Abstract
Background
During senescence, despite the loss of strength (force-generating capability) associated with sarcopenia, muscle endurance may improve for isometric contractions.
Purpose
The purpose of this study was to perform a systematic meta-analysis of young versus older adults, considering likely moderators (ie, contraction type, joint, sex, activity level, and task intensity).
Data Sources
A 2-stage systematic review identified potential studies from PubMed, CINAHL, PEDro, EBSCOhost: ERIC, EBSCOhost: Sportdiscus, and The Cochrane Library.
Study Selection
Studies reporting fatigue tasks (voluntary activation) performed at a relative intensity in both young (18–45 years of age) and old (≥55 years of age) adults who were healthy were considered.
Data Extraction
Sample size, mean and variance outcome data (ie, fatigue index or endurance time), joint, contraction type, task intensity (percentage of maximum), sex, and activity levels were extracted.
Data Synthesis
Effect sizes were (1) computed for all data points; (2) subgrouped by contraction type, sex, joint or muscle group, intensity, or activity level; and (3) further subgrouped between contraction type and the remaining moderators. Out of 3,457 potential studies, 46 publications (with 78 distinct effect size data points) met all inclusion criteria.
Limitations
A lack of available data limited subgroup analyses (ie, sex, intensity, joint), as did a disproportionate spread of data (most intensities ≥50% of maximum voluntary contraction).
Conclusions
Overall, older adults were able to sustain relative-intensity tasks significantly longer or with less force decay than younger adults (effect size=0.49). However, this age-related difference was present only for sustained and intermittent isometric contractions, whereas this age-related advantage was lost for dynamic tasks. When controlling for contraction type, the additional modifiers played minor roles. Identifying muscle endurance capabilities in the older adult may provide an avenue to improve functional capabilities, despite a clearly established decrement in peak torque.
Although it is well recognized that sarcopenic changes in aging adults result in diminished muscle mass and subsequent loss of strength (force-generating capacity),1,2 it is less clear how aging affects the properties of muscle fatigue. A greater understanding of muscle fatigue capabilities across the life span may influence clinical decision making and affect therapeutic exercise prescription.
Although older adults may be commonly perceived as fatiguing more readily, resistance to muscle fatigue actually may improve with age.3 Perceptions of fatigue may be reported as a “feeling of tiredness” or “lack of energy,”4 which can be distinct from muscle fatigue, defined as, “any exercise-induced reduction in the ability to exert muscle force or power, regardless of whether or not the task can be sustained.”5(p691) Several studies have been performed to assess differences in resistance to muscle fatigue in young adults versus old adults. However, to date, these data have not been systematically compiled to determine whether older adults indeed have consistently greater muscle endurance than young adults and which factors may influence these age-related differences.
Muscle fatigue can vary greatly within and between individuals due to the complex nature of fatigue. That is, muscle fatigue capabilities can vary between contraction types (isometric versus isokinetic),6 joints or muscle groups,7 task intensities,8 and position-matching versus force-matching paradigms.9 In addition, muscle fatigue may differ between men and women10,11 or with physical activity status.12 These factors may have the potential to influence age-related muscle fatigue properties. To date, only small-scale reviews have provided insights into subsets of the research available to ascertain age-related differences in muscle fatigue properties.3,13
Despite the number of available studies on muscle fatigue, our understanding of age-related changes in fatigue remains incomplete. Properly indentifying capabilities in older adults may affect dose-response relationships and modify therapeutic exercise interventions. Thus, the purpose of this study was to characterize differences in muscle fatigue between young and old adults using systematic meta-analysis techniques to compile the available literature. Effect sizes were used to assess the degree to which young or old adults were more fatigable considering all data, as well as preplanned subgroupings based on contraction type, sex, joint region, task intensity, and physical activity levels, when possible.
Method
Database Review
A 2-stage systematic review of the literature was used to identify studies on muscle fatigue including both old and young adults. Stage 1 involved searches of the following databases: PubMed (1948 to June 28, 2010), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1937 to June 28, 2010), PEDro (1929 to June 28, 2010), EBSCOhost: ERIC (1966 to June 28, 2010), EBSCOhost: Sportsdiscus (1888 to June 28, 2010), and The Cochrane Library (1993 to June 28, 2010). A total of 11 search terms and key word combinations were used to elicit relevant articles, including: “endurance,” “fatigue,” “aging adult,” “older adult,” “intermittent fatigue,” “isokinetic fatigue,” and “isometric fatigue.” For example, a search performed in PubMed (accessed October 5, 2009) using the key words “aging” and “fatigue” yielded 600 related articles. The inclusion and exclusion criteria (see below) were used to include studies providing young versus old adult muscle fatigue data. Stage 2 involved reviewing bibliographies of studies meeting the inclusion criteria of stage 1 to find additional relevant fatigue studies. All abstracts were first screened for studies that reported the performance of a relative-intensity fatigue task, including young and old adult cohorts. These studies then were retrieved in full text and reviewed by both authors to ensure agreement on inclusion and exclusion criteria, and all entered data were reviewed twice against the original articles to decrease the likelihood of transcription errors.
Inclusion and Exclusion Criteria
The following criteria were used for study inclusion: human participants who were healthy; young cohort mean age between 18 and 45 years and older cohort mean age ≥55 years; sustained isometric, intermittent isometric, isokinetic, or isotonic tasks using relative intensities based on maximum voluntary contraction (% MVC); outcome measures of either time to task failure (ie, endurance time) or reduction in peak torque (ie, fatigue index); single-joint involvement (per fatigue task); and publication in English. Studies were excluded if they used electrical stimulation to elicit fatigue, simultaneous multijoint testing, or functional tasks that did not assess torque as a percentage of the maximum value or that used body or limb weight as the primary resistance (eg, Sorensen test). In addition, if variance information (eg, standard deviation) was not reported or was unattainable from the authors, studies were excluded. Inclusion and exclusion criteria did not account for athletic training status or level of physical activity, but when reported, this information was utilized.
Quality assessment of included studies did not require the traditional approaches used for meta-analyses to assess interventions, such as blinded investigators, placebo-controls, or random assignment. Rather, the powerful statistical application of meta-analysis was used with observational studies to systematically compile the data available to better distinguish fatigue capabilities in the older versus younger adults, considering several possible moderating variables.
Outcome Variables
To characterize differences in muscle fatigue between 2 groups, protocols typically utilize relative-intensity tasks (% MVC) to standardize task demands between individuals. Muscle fatigue properties are assessed indirectly, either by the duration a relative-intensity task can be sustained (ie, endurance time) or the percentage of baseline peak force remaining following the performance of a task for a preset duration. Most studies reported only 1 of these 2 outcome variables, but occasionally those involving intermittent tasks reported both. When this occurred, only the percentage of change in peak force was used in the meta-analysis, as this was the preferred outcome variable reported for intermittent tasks. Greater muscle fatigue is observed as shorter endurance times or lower percentages of baseline torque values. Endurance time usually is reported as the total duration a relative-intensity task can be maintained until the target muscle torque falls to 5% to 10% below target levels. Only acute muscle fatigue was assessed in this study (ie, immediate or short-term outcomes, rather than long-duration decrements in force-producing capability associated with low-frequency fatigue). Relevant endurance or fatigue data reported only in graphic form were extracted using pixel analysis (Adobe Photoshop*) to determine the respective numerical values. Means and standard deviations for young and old cohorts were recorded for each pair of data.
Moderating Variables
Additional study information was recorded for analysis, including sample size, sex, mean age for each cohort (young, old), standardized task intensity (from 1% to 100% of maximum), contraction type (sustained isometric, intermittent isometric, and isokinetic), joint region tested (eg, ankle, back, elbow), joint angle, torque direction (eg, flexion, extension), and physical activity level (when reported). When outcome measures were not reported separately by sex and were unavailable following attempts to contact the corresponding authors, the data were coded simply as “mixed sex.” Contractions were classified as 1 of 3 types: 2 static (isometric with [sustained] or without [intermittent] rest intervals) and 1 dynamic (isokinetic). Task intensity was categorized as low (≤33% of maximum), moderate (34%–66% of maximum), or high (≥67% of maximum), regardless of contraction type. Given the varying methods for quantifying physical activity, data were dichotomized to active or inactive when available.
When necessary, the data for multiple age cohorts (eg, for 20–29 and 30–39 years) were combined using weighted means and pooled standard deviations.14 When multiple task intensities (eg, 30% and 50% of maximum) or joint regions (eg, ankle and elbow) were reported in a single manuscript, data for each fatigue task were included (in separate rows) rather than combining them into a single mean or selecting only one task per study. We chose this strategy to minimize any potential self-selection bias or missing potential effects due to joint or intensity factors. Although this approach allows for multiple measures that are not fully independent, particularly for observational studies, it is challenging to determine whether independence is ensured across publications (by the same authors). That is, the same patient population may be recruited for studies reported in multiple publications.
Statistical Analyses
Effect size is the standardized mean difference between 2 populations (ie, young versus old). Hedges' g was chosen as the best effect size estimate due to its correction for slight overestimations that may occur with small samples.15 Mean effect sizes (and associated variances) across studies were calculated (Comprehensive Meta-Analysis†) using a mixed-effects model determined a priori (random and fixed effects). A random-model approach was chosen under the guise of generalizing results among the older population and inherent inequality of effect sizes across studies. A fixed subgroup analysis assumes results will generalize to the specific variables of interest such as contraction type (eg, sustained isometric, intermittent isometric, isokinetic), intensity, and so on. Results are presented such that positive effect size values indicate older adults are more resistant to fatigue, whereas negative effect size values indicate young adults are more resistant to fatigue.
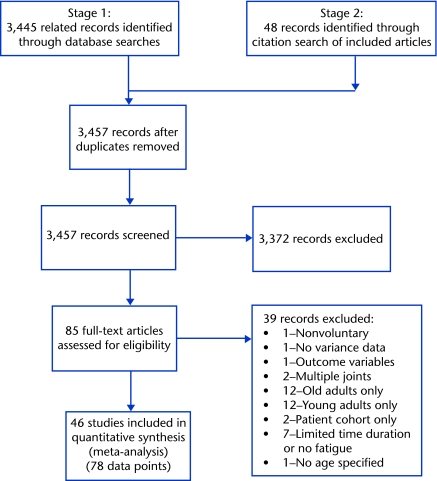
Analyses were stratified into 3 levels (Fig. 1), with preplanned subgrouping categories. A specific subgroup was included in comparisons only if it included data from a minimum of 3, separate published studies; thus, the final set of subgroups was determined by the data available.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the literature search.
The level I analysis determined a single composite effect size for resistance to fatigue for old versus young groups, including all data points with no subgroups. Level II analyses included subgrouping by single individual categories (sex, contraction type, intensity, joint tested, and activity level) to the extent sufficient data were available. Level III analyses involved further subgrouping the contraction types from level II, such as comparing intensity levels, sex, or joints within each contraction type (eg, interaction or moderated effects), when sufficient data were available. Level III subgroupings were considered only when sufficient data were available (ie, 3 or more independent studies).
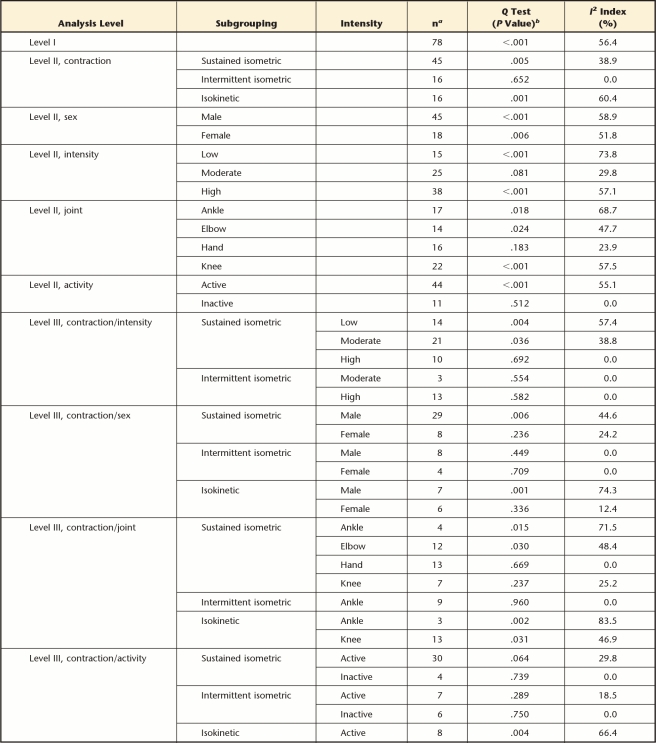
The goal of a meta-analysis is to not only compute summary effect sizes but also determine the extent of variation present in the true effect size (ie, heterogeneity), suggesting whether additional moderating variables are involved. Heterogeneity was quantified via the I2 index and the Q test.15 A significant Q statistic indicates only the presence of heterogeneity among the data included, whereas the I2 index is better able to quantify the magnitude of the heterogeneity.16 We operationally defined the magnitude of heterogeneity as low (I2≤33%), moderate (34%≤I2<67%), and high (I2≥67%), based on previous suggestions.17 Heterogeneity estimates were evaluated at each level of analysis.
Results are reported as mean summary effect sizes (with P values) for each subgroup analysis. A more stringent alpha level than conventionally used (α≤.01) was chosen to minimize both type I and II errors.18 Observational studies are less likely than interventional studies to be adversely affected by publication bias, as identifying a fatigue advantage in either direction would be deemed a valuable scientific contribution. Furthermore, several studies investigated other aspects of muscle fatigue, such that the data relevant to this meta-analysis were not necessarily the primary outcomes (accordingly, lack of age-related fatigue differences would not influence likelihood of publication). Therefore, our analyses were not further extended for publication bias within or between studies.
Results
Database Review
Initial search strategies (stages 1 and 2) resulted in 3,457 potential studies (after duplicates were removed), with 46 studies meeting all inclusion criteria (Fig. 1). Several studies reported on more than one joint or muscle group, contraction type, or task intensity, for a total of 78 young adult versus old adult fatigue comparisons (ie, individual effect sizes) to analyze. The numbers of data points per subgroup comparison are detailed in the Table.
Table.
Summary of Heterogeneity Statistics for Each Subgroup Analysis
a Number of data points per analysis level.
b P values are uncorrected for multiple comparisons.
Level I: Overall Age Effect
The level I analysis, using all 78 individual effect sizes, revealed that older adults were significantly more resistant to acute muscle fatigue (greater muscle endurance) than young adults, with a moderate mean effect size of 0.49 (95% confidence interval=0.35–0.63). Forest plots showing individual effect sizes by contraction type are presented in Figures 2, 3, and 4. Figure 5 illustrates the effect sizes for each analysis level. One isotonic effect size was included in the overall analysis, but was not extended to a separate contraction-type subgroup due to a lack of comparative studies.
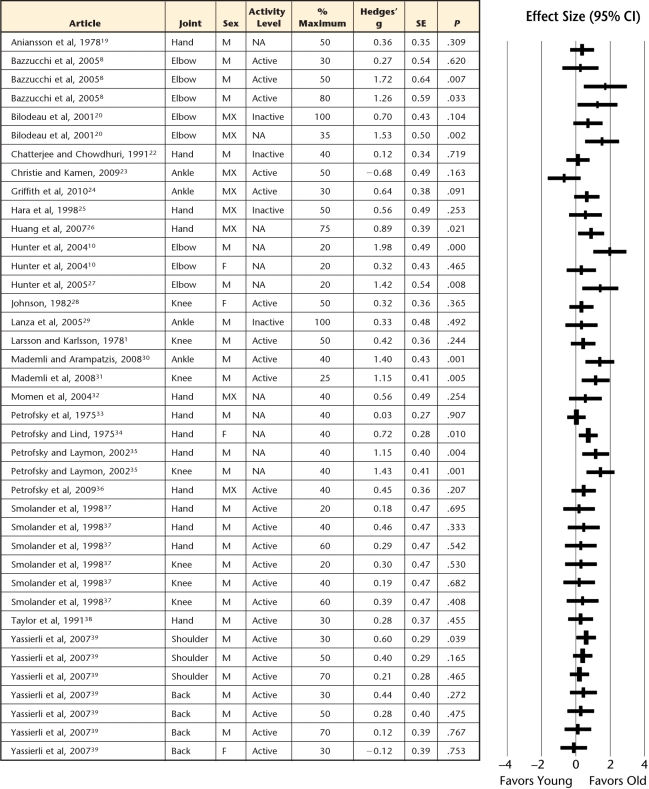
Figure 2.
Forest plot of individual effect sizes for sustained isometric contractions only, with their corresponding subgrouping categories for sex, joint, task intensity, and physical activity level. Positive effect sizes indicate greater fatigue resistance for older adults, whereas negative effect sizes indicate greater fatigue resistance for younger adults. SE=standard error, CI=confidence interval, M=male, F=female, MX=mixed, NA=not available.
Figure 3.
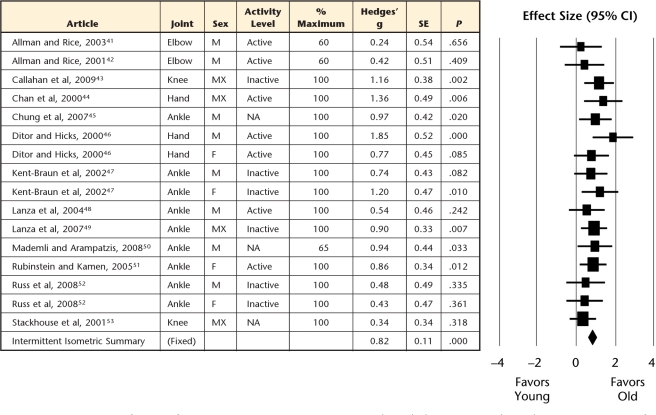
Forest plot of individual effect sizes for intermittent isometric contractions only, with their corresponding subgrouping categories for sex, joint, task intensity, and physical activity level. Positive effect sizes indicate greater fatigue resistance for older adults, whereas negative effect sizes indicate greater fatigue resistance for younger adults. SE=standard error, CI=confidence interval, M=male, F=female, MX=mixed, NA=not available.
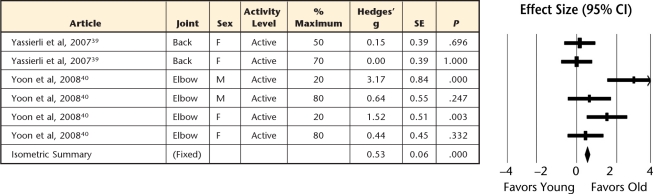
Figure 4.
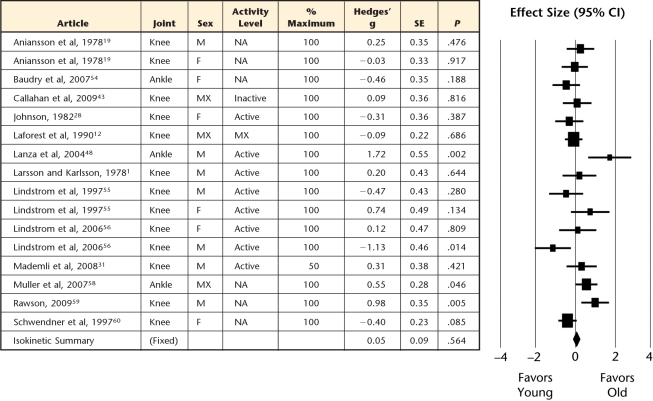
Forest plot of individual effect sizes for isokinetic contractions only, with their corresponding subgrouping categories for sex, joint, task intensity, and physical activity level. Positive effect sizes indicate greater fatigue resistance for older adults, whereas negative effect sizes indicate greater fatigue resistance for younger adults. SE=standard error, CI=confidence interval, M=male, F=female, MX=mixed, NA=not available.
Figure 5.
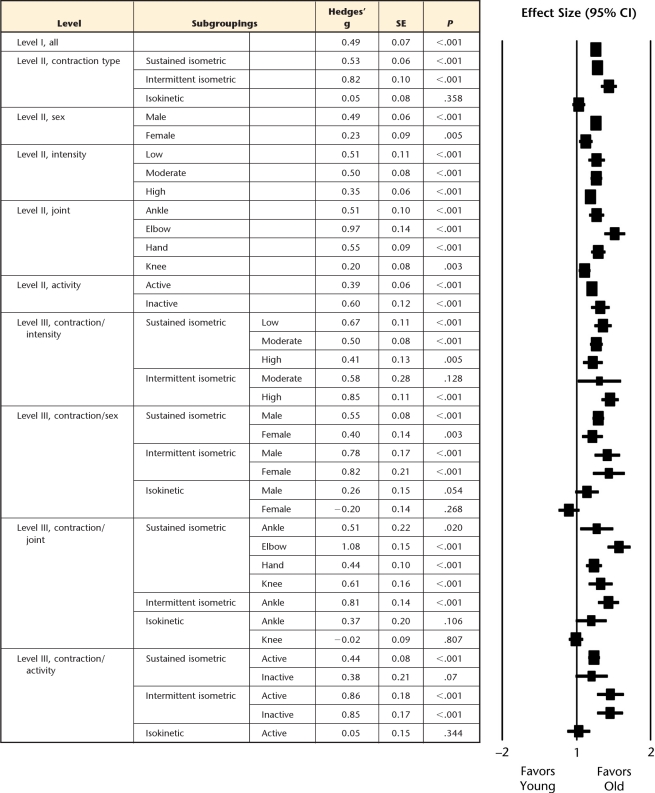
Forest plot of summary effect sizes for each subgrouping category (level I–III). Positive effect sizes indicate greater fatigue resistance for older adults, whereas negative effect sizes indicate greater fatigue resistance for younger adults. SE=standard error, CI=confidence interval.
Level II Subgroups
Contraction type.
Older adults demonstrated greater muscle fatigue resistance (ie, more endurant) for both sustained (Fig. 2) and intermittent (Fig. 3) isometric contractions, but the intermittent tasks showed the greatest age-related advantage compared with sustained tasks (effect size=0.82 versus 0.52, P=.009; Fig. 5). However, for dynamic contractions, no age-related difference in muscle fatigue was observed (effect size=0.05, Fig. 4).
Sex.
Older adults of both sexes were more fatigue resistant than younger adults. This age-related advantage, however, was greater for men than for women (P=.009) when not accounting for any additional moderating factors (Fig. 5).
Joint.
Older adults were significantly more fatigue resistant than young adults across all joint region subgroups assessed (ie, ankle, elbow, hand, and knee joint regions had sufficient data available). However, these effect sizes differed among joints (P<.008), with the exception of the ankle versus the hand joint regions (P=.42). The largest effect size was observed at the elbow and the smallest effect size was observed at the knee, when not accounting for any additional factors (Fig. 5). However, the elbow joint tasks comprised solely static contraction protocols, whereas the knee included both isometric and isokinetic testing (see level III subgrouping below).
Intensity.
Older adults were more resistant to fatigue across all intensity levels (low, moderate, and high) (Fig. 5). Although effect sizes decreased with increasing intensity (ie, the fatigue advantage with advancing age decreased at higher intensities), none of the differences achieved significance (P>.067) (Fig. 5).
Physical activity.
Older adults were more resistant to fatigue across active and inactive cohorts, with the difference in effect sizes between subgroups just beyond significance (P=.063).
Level III Analyses
Contraction type × intensity.
Although task intensity moderated the age-related fatigue advantage overall (see level II above), this effect was lost or reversed when controlling for contraction type (Fig. 5). For sustained isometric contractions, older adults remained more fatigue resistant than young adults across all intensities (low, moderate, and high), with no significant difference exhibited among intensity subgroups (P≥.07). Conversely, for intermittent isometric contractions, no significant age differences occurred for moderate intensities, whereas a large effect size was observed for high intensities (insufficient low-intensity, intermittent task data available). Although these findings demonstrate opposing influences of intensity on intermittent tasks than observed with the sustained isometric tasks (or overall in level II), only 3 of the 16 intermittent tasks were performed at a moderate intensity.
Contraction type × sex.
Although sex was a significant moderator of the age-related endurance advantage in the level II analyses, it was not a significant moderator when considering each contraction type separately. Older adults (both men and women) remained more fatigue resistant for sustained and intermittent isometric tasks, but did not differ between sexes (P>.17) (Fig. 5). Furthermore, no age-related advantage was observed in either men or women (effect sizes not significantly different than zero) for the isokinetic contractions.
Contraction type × joint.
Similarly, further subgrouping contraction type by joint region slightly altered the findings from the previous level II analyses. Within sustained isometric contractions, older adults were more fatigue resistant across the joints considered (elbow, hand, and knee joint regions, with the ankle just surpassing our stringent critical value). However, only the elbow continued to result in significantly larger effect sizes than the remaining joints, with the knee now exhibiting effect sizes similar to those of the hand and ankle (Fig. 5). For intermittent isometric contractions, the ankle (only subgroup possible) demonstrated significant age-related advantages in muscle fatigue. During isokinetic contractions, neither the ankle nor the knee (the only joints with sufficient data) demonstrated any age-related advantage (or disadvantage) in fatigue resistance (P≥.06). Overall, joint region had only mild moderating influences on the fatigue differences observed between young versus old adults when controlling for contraction type.
Contraction type × physical activity.
Physical activity did not substantially alter the previous contraction-type subgroups (Fig. 5). Older adults were significantly more fatigue resistant across each combination of sustained and intermittent isometric contraction types and activity levels except for the inactive, sustained isometric group, which likely was underpowered (n=4, effect size=0.38, P=.07). The age-related fatigue advantage did not differ significantly between the active and inactive groups for isometric or intermittent tasks (P>.40).
Heterogeneity
Overall, heterogeneity was categorized as low to moderate for all levels of the meta-analysis (Tab. 1). The proportion of subgroups categorized with low heterogeneity increased from 28.6% for level II analyses to 60.9% for level III analyses. The increased proportion of low heterogeneity with additional subgroup analyses suggests that several moderators identified in this analysis (eg, contraction type, joint, intensity) contributed to variations in age-related fatigue resistance. Although the level III heterogeneity increased at the ankle for both sustained and isokinetic contractions, the limited number of data points (4 and 3, respectively) demonstrated the difficulty in attaining a consistent summary effect size. Additional data are needed to fully characterize age-related fatigue differences.
Discussion
This is the first study to systematically compile outcomes data to characterize age-related differences in muscle fatigue considering several potential moderating variables: contraction type, intensity, sex, joint region, and activity level. The primary finding of this meta-analysis is that muscle fatigue resistance is enhanced with age for relative-intensity tasks when additional intrinsic and extrinsic factors are not considered (level I analysis). This age-related advantage in fatigue resistance occurred for both sustained and intermittent isometric contractions, but is lost for isokinetic contractions.
Improved fatigue resistance with advancing age is consistent with several reported changes in muscle properties with aging. A preferential atrophy of type II fibers1,61 and preferential loss of fast motor units2 have been observed with advancing age and sarcopenia. These changes would result in a greater proportion of type I or slow, oxidative fibers, which may account for greater fatigue resistance during relative-intensity tasks (ie, tasks standardized to maximum strength). However, this adaptation did not prove beneficial under all conditions (ie, dynamic tasks).
Level II and III analyses revealed older adults were more endurant than young adults for sustained and intermittent isometric (static) contractions, but not for isokinetic (dynamic) contractions. This result is somewhat surprising, as we anticipated the intermittent isometric contractions to behave similarly to isokinetic contractions, as greater muscle reperfusion, replenishment of oxygenated blood, and removal of metabolic wastes might be facilitated under both conditions. To the contrary, the intermittent tasks resulted in the greatest age-related fatigue advantage, whereas isokinetic tasks showed no age-related differences. Thus, the inclusion of rest intervals, and accordingly muscle reperfusion, does not appear to be the key variable, but rather the contraction type itself appears to be of importance in age-related endurance changes. One explanation may be that the proportional shift toward type I fibers and the slowing of both contraction and relaxation times that occurs with aging may cause a leftward shift in the force-frequency curve62 and a leftward and downward shift in the force-velocity curve.63 Thus, although the muscle fibers are slower and rely on greater oxidative energy sources, they may be less able to maintain power (ie, force × velocity) over time. These adaptations may enable the older adult to be more fatigue resistant for isometric contractions (slower, oxidative fibers), but not during dynamic contractions, where impaired power generation would be expected to have its greatest impact.
Anecdotal perceptions of muscle fatigue increasing with advancing age are in opposition to the controlled research findings of greater fatigue resistance with aging. This finding may be partially explained by the differences observed between static and dynamic tasks, as many functional tasks (eg, sit-to-stand maneuver, ambulation) require dynamic rather than static contractions. However, even with dynamic tasks, older adults are not disadvantaged; thus, this potential discrepancy may be further attributed to differences between absolute- and relative-intensity conditions. Functional tasks (eg, stair climbing) require absolute loads that are not proportional to peak strength. As the older adult weakens with age,1,2 functional tasks can require a greater percentage of maximal capacity; thus, tasks are performed at a higher relative intensity.64 Fatigue occurs more rapidly with increasing task intensity; maximum endurance time decreases nonlinearly with increasing task intensity.7 Thus, although resistance to fatigue may improve with age for a relative-intensity (eg, 50% of maximum) task that is standardized among individuals, the increased relative workload for a functional task may offset any age advantage. That is, if a given task requires 40% of maximum strength for a young adult, but 60% for an older adult, the apparent task endurance may be less for the older adult, even if underlying muscle fatigue resistance is greater with age.
Interpretation of the remaining potential moderators (sex, physical activity, intensity, and joint) associated with age-related differences in muscle fatigue is somewhat challenging given the incomplete data available for each possible subgrouping. No significant differences between men and women were consistently observed in this meta-analysis, once contraction type was controlled for, which is in agreement with conclusions drawn from several individual studies19,46,47 but in opposition to others.10 Current comparisons did not assess whether sex differences in muscle fatigue occurred, but rather whether age differences varied by sex. Lastly, greater physical activity did not influence the age-related fatigue advantage. However, these findings are based on smaller subgroup samples, with heterogeneous definitions of active versus inactive individuals, and thus may reflect less stability in effect size estimates.
Although the current meta-analysis was able to identify differences in muscle fatigue properties across contraction types between young and old adults, there are several limitations that should be acknowledged. Several subgrouping comparisons in levels II and III for joint, intensity, and contraction type were not performed due to a lack of available data. The majority of intermittent isometric and isokinetic protocols were performed at intensities of 50% MVC or higher (most at 100%), limiting the interpretation intensity has upon fatigue differences with aging for these contraction types. Intermittent tasks were further limited by the disproportionate number of comparisons including men (8 men versus 4 women) and limited joint regions that have been tested (ankle=9, all others combined=7). Physical activity data were classified simply as active versus sedentary, which may miss subtle influences of varying levels of physical activity. Lastly, we included studies with cohorts aged ≥55 years, thus a relatively “young” older adult minimum age criterion. Secondary analyses demonstrated no significant difference in effect size estimates if we had used only studies with adults over 60 years as our age minimum criterion.
These findings suggest the need for future studies to explicitly report fatigue data by sex and provide physical activity information for both young and old adult cohorts when possible. Additional fatigue studies involving isokinetic and intermittent tasks using the upper extremities and lower intensities would help to minimize the potential bias and interactions present among muscle group, intensity, and contraction type, as observed here. In particular, it is not clear why this age-related advantage is lost during dynamic contractions, which would benefit from research considering potential influences such as: task complexity, passive tissue contributions, and muscle power. Finally, although these findings provide greater insight into age-related changes in muscle fatigue properties, additional research is needed to clarify the magnitude and impact of this potential benefit and whether it can be further altered by therapeutic interventions.
Despite the abundance of acute muscle fatigue research, few studies have attempted to compile all of the available data on age-related differences in fatigue resistance. This meta-analysis supports that aging results in a general muscle fatigue resistance advantage, but this advantage is particularly dependent on contraction type. Dynamic tasks, specifically isokinetic tasks, were not found to exhibit any advantage (or disadvantage) in muscle fatigue for old versus young adults. The underlying mechanisms for these findings remain somewhat unclear, but may be due to a greater loss of muscle power with aging. Ultimately, these age-related fatigue differences may help offset the deleterious effects of sarcopenia and loss of muscle strength. In light of a reduction in strength, therapeutic interventions may target muscle fatigue resistance to affect functional capabilities in the older adult.
Footnotes
Both authors provided concept/idea/project design, writing, data collection and analysis, and project management.
A poster presentation of this research was given at the Combined Sections Meeting of the American Physical Therapy Association; February 17–20, 2010; San Diego, California.
The authors were funded, in part, by National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health grant K01AR056134, National Research Service Award 1 F31 AR056175, and the Foundation for Physical Therapy.
Adobe Systems Inc, 345 Park Ave, San Jose, CA 95110.
Biostat, 14 N Dean St, Englewood, NJ 07631.
References
- 1. Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal-muscle characteristics. Acta Physiol Scand. 1978;104:129–136 [DOI] [PubMed] [Google Scholar]
- 2. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50 Spec. No.:11–16 [DOI] [PubMed] [Google Scholar]
- 3. Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St Clair Gibson A, Baden DA, Lambert MI, et al. The conscious perception of the sensation of fatigue. Sports Med. 2003;33:167–176 [DOI] [PubMed] [Google Scholar]
- 5. Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699 [DOI] [PubMed] [Google Scholar]
- 6. Hunter SK. Aging and Mechanisms of Task-dependent Muscle Fatigue. Kerala, India: Research Signpost; 2009 [Google Scholar]
- 7. Frey Law LA, Avin KG. Endurance time is joint-specific: a modelling and meta-analysis investigation. Ergonomics. 2010;53:109–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bazzucchi I, Marchetti M, Rosponi A, et al. Differences in the force/endurance relationship between young and older men. Eur J Appl Physiol. 2005;93:390–397 [DOI] [PubMed] [Google Scholar]
- 9. Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve. 2005;31:730–740 [DOI] [PubMed] [Google Scholar]
- 10. Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol. 2004;97:1723–1732 [DOI] [PubMed] [Google Scholar]
- 11. Avin KG, Naughton MR, Ford BW, et al. Sex differences in fatigue resistance are muscle group dependent. Med Sci Sports Exerc. 2010;42:1943–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laforest S, St-Pierre DM, Cyr J, Gayton D. Effects of age and regular exercise on muscle strength and endurance. Eur J Appl Physiol Occup Physiol. 1990;60:104–111 [DOI] [PubMed] [Google Scholar]
- 13. Allman BL, Rice CL. Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve. 2002;25:785–796 [DOI] [PubMed] [Google Scholar]
- 14. Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.02. Available at: http://www.cochrane-handbook.org Accessed April 5, 2010
- 15. Borenstein M. Introduction to Meta-analysis. Chichester, West Sussex, United Kingdom: John Wiley & Sons Ltd; 2009 [Google Scholar]
- 16. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garamszegi LZ. Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behavioral Ecology. 2006;17:682–687 [Google Scholar]
- 19. Aniansson A, Grimby G, Hedberg M, et al. Muscle function in old age. Scand J Rehabil Med Suppl. 1978;6:43–49 [PubMed] [Google Scholar]
- 20. Bilodeau M, Erb MD, Nichols JM, et al. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24:98–106 [DOI] [PubMed] [Google Scholar]
- 21. Bilodeau M, Henderson TK, Nolta BE, et al. Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol. 2001;91:2654–2664 [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee S, Chowdhuri BJ. Comparison of grip strength and isomeric endurance between the right and left hands of men and their relationship with age and other physical parameters. J Hum Ergol (Tokyo). 1991;20:41–50 [PubMed] [Google Scholar]
- 23. Christie A, Kamen G. Motor unit firing behavior during prolonged 50% MVC dorsiflexion contractions in young and older adults. J Electromyogr Kinesiol. 2009;9:543–552 [DOI] [PubMed] [Google Scholar]
- 24. Griffith EE, Yoon T, Hunter SK. Age and load compliance alter time to task failure for a submaximal fatiguing contraction with the lower leg. J Appl Physiol. 2010;108:1510–1519 [DOI] [PubMed] [Google Scholar]
- 25. Hara Y, Findley TW, Sugimoto A, Hanayama K. Muscle fiber conduction velocity (MFCV) after fatigue in elderly subjects. Electromyogr Clin Neurophysiol. 1998;38:427–435 [PubMed] [Google Scholar]
- 26. Huang CT, Huang CC, Young MS, Hwang IS. Age effect on fatigue-induced limb acceleration as a consequence of high-level sustained submaximal contraction. Eur J Appl Physiol. 2007;100:675–683 [DOI] [PubMed] [Google Scholar]
- 27. Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve. 2005;31:730–740 [DOI] [PubMed] [Google Scholar]
- 28. Johnson T. Age-related differences in isometric and dynamic strength and endurance. Phys Ther. 1982;62:985–989 [DOI] [PubMed] [Google Scholar]
- 29. Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744 [DOI] [PubMed] [Google Scholar]
- 30. Mademli L, Arampatzis A. Mechanical and morphological properties of the triceps surae muscle-tendon unit in old and young adults and their interaction with a submaximal fatiguing contraction. J Electromyogr Kinesiol. 2008;18:89–98 [DOI] [PubMed] [Google Scholar]
- 31. Mademli L, Arampatzis A, Walsh M. Age-related effect of static and cyclic loadings on the strain-force curve of the vastus lateralis tendon and aponeurosis. J Biomech Eng. 2008;130:011007. [DOI] [PubMed] [Google Scholar]
- 32. Momen A, Leuenberger UA, Handly B, Sinoway LI. Effect of aging on renal blood flow velocity during static exercise. Am J Physiol Heart Circ Physiol. 2004;287:H735–H740 [DOI] [PubMed] [Google Scholar]
- 33. Petrofsky JS, Burse RL, Lind AR. Comparison of physiological responses of women and men to isometric exercise. J Appl Physiol. 1975;38:863–868 [DOI] [PubMed] [Google Scholar]
- 34. Petrofsky JS, Lind AR. Aging, isometric strength and endurance, and cardiovascular responses to static effort. J Appl Physiol. 1975;38:91–95 [DOI] [PubMed] [Google Scholar]
- 35. Petrofsky JS, Laymon M. The effect of ageing in spinal cord injured humans on the blood pressure and heart rate responses during fatiguing isometric exercise. Eur J Appl Physiol. 2002;86:479–486 [DOI] [PubMed] [Google Scholar]
- 36. Petrofsky JS, Prowse M, Remigio W, et al. The use of an isometric handgrip test to show autonomic damage in people with diabetes. Diabetes Technol Ther. 2009;11:361–368 [DOI] [PubMed] [Google Scholar]
- 37. Smolander J, Aminoff T, Korhonen I, et al. Heart rate and blood pressure responses to isometric exercise in young and older men. Eur J Appl Physiol Occup Physiol. 1998;77:439–444 [DOI] [PubMed] [Google Scholar]
- 38. Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. Am J Physiol. 1991;261(5 pt 2):R1061–R1069 [DOI] [PubMed] [Google Scholar]
- 39. Yassierli, Nussbaum MA, Iridiastadi H, Wojcik LA. The influence of age on isometric endurance and fatigue is muscle dependent: a study of shoulder abduction and torso extension. Ergonomics. 2007;50:26–45 [DOI] [PubMed] [Google Scholar]
- 40. Yoon T, De-Lap BS, Griffith EE, Hunter SK. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle Nerve. 2008;37:457–466 [DOI] [PubMed] [Google Scholar]
- 41. Allman BL, Rice CL. Perceived exertion is elevated in old age during an isometric fatigue task. Eur J Appl Physiol. 2003;89:191–197 [DOI] [PubMed] [Google Scholar]
- 42. Allman BL, Rice CL. Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve. 2001;24:1156–1167 [DOI] [PubMed] [Google Scholar]
- 43. Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan KM, Raja AJ, Strohschein FJ, Lechelt K. Age-related changes in muscle fatigue resistance in humans. Can J Neurol Sci. 2000;27:220–228 [DOI] [PubMed] [Google Scholar]
- 45. Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol. 2007;103:1628–1635 [DOI] [PubMed] [Google Scholar]
- 46. Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790 [PubMed] [Google Scholar]
- 47. Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823 [DOI] [PubMed] [Google Scholar]
- 48. Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975 [DOI] [PubMed] [Google Scholar]
- 49. Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583(pt 3):1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mademli L, Arampatzis A. Effect of voluntary activation on age-related muscle fatigue resistance. J Biomech. 2008;41:1229–1235 [DOI] [PubMed] [Google Scholar]
- 51. Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol. 2005;15:536–543 [DOI] [PubMed] [Google Scholar]
- 52. Russ DW, Towse TF, Wigmore DM, et al. Contrasting influences of age and sex on muscle fatigue. Med Sci Sports Exerc. 2008;40:234–241 [DOI] [PubMed] [Google Scholar]
- 53. Stackhouse SK, Stevens JE, Lee SC, et al. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109 [PubMed] [Google Scholar]
- 54. Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525 [DOI] [PubMed] [Google Scholar]
- 55. Lindstrom B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997;52:B59–B66 [DOI] [PubMed] [Google Scholar]
- 56. Lindstrom B, Karlsson JS, Lexell J. Isokinetic torque and surface electromyography during fatiguing muscle contractions in young and older men and women. Isokinet Exerc Sci. 2006;14:225–234 [Google Scholar]
- 57. McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629 [DOI] [PubMed] [Google Scholar]
- 58. Muller F, Dehail P, Bestaven E, et al. Maximal and sustained isokinetic lower-limb muscle strength in hospitalized older people. Muscle Nerve. 2007;35:739–744 [DOI] [PubMed] [Google Scholar]
- 59. Rawson ES. Enhanced fatigue resistance in older adults during repeated sets of intermittent contractions. J Strength Cond Res. 2010;24:251–256 [DOI] [PubMed] [Google Scholar]
- 60. Schwendner KI, Mikesky AE, Holt WS, Jr, et al. Differences in muscle endurance and recovery between fallers and nonfallers, and between young and older women. J Gerontol A Biol Sci Med Sci. 1997;52:M155–M160 [DOI] [PubMed] [Google Scholar]
- 61. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy: total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294 [DOI] [PubMed] [Google Scholar]
- 62. Allman BL, Rice CL. An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol. 2004;96:1026–1032 [DOI] [PubMed] [Google Scholar]
- 63. Raj IS, Bird SR, Shield AJ. Aging and the force-velocity relationship of muscles. Exp Gerontol. 2010;45:81–90 [DOI] [PubMed] [Google Scholar]
- 64. Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460 [DOI] [PubMed] [Google Scholar]