Abstract
Immunoassays are routinely used as research tools to measure intracellular cAMP and cGMP concentrations. Ideally, this application requires antibodies with high sensitivity and specificity. The present work evaluates the cross-reactivity of commercially available cyclic nucleotide analogs with two non-radioactive and one radioactive cAMP and cGMP immunoassay. Most of the tested cyclic nucleotide analogs showed low degree competition with the antibodies; however, with Rp-cAMPS, 8-Br-cGMP and 8-pCPT-cGMP, a strong cross-reactivity with the corresponding cAMP and cGMP, respectively, immunoassays was observed. The determined EIA-binding constants enabled the measurement of the intracellular cyclic nucleotide concentrations and revealed a time- and lipophilicity-dependent cell membrane permeability of the compounds in the range of 10–30% of the extracellular applied concentration, thus allowing a more accurate prediction of the intracellular analog levels in a given experiment.
Electronic supplementary material
The online version of this article (doi:10.1007/s00210-011-0662-6) contains supplementary material, which is available to authorized users.
Keywords: Cyclic nucleotides, Enzyme immunoassay, Lipophilicity, Cell permeability
Introduction
The second messengers cAMP and cGMP control a multitude of cellular processes, such as gene transcription, chemotaxis, proliferation, differentiation and apoptosis. In this regard, cAMP and cGMP enzyme immunoassays (EIA) and radioactive immunoassays (RIA) are routinely used as research tools to estimate the concentration of the cyclic nucleotides in a cell. The cAMP and cGMP immunoassays are based on the competition of free cyclic nucleotide with linked cAMP or cGMP tracer. Routinely, the cyclic nucleotide antibodies are analysed for cross-reactivity with the mono-, di- and triphosphates of several nucleotides (e.g. ADP, GTP and CTP); however, nonspecific binding of cyclic nucleotide derivatives is not determined.
In the last 30 years, a large variety of cyclic nucleotide analogs (e.g. 8-Br-cAMP, 8-pCPT-cGMP and 8-pCPT-2′-O-Me-cAMP; Poppe et al. 2008) as well as purine-based phosphodiesterase inhibitors (IBMX, theophylline) have been applied to study intracellular cAMP and cGMP pathways and to manipulate cyclic nucleotide binding partners like ion channels (Craven and Zagotta 2006), protein kinases (Lohmann and Walter 2005), phosphodiesterases (Omori and Kotera 2007) and exchange proteins directly activated by cAMP (Epac; Gloerich and Bos 2010) in intact cells. Based on their purine-riboside structure, these synthetic derivatives have the potential to interfere with the cAMP- and cGMP-EIAs to give false positive results. We therefore tested the most commonly applied cyclic nucleotides for their competition with the specific cAMP and cGMP antigens in two frequently used commercial EIAs in vitro and with intact cells (Horton et al. 1992). In addition, a set of systematically modified cyclic nucleotide analogs was chosen to map the epitope interaction of the polyclonal antibodies and to predict potential cross-reactivities with novel derivatives.
Due to their polar ionic structure, cAMP and cGMP are not able to penetrate intact cellular membranes. Therefore, cyclic nucleotide analogs with lipophilic substituents are used in intact cell experiments to mimic the intracellular effects of cAMP and cGMP. However, the concentrations needed for a given cell line have to be determined laborious in dose–response experiments. On the basis of the EIA data raised in this study, we are now able to measure the intracellular concentration of extracellularly applied cyclic nucleotides and to predict the bioavailability of the derivatives in accordance to their lipophilicity index.
Methods
Cyclic nucleotide analogs
All cyclic nucleotides and derivatives (Supplementary Figs. S1 and S2) were from Biolog Life Science Institute (Bremen, Germany) and diluted in aqua dest. The PDE inhibitors 3-isobutyl-methyl-xanthine (IBMX), theophylline and erythro-9-(2-Hydroxy-3 nonyl)-adenine hydrochloride (EHNA) (Supplementary Fig. S3) were obtained from Sigma-Aldrich (Deisenhofen, Germany) and diluted in DMSO.
Cyclic nucleotide enzyme immunoassay (EIA) and radio immunoassay (RIA)
For measuring cyclic nucleotide concentrations, the non-radioactive cAMP- and cGMP-EIA kits from ENZO Life Sciences (Loerrach, Germany), the cGMP-EIA from Cayman (IBL International, Hamburg, Germany) and the radioactive cAMP- and cGMP-RIA kits from IBL (Hamburg, Germany) were used following the instructions of the manufacturer. If not stated otherwise, all experiments were performed with the acetylation protocol to achieve maximum sensitivity. The yellow-coloured product formed with the EIAs is inversely proportional to the amount of cyclic nucleotide present in the sample and was detected at 405 nm by a multiple counter (Victor2™ 1420, Wallac/Perkin Elmer, Rodgau, Germany). Radioactivity (I-125) was detected with a Berthold Counter LB 2104 (Bad Wildbad, Germany). All experiments were done three times in duplicate.
Preparation of washed human platelets
All procedures were carried out at room temperature. Blood was drawn from healthy volunteers, who gave informed consent and had been medication-free for 10 days, using venipuncture with a hollow needle into CCD-EGTA-buffer (20 mM sodium citrate, pH 6.5, 1.5 mM citric acid, 10 mM glucose, 4 mM EGTA, final concentration). Platelet-rich plasma was prepared by 20-min centrifugation at 300×g. After 5 min rest, platelets were pelleted by 10-min centrifugation at 380×g, washed once in resupension buffer (10 mM Hepes, pH 7.4, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose) and resuspended at a final concentration of 3 × 108 platelets/ml (washed platelets). Platelet count was determined using a Technicon H.3 RTC blood cell counter (Bayer, Frankfurt, Germany).
Cell culture
Chinese hamster ovarian cells (CHO) were grown on 75-cm2 culture flasks in 15 ml of Dulbecco's modified Eagle's medium (Invitrogen, Karlsruhe, Germany), supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin solution (Sigma-Aldrich, Deisenhofen, Germany). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Cell membrane permeability
Cell membrane permeability of cyclic nucleotides was determined by incubating 300 μl washed platelets with various concentrations of cyclic nucleotides at 37°C for the time points indicated (normally 20 min). After centrifugation (1 min, 720×g) and removal of the analog containing supernatant, cells were resuspended in 300 μl hypertonic buffer (10 mM Hepes, pH 8.1, 10 mM KCl, 1.5 mM EDTA, 200 mM sucrose, 1 mM IBMX) and left on ice for 30 min. For CHO cell permeability measurements, cells were incubated with various analog concentrations in serum-free medium for the time points indicated (normally 20 min), washed with PBS, covered with 300 μl hypotonic buffer, left on ice for 30 min, scraped off and transferred into a reaction tube.
Further cell disruption was done for both cell types in a freeze/thaw cycle with liquid nitrogen followed by needle disruption. After centrifugation (10 min, 20.800×g, 4°C), the cyclic nucleotide containing supernatant was lyophilized and then used in the EIA.
SDS-PAGE and western blot
Ten microgrammes of the protein extracts were separated by 9% SDS-PAGE under reducing conditions and transferred onto nitrocellulose membranes by the wet method. After blocking with 3% non-fat dry milk in 10 mM Tris buffer, pH 7.5, 150 mM NaCl, and 0.1% (w/v) Tween 20, the membrane was incubated with the 16C2 antibody raised against pVASP Ser-239 (PKG site) or 5C6 antibody raised against pVASP Ser-157 (PKA site; both 1:10,000, Nanotools, Freiburg, Germany) followed by incubation with horseradish peroxidase-coupled goat anti-mouse IgG (Biorad, Munich, Germany), diluted 1:5,000 and visualized by ECL (GE-Healthcare, Freiburg, Germany).
Calculations
The binding constants were calculated according to the Hill equation:
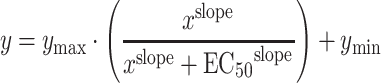 |
where x is the concentration of cyclic nucleotide, y the percentage of binding (percent B/B 0) and EC50 the concentration of the standard or the analogs entailing 50% binding. Binding curves were determined for each derivative with at least six different concentrations in duplicates and with platelets from three different donors. EC50 values were calculated using GraphPad Prism 4 software (GraphPad, San Diego, CA, USA). Binding constants of the cyclic nucleotide analogs were calculated by dividing the EC50 derivative concentrations by the EC50 values of the cAMP or cGMP standards. To define the percentage of cyclic nucleotide analog permeability, the calculated intracellular concentration was divided by the used extracellular concentration and then multiplied by the binding constant (Supplementary Tables 1 and 2).
For calculating intracellular analog concentrations, cell volumes of 5.2 fl for platelets and 1.5 pl for CHO cells were taken as a basis (Eigenthaler et al. 1992; Lu et al. 2003).
Results and discussion
Epitope mapping
A series of systematically modified cAMP and cGMP analogs (Supplementary Figs. S1 and S2) was used to map the essential molecular interactions between cAMP or cGMP, respectively, and the polyclonal antibodies supplied with the ENZO enzyme immunoassay (Supplementary Tables 1 and 2).
In solution, cAMP preferentially arranges in the anti-conformation, while analogs with bulky substituents at the 8-position (8-Br-cAMP) stabilize the molecule in the syn-conformation (Lassota et al. 1984). The reduced affinity of 8-Br-cAMP to the cAMP-EIA paratope indicates binding of the nucleotide to the cAMP antibody in the anti-conformation.
Due to formation of an intra-molecular H-bond between the phosphate ring and the 2-amino group, cGMP is stabilized and bound to the cGMP-EIA antibody in the syn-position. 8-Br-cGMP and 8-pCPT-cGMP further stabilize this conformation and thus exhibit stronger binding constants than cGMP.
Discrimination of cAMP and cGMP by substituents in 1- or 6-position is less pronounced as expected. Apparently, cGMP is recognized by the hydrogen bond accepting oxygen at the 6-position (6-SH-cGMP and 2-NH2-cPuMP show considerably reduced binding), while the hydrogen-bond donor NH2-group at position 6 in the cAMP molecule is not involved in antibody binding (only 20% reduced binding of cPuMP).
The Rp- and Sp-phosphorothioates (Rp/Sp-cGMPS; Sp-cAMPS) with an exocyclic sulphur, a substitution leading to increased size, polarisability and altered distribution of the negative charge (Frey and Sammons 1985), show a less pronounced binding than cGMP and cAMP, demonstrating the importance of the ionic interaction between antibody and cyclic phosphate moiety. Surprisingly, Rp-cAMPS (Supplementary Fig. S1, compound 7) does not show reduced binding (compare cAMP with Rp-cAMPS and 8-Br-cAMP with Rp-8-Br-cAMPS, respectively). It is concluded that the equatorial oxygen in cAMP and the corresponding sulphur in Rp-cAMPS/Rp-8-Br-cAMPS are recognized by the cAMP-EIA with similar, presumably weak and non-essential molecular interactions. In contrast, the axial sulphur in Sp-cAMPS obviously generates significant steric or electrostatic repulsion in a distinct region of the binding pocket. The decreased affinities observed for 2′-dcAMP and 2′-dcGMP are conditional upon the method. The 2′-deoxy modification blocks the acetylation desired at this position and therefore leads to reduced assay sensitivity. Without acetylation, 2′-dcGMP shows a moderately increased binding affinity (2.2-fold) compared to cGMP in the non-acetylated cGMP-EIA, while the relative binding values for 8-Br-cGMP and 8-Br-PET-cGMP were not affected (Table 1). A similar observation of reduced binding difference was made for cAMP and 2′-dcAMP with the non-acetylated cAMP-EIA (Table 1). Overall, acetylation enhances the sensitivity of the EIAs but does not affect the relative binding values obtained without acetylation.
Table 1.
Binding specificities of non-acetylated cGMP and cAMP analogs measured with the cGMP- and cAMP-EIA, respectively, from ENZO
| Analog | Absolute binding EC50 [M] non-acetylated | Relative binding EC50cGMP/EC50Analog | Relative binding EC50cAMP/EC50Analog |
|---|---|---|---|
| cGMP | 1.17 × 10−08 | 1.00 | |
| 2′-dcGMP (17) | 5.28 × 10−09 | 2.21 | |
| 8-Br-cGMP (15) | 1.83 × 10−09 | 6.40 | |
| 8-Br-PET-cGMP (22) | 1.36 × 10−07 | 0.08 | |
| cAMP | 4.2 × 10−09 | 1.00 | |
| 2′-dcAMP (6) | 1.9 × 10−08 | 0.22 |
The EC50 values represent means (SEM) of three experiments in duplicate
Cross-reactivities
The chemical structures of the cyclic nucleotide derivatives tested are shown in Supplementary Fig. S1 (cAMP analogs), Supplementary Fig. S2 (cGMP analogs) and Supplementary Fig. S3 (PDE inhibitors). Dose–response curves were prepared using different concentrations of cyclic nucleotide analogs (Supplementary Fig. S4).
In the cAMP-EIA, significant cross-reactivity was observed only with cPuMP and the PKA inhibitor Rp-cAMPS (Supplementary Table 1). A similar interference was also mentioned by Grazul-Bilka and colleagues. In their study, Rp-cAMPS and dibutyryl-cAMP, both were recognized by a cAMP-RIA from DuPont/NEN (Grazul-Bilska et al. 1996). Due to its low lipophilicity, Rp-cAMPS is not frequently used for in vivo experiments; however, studies with Rp-cAMPS in combination with cAMP determination are reported (Fan et al. 2009).
The most prominent competitors in the ENZO cGMP-EIA with superior binding constants compared to cGMP are 8-Br-cGMP and 8-pCPT-cGMP (Table 2 and Supplementary Table 2), two PKG activators frequently used in intact cells. The PKG activator 8-Br-PET-cGMP still exhibits 10% of the cGMP affinity to the antibody used in the assay. Occasionally, these compounds are used to investigate effects of PKG on PDE activity. In some studies, the measured intracellular cGMP levels are far too high and imply cross-reactivity of the applied cGMP analogs with the used immunoassays (Yoshioka et al. 2000; Bosgraaf et al. 2002). With regard to our data, the measurement of cGMP concentrations in the presence of 8-Br-cGMP or 8-pCPT-cGMP should be avoided with the ENZO cGMP-EIA, while the use of 8-Br-PET-cGMP is recommended. Conversely, this kit can be used to determine cellular uptake or secretion of 8-Br-cGMP and 8-pCPT-cGMP.
Table 2.
Relative binding specificities of cyclic nucleotide analogs measured with various cGMP and cAMP immunoassays as described under “Methods” section
| Analog | Specificity ENZO cAMP-EIA | Specificity IBL cAMP-RIA | Specificity ENZO cGMP-EIA | Specificity IBL cGMP-RIA | Specificity Cayman cGMP-EIA |
|---|---|---|---|---|---|
| cAMP | 1.00 | 1.00 | |||
| cGMP | 1.00 | 1.00 | 1.00 | ||
| 2′-dcGMP | 0.052 | ||||
| Rp-cGMPS | 0.0027 | 0.10 | |||
| 2′-dcAMP | 0.034 | ||||
| 8-Br-cGMP (15) | 4.90 | 0.20 | 0.005 | ||
| Rp-cAMPS (7) | 0.68 | 0.67 | |||
| 8-Br-cAMP (4) | 0.004 | 0.026 | |||
| Rp-8-Br-cAMPS (12) | 0.003 | ||||
| 6-MB-cAMP (1) | 0.004 | 0.012 | |||
| 6-Bnz-cAMP (2) | 0.006 | 0.009 | |||
| 8-pCPT-cGMP (16) | 2.40 | 0.30 | 0.0008 | ||
| 8-pCPT-cAMP (5) | 0.0005 | ||||
| 8-Br-PET-cGMP (22) | 0.10 | 0.015 | 0.016 | ||
| Rp-8-Br-PET-cGMPS (23) | 0.002 | 0.011 | |||
| 8-pCPT-2′-OMe-cAMP (10) | 0.0003 | 0.0066 | 0.0002 | <10−4 | |
| Sp-5,6-DCl-cBIMPS (11) | <10−4 | 0.005 | |||
| IBMX (26) | <10−5 | <10−4 | <10−6 | <10−6 | <10−5 |
The analogs are ordered according to their lipophilicity (see Table 4). Specificity is expressed as EC50cGMP or cAMP/EC50Analog. The absolute EC50 binding constants for acetylated cAMP in [M] are 7.62 × 10−10 (ENZO) and 5.8 × 10−9 (IBL); for acetylated cGMP in [M]: 2.27 × 10−10 (ENZO), 1.8 × 10−9 (IBL) and 3.9 × 10−10 (Cayman)
In consequence, we tested some of the interfering derivatives with a second cGMP-EIA (Cayman Chemical Company) and two radioactive immunoassays (IBL International). While the Cayman EIA assay is less sensitive to cGMP (1.0 pmol/ml) than the ENZO cGMP-EIA (0.01 pmol/ml), the specificity concerning cGMP analogs is superior: cGMP 1.00, 8-Br-PET-cGMP 0.16, 8-Br-cGMP 0.05 and 8-pCPT-cGMP 0.0008 (Table 2). The Cayman assay is therefore advantageous when measuring cGMP in the presence of 8-Br-cGMP and 8-pCPT-cGMP.
The cAMP- and cGMP-RIA from IBL exhibit lower cross-reactivities with the selected cNMP analogs than the ENZO assays; however, binding constants in the range of 20% for 8-Br-cGMP and 30% for 8-pCPT-cGMP with the cGMP-RIA as well as 66% for Rp-cAMPS with the cAMP-RIA are still far too high for measuring cAMP and cGMP concentrations in intact cells in the presence of theses cNMP analogs (Table 2).
The two nonspecific PDE inhibitors theophylline and IBMX as well as the PDE 2 inhibitor EHNA (Supplementary Fig. S3) do not interfere with any of the tested immunoassays (Table 2 and Supplementary Tables 1 and 2) at all. Therefore, measurement of cGMP and/or cAMP is feasible even if these inhibitors are used in the millimolar range sometimes required for experiments in intact cells for efficient PDE inhibition.
Also, the Epac activator 8-pCPT-2′-O-Me-cAMP does not cross-react with either immunoassay; however, inhibition of certain phosphodiesterases by 8-pCPT-2′-O-Me-cAMP can indirectly affect endogenous cGMP levels (Poppe et al. 2008).
Cell membrane permeability
Passive diffusion is the major route for cell permeation of most drugs and predominantly governed by lipophilicity, polarity, charge and size of the respective molecule. We have selected cyclic nucleotide analogs covering a wide range in lipophilicity (log K w; Krass et al. 1997; http://www.biolog.de/technical-info/lipophilicity-data/) and determined their cell membrane permeability with human platelets and CHO cells (Table 3).
Table 3.
Cell membrane permeability and activation constants for selected cyclic nucleotide analogs
| Analog | cNMP conc. inside platelets (% of external) | cNMP conc. inside CHO cells (% of external) | Lipophilicitya log K w | Activationb PKG Iβ K a (μM) | Activationb PKA II K a (μM) |
|---|---|---|---|---|---|
| cGMP | 0.77 | 0.9 | |||
| 2′-dcGMP (17) | 0.5 ± 0.1 | 0.3 ± 0.5 | 0.66 | 21 | |
| 8-Br-cGMP (15) | 12.1 ± 3.5 | 13.5 ± 11.5 | 1.17 | 1.0 | |
| 8-pCPT-cGMP (16) | 19.6 ± 4.7 | n/d | 2.52 | 0.9 | |
| 8-Br-PET-cGMP (22) | 30.9 ± 5.7 | 24.1 ± 9.7 | 2.83 | 0.009 | |
| cAMP | 1.09 | 0.08 | |||
| 2′-dcAMP (6) | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.97 | n/d | |
| 8-Br-cAMP (4) | n/d | n/d | 1.35 | 0.09 | |
| Sp-5,6-DCl-cBIMPS (11) | n/d | n/d | 2.99 | 0.12 |
For cyclic nucleotides with a molecular weight of about 350 ± 150, membrane diffusion without restriction is assumed (Camenisch et al. 1998). In general, the intracellular cyclic nucleotide concentrations measured confirm the expected correlation of lipophilicity and permeability (Table 4). Compounds with low lipophilicity (log K w < 1) are too hydrophilic for any diffusion into the cell (2′-dcGMP, 2′-dcAMP). At a log K w ≈ 1–2, about 12% of the extracellularly applied cyclic nucleotide concentration is detected inside platelets and CHO cells (8-Br-cGMP and Rp-cAMPS). At higher lipophilicity (log K w ≈ 2.5), passive diffusion increases, and intracellular cyclic nucleotide concentration raises up to 20% (8-pCPT-cGMP) and even 30% (8-Br-PET-cGMP; log K w = 2.83) of the external concentration. The relative lipophilicities of the analogs calculated from the experimentally obtained log K w data (Table 4) correlate linearly with the amount permeated (r 2 = 0.99; shown for cGMP scale in Fig. 1a). Extrapolation of this linear approximation predicts 45% diffusion for Rp-8-Br-PET-cGMPS, a compound with log K w ≈ 3 (relative lipophilicity, 200). However, this analog does not bind to the cGMP antibody in the EIA and therefore could not be tested to substantiate/confirm this assumption. With this linear model of relative lipophilicities, the actual intracellular concentration of a cyclic nucleotide can be predicted from its known lipophilicity.
Table 4.
Lipophilicity and cell membrane permeability of cyclic nucleotide analogs
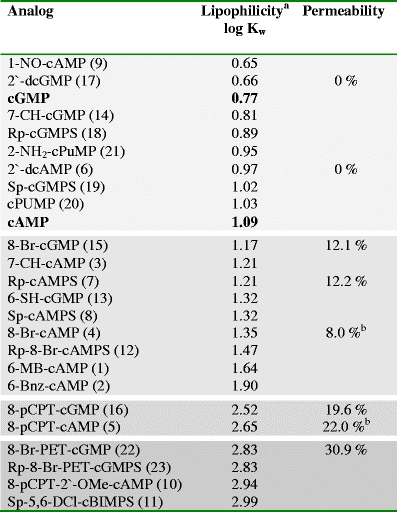
Fig. 1.
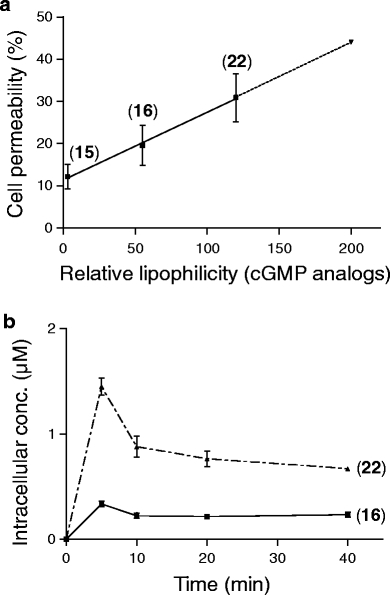
a Effect of cyclic nucleotide lipophilicity on cell membrane permeability in platelets. Error bars represent SD of three independent experiments. Shown are the data for 8-Br-cGMP (analog 15), 8-pCPT-cGMP (analog 16) and 8-Br-PET-cGMP (analog 22). b Time-dependent influx of cyclic nucleotides. Platelets were incubated with 3nM 8-pCPT-cGMP (analog 16) or 8-Br-PET-cGMP (analog 22) for the time points indicated, and the intracellular analog concentration was measured. Error bars represent SD of two independent experiments in duplicate
Comparable data were obtained in an earlier study analyzing cAMP analog permeation in rat C6 glioma cells. Incubation with 8-Br-cAMP (log K w = 1.35) revealed an intracellular concentration of about 8%, while the more lipophilic 8-pCPT-cAMP (log K w = 2.65) reached 22% of the applied extracellular concentration (Bartsch et al. 2003).
The equilibrium of inside/outside concentration of cNMPs fairly resistant to intracellular metabolism like 8-Br-cGMP (16) or 8-pCPT-cGMP (22) is achieved after 10–20 min (Fig. 1b). Longer incubation times (up to 60 min) did not considerably increase the intracellular concentration of the analog (not shown). Hence, incubation times of 20 min are sufficient to ensure adequate loading; however, we never observed an intracellular concentration close to the external concentration (assuming a bidirectional analog passage). The molecular basis for this apparent imbalance might be explained by an active transport of cyclic nucleotide analogs from the cytosol into the extracellular environment (Boadu et al. 2001). This process of cellular cAMP and cGMP secretion by an apical plasma membrane transporter or by members of the multidrug resistance protein family (MRP4 and MRP5) against concentration gradients was reported in various cells like hepatocytes, vascular smooth muscle cells, epithelial cells, neuronal cells and platelets (Sager and Ravna 2009). This unidirectional ATP-activated process is analog- and concentration-dependent, and thus might explain for the observed intracellular cNMP accumulation during the first 5 min (Fig. 1b) before active extrusion commences.
Overall, the intracellular levels of externally applied cyclic nucleotides depend on more than simple membrane permeability; however, independent of the occurring membrane processes, our data depict a correlation between cNMP lipophilicity and intracellular accumulation that can be used for the design of in vivo experiments.
Western blot analysis
Western blot analysis of vasodilator-stimulated phosphoprotein (VASP), which is highly expressed in platelets, was selected to prove the correlation between lipophilicity and cell membrane permeability of cyclic nucleotide analogs. VASP is one of the most prominent substrates of cAMP- and cGMP-dependent protein kinase (PKA II and PKG Iβ, respectively; Butt et al. 1994), which in turn are major targets of cAMP and GMP in platelets.
8-pCPT-cGMP (K a = 0.9 μM) and 8-Br-cGMP (K a = 1.0 μM) show similar PKG Iβ activation constants but exhibit different lipophilicities (Table 3). After 20 min, maximal VASP phosphorylation is achieved with 50 μM 8-pCPT-cGMP (log K w = 2.52; permeability ≈ 20%), while for 8-Br-cGMP (log K w = 1.17, permeability ≈ 10%), 200 μM is required to achieve the same effect (Fig. 2a). For 8-Br-PET-cGMP (permeability ≈ 30%; K a = 0.009 μM), even 0.25 μM are sufficient to activate PKG in human platelets (Fig. 2a). In contrast, no VASP phosphorylation is observed with 2′-dcGMP (K a = 21 μM, log K w = 0.66) at concentrations up to 2 mM due to the lack of cell membrane permeability of this compound (Fig. 1b).
Fig. 2.
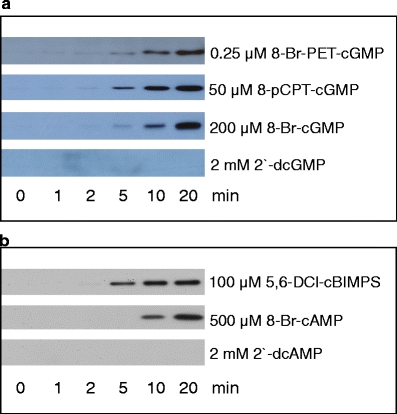
Cyclic nucleotide stimulated VASP phosphorylation in intact human platelets. Cells were incubated with different concentrations of cyclic nucleotide analogs for the time points indicated. Proteins were separated by SDS-PAGE and subjected to western blot analysis for VASP phosphorylation with PKG-specific pVASP antibody 16C2 (a) or PKA-specific pVASP antibody 5C6 (b)
For PKA activation experiments, the compounds Sp-5,6-DCl-cBIMPS and 8-Br-cAMP were selected. Both analogs exhibit similar activation constants but different lipophilicities (Table 3). To detect comparable VASP phosphorylation, only 100 μM of the more lipophilic compound Sp-5,6-DCl-cBIMPS (K a = 0.12 μM, log K w = 2.99) is needed, while for 8-Br-cAMP (K a = 0.09 μM, log K w = 1.35), 500 μM has to be applied (Fig. 2b). Similar to 2′-dcGMP, no VASP phosphorylation is seen with the impermeable compound 2′-dcAMP (Fig. 2b).
In summary, with the exception of Rp-cAMPS, 8-Br-cGMP and 8-pCPT-cGMP, all analogs used in this study do not interfere with the tested cAMP and cGMP enzyme immunoassays from ENZO. For higher specificity, the Cayman cGMP-EIA is suggested. Overall, when assessing cAMP and cGMP levels in the presence of cNMP analogs, cross-reactivity tests with the used immunoassays used are strongly recommended.
Data acquired with immunoassays for cyclic nucleotides in the presence of cyclic nucleotide derivatives should be taken with great care, and conclusions based on these data carefully reappraised in the light of this study.
The amount of intracellularly accumulated analog was found to be 10–30% of the extracellular applied concentration, predominantly depending on the lipophilicity index of the respective compound. Experiments with cyclic nucleotide analogs in living cells should be designed, taking these observations into account. Our data provide a useful basis for scientists in the field of cyclic nucleotide regulation in living cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 159 kb)
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bartsch M, Zorn-Kruppa M, Kuhl N, Genieser HG, Schwede F, Jastorff B. Bioactivatable, membrane-permeant analogs of cyclic nucleotides as biological tools for growth control of C6 glioma cells. Biol Chem. 2003;384(9):1321–1326. doi: 10.1515/BC.2003.148. [DOI] [PubMed] [Google Scholar]
- Boadu E, Vaskinn S, Sundkvist E, Jaeger R, Sager G. Inhibition by guanosine cyclic monophosphate (cGMP) analogues of uptake of [(3)H]3′,5′-cGMP without stimulation of ATPase activity in human erythrocyte inside-out vesicles. Biochem Pharmacol. 2001;62(4):425–429. doi: 10.1016/S0006-2952(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Russcher H, Snippe H, Bader S, Wind J, Van Haastert PJ. Identification and characterization of two unusual cGMP-stimulated phoshodiesterases in dictyostelium. Mol Biol Cell. 2002;13(11):3878–3889. doi: 10.1091/mbc.E02-05-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269(20):14509–14517. [PubMed] [Google Scholar]
- Camenisch G, Alsenz J, van de Waterbeemd H, Folkers G. Estimation of permeability by passive diffusion through Caco-2 cell monolayers using the drugs' lipophilicity and molecular weight. Eur J Pharm Sci. 1998;6(4):317–324. [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- Eigenthaler M, Nolte C, Halbrugge M, Walter U. Concentration and regulation of cyclic nucleotides, cyclic-nucleotide-dependent protein kinases and one of their major substrates in human platelets. Estimating the rate of cAMP-regulated and cGMP-regulated protein phosphorylation in intact cells. Eur J Biochem. 1992;205(2):471–481. doi: 10.1111/j.1432-1033.1992.tb16803.x. [DOI] [PubMed] [Google Scholar]
- Fan P, Jiang Z, Diamond I, Yao L. Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol. 2009;76(3):526–533. doi: 10.1124/mol.109.057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey PA, Sammons RD. Bond order and charge localization in nucleoside phosphorothioates. Science. 1985;228(4699):541–545. doi: 10.1126/science.2984773. [DOI] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Reynolds LP, Kirsch JD, Redmer DA. Gap junctional intercellular communication of bovine luteal cells from several stages of the estrous cycle: effects of cyclic adenosine 3′,5′-monophosphate. Biol Reprod. 1996;54(3):538–545. doi: 10.1095/biolreprod54.3.538. [DOI] [PubMed] [Google Scholar]
- Horton JK, Martin RC, Kalinka S, Cushing A, Kitcher JP, O'Sullivan MJ, Baxendale PM. Enzyme immunoassays for the estimation of adenosine 3′,5′ cyclic monophosphate and guanosine 3′,5′ cyclic monophosphate in biological fluids. J Immunol Methods. 1992;155(1):31–40. doi: 10.1016/0022-1759(92)90268-X. [DOI] [PubMed] [Google Scholar]
- Krass JD, Jastorff B, Genieser HG. Determination of lipophilicity by gradient elution high performance liquid chromatography. Anal Chem. 1997;69:2575–2581. doi: 10.1021/ac961246i. [DOI] [PubMed] [Google Scholar]
- Lassota P, Stolarski R, Shugar D. Conformation about the glycosidic bond and susceptibility to 5′-nucleotidase of 8-substituted analogues of 5′-GMP. Zeitschrift fur Naturforschung. 1984;39(1–2):55–63. doi: 10.1515/znc-1984-1-210. [DOI] [PubMed] [Google Scholar]
- Lohmann SM, Walter U. Tracking functions of cGMP-dependent protein kinases (cGK) Front Biosci. 2005;10:1313–1328. doi: 10.2741/1621. [DOI] [PubMed] [Google Scholar]
- Lu S, Sun X, Shi C, Zhang Y. Determination of tricarboxylic acid cycle acids and other related substances in cultured mammalian cells by gradient ion-exchange chromatography with suppressed conductivity detection. J Chromatogr A. 2003;1012(2):161–168. doi: 10.1016/S0021-9673(03)01134-8. [DOI] [PubMed] [Google Scholar]
- Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100(3):309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5(4):277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Sager G, Ravna AW. Cellular efflux of cAMP and cGMP—a question about selectivity. Mini Rev Med Chem. 2009;9(8):1009–1013. doi: 10.2174/138955709788681654. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Yamaya Y, Saiki S, Kanemoto M, Hirose G, Pleasure D. Cyclic GMP/cyclic GMP-dependent protein kinase system prevents excitotoxicity in an immortalized oligodendroglial cell line. J Neurochem. 2000;74(2):633–640. doi: 10.1046/j.1471-4159.2000.740633.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 159 kb)


