. After incubation, the cells were washed twice with ice-cold PBS and incubated with an anti-rabbit or -mouse secondary antibody conjugated to Alexa Fluor 488 or 568 (Invitrogen–Molecular Probes, Carlsbad, CA, USA) at room temperature for 1 h. Immunofluorescence images were acquired using an inverted fluorescent microscope Zeiss Axiovert 200 M (Carl Zeiss, Thornwood, NY, USA).
Quantitative real-time PCR
For mRNA quantification, total RNA was extracted using the RNAqueous kit and the cDNA was synthesized 1 μg of total RNA using High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Quantitative real-time PCR was performed using specific primers for GAPDH (Mm99999915_g1), IL-6 (Mm00446190_m1), TNF-α (Mm00443258_m1), IL-10 (Mm00439616_m1) and IL-1β (Mm00434228_m1) in a 7500 Real-Time PCR System (Applied Biosystems). Thermocycling conditions consisted of an initial denaturation of 20 s at 95 °C, followed by 60 cycles of 95 °C for 30 s and 60 °C for 30 s. The values obtained for the target gene expression were normalized to GAPDH and quantified relative to the expression in control samples. For the calculation of relative quantification, the 2−ΔΔCT formula was used, where −ΔΔCT=(CT,target–CT,GAPDH) experimental sample−(CT,target−CT,GAPDH) control sample.
FACS analysis for immune cell populations
Immune cell populations in the whole blood, spleen and tumor were analyzed by FACS analysis. One hundred microliters of whole blood was collected using a hematocrit capillary tube and blocked with Fc block (eBioscience, San Diego, CA, USA) to reduce nonspecific antibody binding for 3 min at room temperature. Cells were then incubated in the dark with 10 μl of the appropriate fluorochrome-conjugated antibodies from eBioscience—T cells (anti-CD3-FITC, 1:25), B cells (anti-CD19-PE, 1:25) and NK cells (anti-CD49-APC, 1:50) for 20 min at 4 °C. Cells were washed with 500 μl of FACS buffer containing 0.02% sodium azide and 2% FBS in PBS. The red blood cells were lysed for 5 min with FACS lysis buffer (BD Bioscience, Franklin Lakes, NJ, USA) at room temperature, and then re-washed with FACS buffer. Finally, each sample was fixed with 1% paraformaldehyde until further analysis. Flow cytometry analysis was performed on the FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA). Control samples were matched for each fluorochrome. Data were analyzed using the CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA). For immune cell population analysis in the spleens and tumor, tissues were disrupted by forcing them through 70-μm cell strainer into 10 ml of cold PBS using a rubber-tipped syringe plunger. The cell suspensions were then centrifuged at 1500 r.p.m. for 10 min and the supernatants were discarded. The cells were resuspended in 3 ml ACK lysing buffer (Lonza, Walkersville, MD, USA) for 3 min and the debris was sedimented by centrifuging at 1500 r.p.m. for 10 min. Cell concentrations were determined by hemocytometer counting using Trypan blue dye exclusion, and were adjusted to 1 × 106 cells per ml. Single-cell suspensions were stained with the following fluorochrome-conjugated antibodies from BD Biosciences: B cells (anti-B220-APC, 1:100), T cells (anti-CD3-FITC, 1:400), NK cells (anti-CD49-APC, 1:50) and cytotoxic T cells (anti-CD8-FITC, 1:100). Flow cytometry and data analysis was carried out as mentioned above in the blood.
Statistical analysis
The data were analyzed using the GraphPad Prism 4 ver. 4.03 software (La Jolla, CA, USA). Data are presented as means±s.d. The homogeneity of variances was assessed using the Bartlett test in tumor incidence test. The differences in all data were assessed by one-way analysis of variance. When the P-value in the analysis of variance test indicated statistical significance, the differences were assessed by the Dunnett's test. A value of P<0.05 was considered to be statistically significant.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (MRC, R13-2008-001-00000-00) and the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0920080). D Yoon was partially from National Research Foundation of Korea (2009-0093824, 2010-0019306) and D Yu from KRIBB Research Initiative Program Grant. We thank Dr Howard P Glauert (University of Kentucky) who provided invaluable assistance with English legal manuscripts.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Araki S, Omorin Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- Ban JO, Oh JH, Hwang BY, Moon DC, Jeong HS, Lee S, et al. Inflexinol inhibits colon cancer cell growth through inhibition of nuclear factor-kappaB activity via direct interaction with p50. Mol Cancer Ther. 2009;8:1613–1624. doi: 10.1158/1535-7163.MCT-08-0694. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signaling hubs in inflammation-mediated tumor promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Cacev T, Radosevic S, Krizanac S, Kapitanovic S. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis. 2008;29:1572–1580. doi: 10.1093/carcin/bgn164. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126:535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Bae S, Hong J, Ryoo S, Jhun H, Hong K, et al. Paradoxical effects of constitutive human IL-32gamma in transgenic mice during experimental colitis. Proc Natl Acad Sci USA. 2010;107:21082–21086. doi: 10.1073/pnas.1015418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Knag HB, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2002;69:583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- Dinarello CA, Kim SH. A novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65:iii61–iii64. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Aoki I, Toyoki H, Khatun S, Tamaya T. Clinical implications of expression of ETS-1 related to angiogenesis in uterine cervical cancer. Ann Oncol. 2002;13:1598–1604. doi: 10.1093/annonc/mdf248. [DOI] [PubMed] [Google Scholar]
- Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, et al. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groscurth P, Diener S, Stahel R, Jost L, Kagi D, Hengartner H. Morphologic analysis of human lymphokine-activated killer (LAK) cells. Int J Cancer. 1990;45:694–704. doi: 10.1002/ijc.2910450422. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, van den Brink GR, Offerhaus GJ, van Deventer SJ, Peppelenbosch MP. NF-kappaB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene. 2001;20:819–827. doi: 10.1038/sj.onc.1204162. [DOI] [PubMed] [Google Scholar]
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- Hou T, Ray S, Lee C, Brasier AR. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. J Biol Chem. 2008;283:30725–30734. doi: 10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. 1996;2:1969–1979. [PubMed] [Google Scholar]
- Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin-6 in human basal cell carcinoma cell lines increases anti-apoptotic activity and tumorigenic potency. Oncogene. 2001;20:198–208. doi: 10.1038/sj.onc.1204076. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M. The threshold of 130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–3520. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]
- Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH, Lim JS, et al. A proinflammatory cytokine interleukin-32β promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128:e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shim JH, Seo EH, Cho MC, Kang JW, Kim SH, et al. Interleukin-32 monoclonal antibodies for immunohistochemistry, Western blotting, and ELISA. J Immunol Methods. 2008;333:38–50. doi: 10.1016/j.jim.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. IL-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fenton BM, Koch CJ, Frelinger JG, Lord EM. Interleukin 2 expression by tumor cells alters both the immune response and the tumor microenvironment. Cancer Res. 1998;58:1478–1485. [PubMed] [Google Scholar]
- Lee S, Kim S, Bae S, Choi J, Hong J, Ryoo S, et al. Interleukin-32 gamma specific monoclonal antibody and developing IL-32 specific ELISA. Hybridoma. 2010;29:501–509. doi: 10.1089/hyb.2010.0059. [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- Lu C, Soria JC, Tang X, Xu XC, Wang L, Mao L, et al. Prognostic factors in resected stage I non-small-cell lung cancer: a multivariate analysis of six molecular markers. J Clin Oncol. 2004;22:4575–4583. doi: 10.1200/JCO.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184:4898–4906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420–422. doi: 10.1016/s0167-5699(00)01673-x. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci USA. 2009;106:3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Lee C, Hou T, Boldogh I, Brasier AR. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008;36:4510–4520. doi: 10.1093/nar/gkn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Seo EH, Kang J, Kim KH, Cho MC, Lee S, Kim HJ, et al. Detection of expressed IL-32 in human stomach cancer using ELISA and immunostaining. J Microbiol Biotechnol. 2008;18:1606–1612. [PubMed] [Google Scholar]
- Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies. Cancer Metast Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- Son DJ, Park MH, Chae SJ, Moon SO, Lee JW, Song HS, et al. Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor kappaB. Mol Cancer Ther. 2007;6:675–683. doi: 10.1158/1535-7163.MCT-06-0328. [DOI] [PubMed] [Google Scholar]
- Sorrentino C, Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. 2009;180:769–779. doi: 10.1164/rccm.200903-0400OC. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AG, Moon HB, Lee MR, Hwang CY, Kwon KS, Yu SL, et al. Gender-dependent hepatic alterations in H-ras 12V transgenic mice. J Hepatol. 2005;43:836–844. doi: 10.1016/j.jhep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol. 2003;171:3194–3201. doi: 10.4049/jimmunol.171.6.3194. [DOI] [PubMed] [Google Scholar]
- Westbrook AM, Wei B, Braun J, Schiestl RH. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–4834. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Suares G, Sha J, Sierra JC, Peterson JW, Chopra AK. Phospholipase A2-activating protein (PLAA) enhances cisplatin-induced apoptosis in HeLa cells. Cell signal. 2009;21:1085–1099. doi: 10.1016/j.cellsig.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.