Abstract
Background
The incidence of obesity is increasing in older adults, with associated worsening in the burden of disability. Little is known about the impact of body mass index (BMI) on self-report and performance-based balance and mobility measures in older adults.
Objective
The purposes of this study were (1) to examine the association of BMI with measures of balance and mobility and (2) to explore potential explanatory factors.
Design
This was a cross-sectional, observational study.
Methods
Older adults (mean age=77.6 years) who participated in an ongoing observational study (N=120) were classified as normal weight (BMI=18.5–24.9 kg/m2), overweight (BMI=25.0–29.9 kg/m2), moderately obese (BMI=30.0–34.9 kg/m2), or severely obese (BMI≥35 kg/m2). Body mass index data were missing for one individual; thus, data for 119 participants were included in the analysis. Mobility and balance were assessed using self-report and performance-based measures and were compared among weight groups using analysis of variance and chi-square analysis for categorical data. Multiple linear regression analysis was used to examine the association among BMI, mobility, and balance after controlling for potential confounding variables.
Results
Compared with participants who were of normal weight or overweight, those with moderate or severe obesity were less likely to report their mobility as very good or excellent (52%, 55%, 39%, and 6%, respectively); however, there was no difference in self-report of balance among weight groups. Participants with severe obesity (n=17) had the lowest levels of mobility on the performance-based measures, followed by those who were moderately obese (n=31), overweight (n=42), and of normal weight (n=29). There were no differences on performance-based balance measures among weight groups. After controlling for age, sex, minority status, physical activity level, education level, and comorbid conditions, BMI still significantly contributed to mobility (β=−.02, adjusted R2=.41).
Conclusions
Although older adults with severe obesity were most impaired, those with less severe obesity also demonstrated significant decrements in mobility.
Obesity is a major public health problem in the United States and around the world. There has been a substantial increase in the prevalence of obesity globally, even in developing countries.1 In the United States, it is estimated that more than 65% of adults are overweight, defined as having a body mass index (BMI) of 25.0 kg/m2 or higher, with more than 30% considered obese (BMI≥30 kg/m2). Despite increased attention to this epidemic, the prevalence of obesity continues to rise.2,3 This increasing prevalence is of great concern because the health and economic burdens of obesity are vast. Numerous chronic diseases, including hypertension, cardiovascular disease, type 2 diabetes, osteoarthritis, and certain forms of cancer, are strongly associated with excess body weight.4,5 Obesity is estimated to account for nearly 10% of all medical spending in the United States.6,7 For these reasons, it is imperative that health care professionals be able to effectively evaluate and treat people with conditions related to overweight and obesity.
The prevalence of obesity is increasing in older adults, with an estimated 31% of those aged 60 years or older reported to be obese in 2003–2004.3,8 The increased prevalence of obesity in older adults is especially concerning given the association between obesity and impaired physical function.9–16 Physical function refers to a person's ability to perform basic and instrumental activities of daily living and mobility tasks. Impairments in physical function, such as the components of mobility and balance, have been linked to the development of disability.17,18 Analysis of recent trends has shown that obesity-related disability is on the rise,19 reinforcing the need for a better understanding of the impact of obesity on mobility and balance.
The BMI is the most common method to quantify weight across a range of body sizes in adults.20 The BMI is calculated by dividing an individual's weight (in kilograms) by his or her height (in meters squared). Using the BMI, individuals can be classified as underweight (<18.5 kg/m2), of normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), class I obese (30–34.9 kg/m2), class II obese (35–39.9 kg/m2), or class III obese (≥40 kg/m2). These categories of BMI were developed by the World Health Organization based on associated health risks.21 Guidelines from the National Institutes of Health suggest this anthropometric index should be utilized in the initial assessment of overweight and obesity.22
The BMI is an inexpensive and easy-to-use clinical measure that can be administered with minimal training.22 Health care professionals, such as physical therapists, may utilize this simple measure to screen patients and determine risks for diseases associated with obesity. Although BMI is an important indicator of body size for use in the primary care and public health domain, it is an indirect surrogate measure of adiposity and thus has several limitations. The BMI may overestimate body fat in individuals with larger muscle mass, such as athletes, and may underestimate body fat in those who have lost muscle mass (eg, older adults).23 Furthermore, the BMI guidelines were established independent of race, age, and sex. Studies have shown these factors influence the relationship between BMI and percentage of body fat, suggesting the need for population-specific BMI classifications.24,25 For these reasons, it has been suggested that the BMI be used as an initial step in the determination of health risks and that this measure be used in conjunction with waist circumference and assessment for the presence of concomitant risk factors.26
The determination of an individual's BMI may assist the clinician in the identification of risk status and consequently result in an intervention to reduce weight or disease risk. In addition to dietary restriction and behavioral therapy, exercise is a primary treatment for obesity. The public health recommendation for physical activity for adults (men and women who are healthy and 18–65 years of age) and older adults (men and women ≥65 years of age) is a minimum of 30 minutes of moderate-intensity activity on 5 days of the week (150 min/wk).27,28 However, there is evidence that higher levels of exercise are needed for achieving weight loss (150–250 min/wk) and for maintaining weight loss (>250 min/wk).29,30 In older adults with obesity, the benefits of moderate weight loss achieved through diet and exercise include improvements in self-report and performance-based mobility and balance measures.31 In addition, studies have shown that exercise, even in the absence of weight loss, leads to improvement in adverse health consequences associated with obesity.32–34
In view of the widespread prevalence of obesity and the critical role of exercise and physical activity in weight loss and health risk reduction, physical therapists are well positioned to have a substantial impact on this significant public health problem. In a recent study of physical therapists' knowledge of obesity, the majority believed that identifying obesity was within their scope of practice.35 Furthermore, most therapists recognized that exercise and diet are key components of a weight loss program. Despite these findings, the researchers concluded that physical therapists lacked the knowledge about the use of the BMI as an indicator for identifying obesity and estimating associated health risks.35 To effectively manage individuals who are overweight, physical therapists must be able to utilize and interpret obesity measures.
Despite evidence relating obesity to impaired physical function, there are several limitations in the current body of research. Studies investigating the relationship between BMI and mobility have focused on individuals with severe obesity,36,37 and few studies have examined the relationship between BMI and balance.38 Thus, little is known about the impact of BMI on balance and mobility across the broader continuum of weight ranges. This information would enable health care professionals to better estimate the functional consequences of excess weight. Furthermore, it is not known how commonly used clinical measures of mobility and balance are affected by BMI. This knowledge would assist physical therapists in determining whether it is necessary to alter tests and measures for patients who are obese. The purposes of this study were: (1) to assess differences in mobility and balance on self-report and performance-based measures across the spectrum of weight categories, (2) to describe how mobility and balance measures are affected by BMI, and (3) to examine other factors that might explain the association between BMI and mobility and balance.
Method
Participants
This cross-sectional study examined older adults who participated in the baseline data collection of an ongoing observational study at the Claude D. Pepper Older Americans Independence Center, Pittsburgh, Pennsylvania (N=120). Individuals were recruited from a research registry of older adults who previously consented to be contacted for studies of balance and mobility. Participants were included in the study if they were 65 years of age or older and had the ability to walk a minimum of a household distance with or without an assistive device and without the assistance of another person. Participants were excluded if they had any of the following conditions that might affect their safety during testing: neuromuscular disorders that impair movement, cancer with active treatment, hospitalization for a life-threatening illness or major surgery in the previous 6 months, severe pulmonary disease, chest pain with activity, or a cardiac event such as a heart attack in the previous 6 months. Body mass index data were missing on one individual; thus, 119 participants were included in the data analysis.
Body Mass Index
Height and weight were measured using a Tanita BWB-800 scale and HR-200 wall-mounted height rod.* Participants were measured while wearing indoor clothing and socks without shoes. Assistance was given to obtain the position for both height and weight measurements, including cues to stand up straight with heels against the wall for assessment of height, but measurements then were recorded in unsupported stance. Weight was recorded to the nearest tenth of a kilogram, and height was measured to the nearest tenth of a centimeter with the height rod at the top of the participant's head in midline. Height and weight measurements were used to determine BMI.
The BMI classifications used in this study were based on the World Health Organization's definitions of normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI≥25 to <30 kg/m2), class I obesity (BMI≥30 to <35 kg/m2), class II obesity (BMI≥35 to <40 kg/m2), and class III obesity (BMI≥40 kg/m2). Because of the limited number of participants with class III obesity (n=3), participants in the obese categories were classified based on obesity severity into moderately obese (BMI≥30 to <35 kg/m2) and severely obese (BMI≥35 kg/m2) weight groups.39,40
Self-Report Measures of Mobility and Balance
A 5-point Likert scale was used to obtain a global rating of mobility and balance for each participant. Participants were asked to rate their current level of mobility and balance as excellent, very good, good, fair, or poor. Self-report measurements of balance and mobility were collected prior to the performance-based measures so that the participants' performance on the tests would not influence their self-report.
Performance-Based Measures of Mobility
Figure-of-8 Walk Test.
The Figure-of-8 Walk Test (F8W) has been shown to be a valid measure of walking skill in older adults based on correlations with gait speed, measures of physical function (Late Life Function and Disability Instrument), and activities of daily living (Physical Performance Test).41 In a study of older adults, the interrater reliability of this measure was determined to be high (intraclass correlation coefficient [ICC]=.85–.92).42 Participants were asked to walk in a figure-of-8 pattern around 2 cones placed 1.524 m (5 ft) apart on the floor. The number of steps taken to complete the course and the total elapsed time in seconds were measured.
Gait speed.
Gait speed is a valid measure to predict health-related outcomes in older adults, including falls and disability.43,44 Studies of older adults who were healthy and those with disease have shown the test-retest reliability of this measure to be high (ICC>.90).43–45 The GaitMat II system† was used to measure gait speed.46 The GaitMat II consists of an approximately 4-m-long, pressure-sensitive walkway controlled by a computer system that processes the data to generate both spatial and temporal variables of walking. On either end of the 4-m-long active walkway, nearly 2 m of inactive surface was available so that acceleration and deceleration were not captured in the timed walk. Gait speed was determined by dividing the distance traversed by the time between the first and last steps (eg, switch closure) and was recorded in meters per second. After 2 practice passes, each participant completed 4 passes at his or her self-selected walking speed for data collection. The mean of the 4 passes was used as the measure of gait speed.
Timed “Up & Go” Test.
The Timed “Up & Go” Test has been used as a test of basic mobility in older adults and has been shown to have high intrarater and interrater reliability (ICC>.90).47,48 For this test, the time required for each participant to stand up from a chair, walk 3 m, turn, walk back, and sit down was measured and recorded.
Six-Minute Walk Test.
The 6-Minute Walk Test has been used as a measure of mobility and aerobic endurance in older adults with and without disease and has shown to be a reliable measure (ICC>.90).49 Each participant was asked to walk as far as possible in 6 minutes, taking standing rest periods as needed. A straight path of 15.24 m (50 ft) was used. The total distance walked back and forth in 6 minutes was recorded. Each participant's heart rate, blood pressure, rating of perceived exertion, and signs and symptoms were monitored before and after testing.
Timed chair stands.
Chair stands have been utilized as a performance-based measure of lower body function and have been shown to have good reliability in older adults (ICC>.80).50 Participants were seated in a rigid chair, asked to fold their arms across their chest, and stand up straight as quickly as possible 5 times. The time to complete 5 repeated stands from the chair was recorded.
Performance-Based Measures of Balance
Timed balance measures.
Participants were asked to maintain their balance for up to 30 seconds under each of the following conditions: standing with eyes closed while the feet were positioned as close together as possible, tandem stance in which the heel of one foot was directly in front of and touching the toes of the other foot, and single-leg stance where the participants were asked to lift one foot off the ground and maintain their balance on the remaining leg. The first 2 tests are a modification of standard balance tests used in the Established Populations for Epidemiologic Studies of the Elderly (EPESE) project.51 In the current study, times for each trial were extended from 10 to 30 seconds, and no support was provided to attain the test position. Interrater reliability (ICC>.9) and test-retest reliability (ICC=.7) have been demonstrated for the EPESE battery of tests.52 In a previous study of older adults who were high functioning, change of the reliability coefficient of single-leg stance was shown to be .69.50
Postural responses.
The postural stress test was used to test postural responses to a destabilizing force applied manually by an examiner in 3 different directions (posteriorly, right, and left). Previous research has shown that older adults classified as “fallers” score lower on postural response tests compared with older “nonfallers” and young adults.53 The ability of the participants to remain upright and their response when nudged at the pelvis in various directions were graded. The response was graded using the following scale: 0=responds (single step); 1=responds (multiple steps); 2=responds but requires support to stabilize; and 3=no obvious response, individual must be supported. The responses to 6 perturbations, 2 in each of the 3 directions, were totaled, with higher scores indicating greater impairment.
Narrow walk test.
As previously described by Bandinelli et al,54 participants were asked to walk a distance of 4 m at their usual walking pace within a 15-cm-wide path marked on the floor with tape. The time taken to complete the task was recorded. The number of deviations from the 15-cm-wide path was also recorded. Individuals who could not complete the test independently, or who stepped outside the walkway more than 10 times, were classified as “unable.” The test-retest reliability of this measure (ICC=.76) has been demonstrated in a sample of older adults.54 Concurrent validity of the narrow walk test has been established based on moderate to strong correlations with other measures of physical performance, such as gait speed and the 400-m corridor walk, in a sample of older adults.55
Obstacle walk test.
As previously described by Bandinelli et al,54 participants were asked to walk a 7-m course at their usual walking pace and step over 2 obstacles of different heights. One obstacle was 6 cm tall and positioned 2 m from the starting line, and the other obstacle was 30 cm tall and positioned 4 m from the starting line. The time taken to complete this task was recorded. The obstacle walk test has shown to be a reliable measure (ICC=.89) in older adults.54
Three licensed physical therapists and one research assistant with extensive experience in geriatric research were responsible for data collection, including measurement of height and weight, as well as administration of the battery of balance and mobility tests. All testers received training in conducting the measurements and were blinded to the purposes of the study but not to study outcomes. Participants' BMIs were calculated after data collection was completed.
Additional Information
Demographics.
Data were collected on the following demographic factors: age, sex, ethnicity, and education level.
Comorbidities Index.
This measure is a self-report of common physician-diagnosed medical conditions, including cardiovascular disease (angina, congestive heart failure, or heart attack), neurologic conditions (stroke or Parkinson disease), lung disease, musculoskeletal conditions (arthritis, osteoporosis, fracture, or joint replacement), general conditions (depression, sleep problems, or chronic pain syndrome), cancer, diabetes, or visual conditions (glaucoma or cataracts).56 For each medical condition, participants were asked whether they had ever been told by a physician that they had the condition. The number of affirmative responses was summed to yield a total score.
Fall history questionnaire.
Participants were asked to respond to the following questions: (1) Are you afraid of falling? and (2) Have you had a fall in the previous year? Responses to the questions were recorded as “yes” or “no.”
Physical activity habits.
During administration of the Survey of Activities and Fear of Falling in the Elderly,57 participants were asked whether they currently walk for exercise. Because walking is the most common form of exercise for older adults,58 those who responded affirmatively to the question were considered to be more physically active than those who responded negatively.
Data Analysis
Individuals were classified as being of normal weight, overweight, moderately obese, or severely obese based on their BMI. For continuous data, descriptive statistics are presented as means and standard deviations, and categorical data are presented as frequencies (percentages). Mobility and balance were compared among weight groups using analysis of variance (ANOVA). Post hoc pair-wise comparisons were conducted for continuous data. Chi-square analyses were conducted for categorical data. One-tailed tests were used because there was a directional hypothesis that mobility and balance would be poorer as BMI increased.
Multiple linear regression analysis was used to examine the association between BMI and mobility and balance during standing and walking while controlling for age, sex, minority status, education level, physical activity level, and number of comorbid conditions.
The level of significance was set at .05. Data analyses were performed with the SAS statistical package (version 9.2).‡
Role of the Funding Source
This research was funded by The University of Pittsburgh Older Americans Independence Center (grant P30 AG024827). Dr Brach was supported by a Paul B. Beeson Career Development Award (K23 AG026766). Dr Studenski was supported by the National Institute on Aging (grant K07 AG023641).
Results
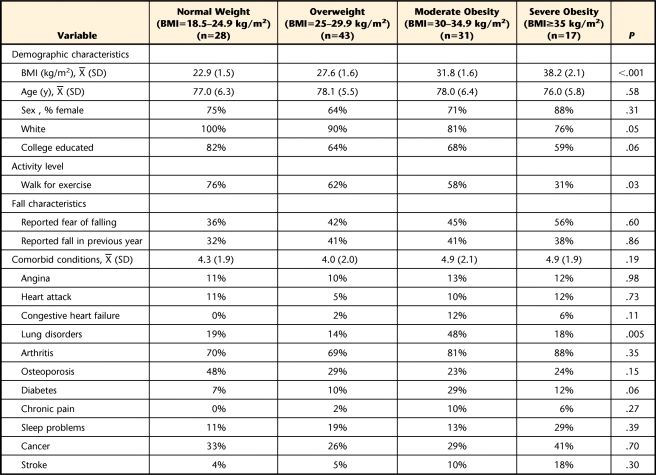
Table 1 provides a summary of the demographic variables, behavioral risk factors, fall characteristics, and prevalent chronic conditions for all participants stratified by weight group. Of the 119 participants, 28 (24%) were of normal weight (BMI=18.5–24.9 kg/m2), 43 (36%) were overweight (BMI=25–29.9 kg/m2), 31 (26%) were moderately obese (BMI=30–34.9 kg/m2), and 17 (14%) were severely obese (BMI≥35 kg/m2). The mean age of the participants was 77.6 years (SD=5.9). There were more women (72%) in our sample than men, and most participants classified their race as white (87%). Several characteristics of the participants were associated with higher BMI levels. Individuals with higher BMI levels were more likely to be black or Hispanic and less likely to report walking for exercise compared with the normal weight group (P≤.05). There were no differences among weight groups in the total number of comorbid health conditions reported. However, compared with the other weight groups, those who were moderately obese were more likely to report having lung disorders (P=.005). There were no differences in the number of falls or fear of falling among weight groups.
Table 1.
Participant Demographics Stratified by Weight Groupa
BMI=body mass index.
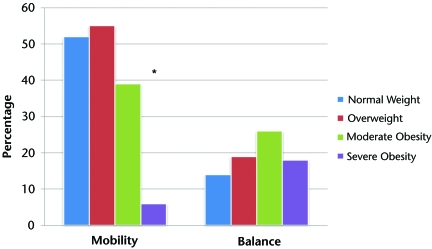
The Figure illustrates self-reported global mobility and balance ratings stratified by weight group. Compared with participants who were of normal weight and those who were overweight, those with moderate and severe obesity were less likely to report their mobility as very good or excellent (52%, 55%, 39%, and 6%, respectively; P=.005). There were no differences in self-reported ratings of balance among weight groups.
Figure.
Global rating of mobility and balance as excellent or very good, stratified by weight group. Asterisk indicates P=.005.
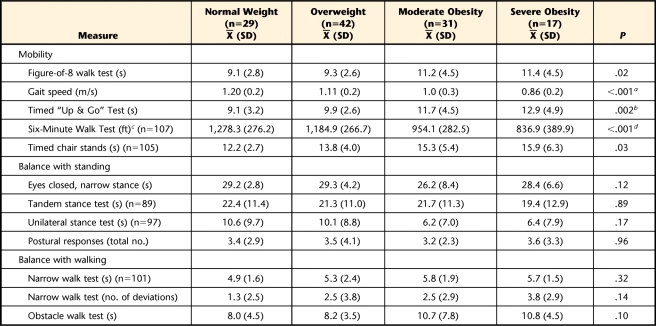
Table 2 provides a description of performance-based mobility and balance measures stratified by weight group. Participants with severe obesity (n=17) had the lowest levels of mobility on the performance-based measures, followed by those who were moderately obese (n=31), overweight (n=42), and of normal weight (n=29). Higher BMI category was not associated with differences in balance. For the mobility measures, post hoc pair-wise comparisons revealed that individuals who were of normal weight and those who were overweight were similar in performance; however, individuals with obesity performed more poorly compared with the other weight groups.
Table 2.
Description of Performance-Based Mobility and Balance Measures Stratified by Weight Group
a Difference between normal weight and moderate obesity=0.21 s, 95% confidence interval (CI)=0.03–0.38, P<.05; difference between normal weight and severe obesity=0.34 s, 95% CI=0.13–0.54, P<.05.
b Difference between normal weight and severe obesity=3.82 s, 95% CI=0.65–7.0, P<.05; difference between overweight and severe obesity=3.02 s, 95% CI=0.03–6.01, P<.05.
c 1 ft=0.3048 m.
d Difference between normal weight and moderate obesity=324 ft, 95% CI=96–553, P<.05; difference between normal weight and severe obesity=441 ft, 95% CI=174–709, P<.05; difference between overweight and moderate obesity=231 ft, 95% CI=20–441, P<.05; difference between overweight and severe obesity=348 ft, 95% CI=95–601, P<.05.
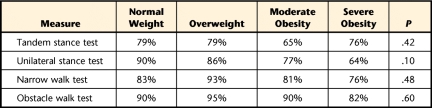
Table 3 provides the results for the percentage of participants who were able to complete the performance-based measures of balance. Compared with those who were overweight and those who were of normal weight, a trend was observed with a greater percentage of participants with moderate and severe obesity unable to complete the unilateral stance test (P=.10).
Table 3.
Percentage of Completion for Performance-Based Measures of Balance by Weight Group
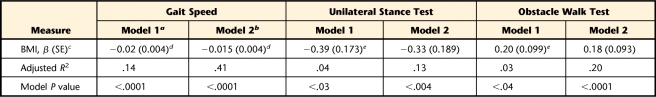
Table 4 provides the results for the series of linear regressions examining the association between BMI and mobility and between BMI and balance during standing and walking. In unadjusted analyses (model 1), BMI was most strongly related to mobility (gait speed, adjusted R2=.14, P<.0001) and to a lesser extent related to balance during standing (unilateral stance test, adjusted R2=.04. P<.03) and balance during walking (obstacle walk test, adjusted R2=.03, P<.04). After adjusting for age, sex, minority status, physical activity level, and total number of comorbid conditions (model 2), BMI remained significantly related to mobility (β=−.015, standard error [SE]=.004, P<.0001). Body mass index approached having a significant association with balance during standing (β=−.331, SE=.189, P<.08) and balance during walking (β=.181, SE=.093, P< .06).
Table 4.
Relationship Between Body Mass Index (BMI) and Mobility and Between BMI and Balance During Standing and Walking
a Unadjusted linear regression.
b Linear regressions adjusted for age, sex, minority status, education level, physical activity level, and total number of comorbid conditions.
c SE=standard error.
d P<.001.
e P<.05.
Discussion
When examining balance and mobility across weight groups in community-dwelling older adults, there were more differences in mobility than in balance. Individuals who were classified as being of normal weight and those classified as overweight were similar in mobility, but individuals with moderate obesity and those with severe obesity demonstrated consistently lower performance than the other groups. The observed relationship between BMI and poor mobility suggests that mobility in older adults is impaired at all levels of obesity.
In our study, self-report of mobility, but not balance, was different for participants with obesity. Individuals who were of normal weight and those who were overweight had a similar perception of mobility. Although self-reported mobility declined for participants who were moderately obese, those with more severe obesity were much less likely to report mobility as very good or excellent. The finding of more frequent self-reported mobility limitation in individuals with obesity is consistent with the findings of previous studies in older adults.10,14,59 In a study of 6,981 older men and women, LaCroix et al14 found that there was a strong association between loss of mobility and high BMI levels. Launer et al10 examined the association between BMI and self-reported mobility disability in the NHANES I Epidemiological Follow-up Study and found that BMI was related to mobility disability in community-dwelling older women; specifically, individuals in the high tertile for BMI had greater risk of impairment compared with those in the low tertile for BMI.
Higher BMI levels were associated with poorer mobility on performance-based measures. Thus, our participants' self-reports of poor mobility were consistent with the findings on the performance-based measures. We found that mobility worsened with increased BMI level; however, post hoc pair-wise comparisons revealed the groups with moderate and severe obesity differed from the other weight groups on most of the measures.
In our sample, only the normal weight group achieved a desirable gait speed (≥1.2 m/s) based on a previous study in older adults.60 For participants who were overweight and those who were obese, the mean gait speed was <1.2 m/s, which may have implications for these individuals to function successfully in the community. For example, in order to safely negotiate through a traffic intersection, an individual must be able to walk at a speed of 1.2 m/s.61 Furthermore, the gait speeds found in the participants classified as moderately or severely obese (1.0 and 0.86 m/s) are not just indicative of impaired functioning, but also have been found to be associated with higher risk for adverse health events, including nursing home admission, falls, and disability.60,62 These findings underscore the detrimental impact that excess weight has on mobility in older adults, even in those with less severe obesity.
Higher BMI levels were not associated with poorer performance on measures of balance during standing, which is consistent with participants' self-reported balance. This finding was not surprising given that there were no differences in fall history among the weight groups; however, our findings differ from those of previous studies that demonstrated more postural instability38 and greater risk of falls in individuals with obesity.63 Although increased BMI in older adults may influence balance, other factors associated with aging also may contribute to postural instability. These factors include sarcopenia, defined as the age-related loss of skeletal muscle mass and strength (force-generating capacity)64; changes in body fat distribution, specifically an increase in visceral abdominal fat and a decrease in subcutaneous fat65; and a decline in the quality of skeletal muscle.66 It is plausible that changes in skeletal muscle and body fat distribution may be related more to postural instability than to BMI alone, which may explain the lack of a stronger relationship between balance and BMI in the current study.
Although not statistically significant, differences in static balance among weight groups may be clinical meaningful. For example, individuals who were of normal weight and those who were overweight had similar performance on the unilateral stance test (10.6 and 10.1 seconds, respectively) compared with individuals with moderate obesity and those with severe obesity, who had poorer performance on the unilateral stance test (6.2 and 6.4 seconds, respectively). A previous study of falls in older adults who were obese showed no difference in performance on standing balance tests in individuals with obesity compared with those of normal weight; however, in contrast to our study, individuals with obesity reported a higher prevalence of falls.63 Interestingly, all measures of balance were static, and no measures of balance during walking were included. Further investigation of the impact of obesity on balance in older adults is warranted and should take into account skeletal muscle mass, strength, and body fat distribution.
A greater number of individuals with moderate obesity and severe obesity were unable to complete the performance-based measures of balance compared with those who were of normal weight and those who were overweight. Lack of completion of balance measures in participants with higher BMI was related to inability to assume the test positions (eg, tandem stance) and difficulty performing certain movements (eg, narrow walk test). Thus, had all participants with obesity been able to complete the balance measures, our results may have differed. These findings reinforce the need for the identification of balance measures most appropriate for use in individuals with higher BMIs, specifically those that incorporate balance during dynamic activities and those that allow individuals with differing body sizes to assume the position required for the test.
Obesity is associated with increased burden of chronic disease and decreased physical activity level, both of which have been shown to negatively affect mobility.14,67,68 In the current study, individuals with obesity were more likely to have lung disorders and were less likely to engage in physical activity compared with those who were overweight and those of normal weight. The association between BMI and mobility was partially explained by these factors; however, the data suggest that even after adjusting for many potential confounding factors, BMI still was independently related to mobility.
The recommendation for weight loss in older adults is controversial because there is a risk of accelerating the age-related decline in lean mass and bone density, thereby leading to poorer physical function.69,70 However, interventions that facilitate weight loss through diet and exercise have been shown to improve mobility, balance, and health-related quality of life in older adults.31,71–73 Villareal et al8 suggested that weight loss programs for older adults include strategies to minimize muscle and bone loss and should include the adoption of resistance exercise and regular physical activity. This recommendation should be taken into account by physical therapists who are frequently involved in exercise prescription for older adults with obesity.
There are several limitations to the current study. First, the results of the study may not apply to the general community-dwelling older population because our sample was a volunteer sample of predominantly white women. Furthermore, there were unequal numbers of participants in each weight group, and not all participants were able to complete the physical performance tests. Individuals in the moderately and severely obese categories had the lowest completion rates compared with those in the normal weight or overweight categories. Our results might have differed had there been equal numbers of participants in the weight groups and had completion rates been higher across the weight groups. Several testers were responsible for data collection in this study, which could have influenced the differences among the weight groups. However, we believe this potential limitation is unlikely in that participant assignment to testers was completely random and all testers evaluated participants from each of the BMI groupings. In addition, although not established in our study, the interrater reliability of many of the measures that we used has been established in other studies and is quite good.
An additional limitation of the current study was the use of the BMI as an indicator of body size. Older individuals typically have less lean mass and more fat mass than younger adults, and as a result, the BMI may underestimate body fat in these individuals.74 Furthermore, there is debate about whether the fat redistribution and relative loss of fat-free mass that occur with aging may exert more influence than the BMI in determining health risks associated with obesity in older adults.75 More sophisticated measures of total body fat are available, including dual-energy x-ray absorptiometry (DXA) and electron beam computed tomography (EBT). Even so, prior research has shown a strong relationship between BMI and total body fat determined by EBT in older women (r=.89, P<.0001), suggesting that BMI may be an acceptable initial screening tool in the clinical setting.76 In addition, DXA and EBT may not be practical measures of total body fat in the clinical setting because these tests are more time-consuming, require more complex training, and are expensive to use. We recognize the limitations of the BMI; however, in the absence of a more suitable measure, the use of the BMI may provide an opportunity for physical therapists to incorporate health promotion into clinical practice. When utilizing the BMI, care should be taken to interpret results along with assessment of regional fat distribution as well as visual inspection of fat and muscle mass to decrease risk of misclassification of body size.
Conclusion
This study used a comprehensive battery of self-report and performance-based measures to characterize mobility and balance in older adults who were of normal weight, overweight, moderately obese, and severely obese. Higher BMI levels were associated with poorer mobility but not balance. Furthermore, individuals classified as being of normal weight and those classified as overweight were similar in mobility, whereas individuals with obesity had greater impairments in mobility. Although those participants with severe obesity (BMI≥35 kg/m2) were most impaired, older adults with less severe obesity (BMI=30–34.9 kg/m2) also demonstrated significant decrements in mobility. Body mass index should be considered when selecting measures of mobility and balance because older adults with obesity may be unable to achieve the position for some tests. When treating older adults with obesity, physical therapists are in a unique position to prescribe exercise to address associated medical complications as well as the functional consequences of obesity.
Footnotes
Dr Hergenroeder and Dr Brach provided concept/idea/research design and data analysis. All authors provided writing. Mr Wert, Dr Hile, and Dr Brach provided project management. Dr Studenski and Dr Brach provided fund procurement and facilities/equipment. Dr Brach provided participants. Mr Wert and Dr Studenski provided consultation (including review of manuscript before submission).
This study was approved by the Institutional Review Board of the University of Pittsburgh.
This work was presented on at Physical Therapy 2009: APTA's Annual Conference & Exposition; June 12, 2009; Baltimore, Maryland.
This research was funded by The University of Pittsburgh Older Americans Independence Center (grant P30 AG024827). Dr Brach was supported by a Paul B. Beeson Career Development Award (K23 AG026766). Dr Studenski was supported by the National Institute on Aging (grant K07 AG023641).
Tanita Corporation of America Inc, 2625 S Clearbrook Dr, Arlington Heights, IL 60005.
E.Q. Inc, PO Box 16, Chalfont, PA 18914-0016.
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513-2414.
References
- 1. Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr. 2005;81:714–721 [DOI] [PubMed] [Google Scholar]
- 2. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850 [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 4. Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 5. Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119(7 pt 2):655–660 [DOI] [PubMed] [Google Scholar]
- 6. Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31(11 suppl):S663–S667 [DOI] [PubMed] [Google Scholar]
- 7. Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who's paying? Health Aff (Millwood). 2003. Suppl Web Exclusives:W3–219–26 [DOI] [PubMed] [Google Scholar]
- 8. Villareal DT, Apovian DT, Kushner RF. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863 [DOI] [PubMed] [Google Scholar]
- 9. Han TS, Tijhuis MA, Lean ME, Seidell JC. Quality of life in relation to overweight and body fat distribution. Am J Public Health. 1998;88:1814–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women: the epidemiologic follow-up study of NHANES I. JAMA. 1994;271:1093–1098 [PubMed] [Google Scholar]
- 11. Apovian CM, Frey CM, Wood GC, et al. Body mass index and physical function in older women. Obes Res. 2002;10:740–747 [DOI] [PubMed] [Google Scholar]
- 12. Himes CL. Obesity, disease, and functional limitation in later life. Demography. 2000;37:73–82 [PubMed] [Google Scholar]
- 13. Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50:918–923 [DOI] [PubMed] [Google Scholar]
- 14. LaCroix AZ, Guralnik JM, Berkman LF, et al. Maintaining mobility in late life; II: smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869 [DOI] [PubMed] [Google Scholar]
- 15. Stenholm S, Rantanen T, Alanen E, et al. Obesity history as a predictor of walking limitation at old age. Obesity (Silver Spring). 2007;15:929–938 [DOI] [PubMed] [Google Scholar]
- 16. Blaum CS, Xue QL, Michelon E. The association between obesity and the frailty syndrome in older women: the Women's Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934 [DOI] [PubMed] [Google Scholar]
- 17. Mendes de Leon CF, Guralnik JM, Bandeen-Roche K. Short-term change in physical function and disability: the Women's Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 2002;57:S355–S365 [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Simonsick EM. Physical disability in older Americans. J Gerontol. 1993;48 Spec. No.:3–10 [DOI] [PubMed] [Google Scholar]
- 19. Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027 [DOI] [PubMed] [Google Scholar]
- 20. Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–153 [PubMed] [Google Scholar]
- 21. Physical status: The Use and Interpretation of Anthropometry. Geneva, Switzerland: World Health Organization; 1995. WHO Technical Report Series 854 [Google Scholar]
- 22. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary; Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917 [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95 [DOI] [PubMed] [Google Scholar]
- 24. Blew RM, Sardinha LB, Milliken LA, et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res. 2002;10:799–808 [DOI] [PubMed] [Google Scholar]
- 25. Evans EM, Rowe DA, Racette SB, et al. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes (Lond). 2006;30:837–843 [DOI] [PubMed] [Google Scholar]
- 26. Seidell JC, Kahn HS, Williamson DF, et al. Report from a Centers for Disease Control and Prevention workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr. 2001;73:123–126 [DOI] [PubMed] [Google Scholar]
- 27. Nelson ME, Rejeski W, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445 [DOI] [PubMed] [Google Scholar]
- 28. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434 [DOI] [PubMed] [Google Scholar]
- 29. Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine position stand: appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33:2145–2156 [DOI] [PubMed] [Google Scholar]
- 30. Saris WH, Blair SN, van Baak M, et al. How much physical activity is enough to prevent unhealthy weight gain: outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–114 [DOI] [PubMed] [Google Scholar]
- 31. Villareal DT, Banks M, Sinacore DR, et al. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866 [DOI] [PubMed] [Google Scholar]
- 32. Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112 [DOI] [PubMed] [Google Scholar]
- 33. Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–325 [DOI] [PubMed] [Google Scholar]
- 34. Dekker MJ, Lee S, Hudson R, et al. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56:332–338 [DOI] [PubMed] [Google Scholar]
- 35. Sack S, Radler DR, Mairella KK, et al. Physical therapists' attitudes, knowledge, and practice approaches regarding people who are obese. Phys Ther. 2009;89:804–815 [DOI] [PubMed] [Google Scholar]
- 36. Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. 2006;100:390–398 [DOI] [PubMed] [Google Scholar]
- 37. de Souza SA, Faintuch J, Valezi AC, et al. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15:1238–1242 [DOI] [PubMed] [Google Scholar]
- 38. Corbeil P, Simoneau M, Rancourt D, et al. Increased risk for falling associated with obesity: mathematical modeling of postural control. IEEE Trans Neural Syst Rehabil Eng. 2001;9:126–136 [DOI] [PubMed] [Google Scholar]
- 39. Kuczmarski RJ, Carroll MD, Flegal KM, Trojano RP. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994). Obes Res. 1997;5:542–548 [DOI] [PubMed] [Google Scholar]
- 40. Andreyeva T, Sturm R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes Res. 2004;12:1936–1943 [DOI] [PubMed] [Google Scholar]
- 41. Hess RJ, Brach JS, Piva SR, Van Swearingen JM. Walking skill can be assessed in older adults: validity of the Figure-of-8 Walk Test. Phys Ther. 2010;90:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Swearingen JM, Brach JS, Hess RJ, et al. Clinical correlates of motor control in walking; the Figure-of-8 Walk Test. Paper presented at: 59th Annual Scientific Meeting of the Gerontological Society of America; November 16–20, 2006; Dallas, Texas [Google Scholar]
- 43. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism [erratum in: Phys Ther. 2010;90:462]. Phys Ther. 2008;88:733–746 [DOI] [PubMed] [Google Scholar]
- 44. Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26:15–19 [DOI] [PubMed] [Google Scholar]
- 45. Brach JS, Perera S, Studenski SA, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walsh JP. Foot fall measurement technology. In: Craik RL, Oatis CA, eds. Gait Analysis: Theory and Application. 1995:125–142 [Google Scholar]
- 47. Nordin E, Rosendahl E, Lundin-Olsson L. Timed “Up & Go” Test: reliability in older people dependent in activities of daily living: focus on cognitive state. Phys Ther. 2006;86:646–655 [PubMed] [Google Scholar]
- 48. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148 [DOI] [PubMed] [Google Scholar]
- 49. King MB, Judge JO, Whipple R, Wolfson L. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Phys Ther. 2000;80:8–16 [PubMed] [Google Scholar]
- 50. Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–742 [DOI] [PubMed] [Google Scholar]
- 51. Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established Populations for Epidemiologic Studies of the Elderly: study design and methodology. Aging (Milano). 1993;5:27–37 [DOI] [PubMed] [Google Scholar]
- 52. Studenski SA, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322 [DOI] [PubMed] [Google Scholar]
- 53. Chandler JM, Duncan PW, Studenski SA. Balance performance on the postural stress test: comparison of young adults, healthy elderly, and fallers. Phys Ther. 1990;70:410–415 [DOI] [PubMed] [Google Scholar]
- 54. Bandinelli S, Pozzi M, Lauretani F, et al. Adding challenge to performance-based tests of walking: the Walking InCHIANTI Toolkit (WIT). Am J Phys Med Rehabil. 2006;85:986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649 [DOI] [PubMed] [Google Scholar]
- 56. Rigler SK, Studenski SA, Wallace D, et al. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:420–428 [DOI] [PubMed] [Google Scholar]
- 57. Lachman ME, Howland J, Tennstedt S, et al. Fear of falling and activity restriction: the Survey of Activities and Fear of Falling in the Elderly (SAFE). J Gerontol B Psychol Sci Soc Sci. 1998;53:P43–P50 [DOI] [PubMed] [Google Scholar]
- 58. Eyler AA, Brownson RC, Bacak SJ, Housemann RA. The epidemiology of walking for physical activity in the United States. Med Sci Sports Exerc. 2003;35:1529–1536 [DOI] [PubMed] [Google Scholar]
- 59. Galanos AN, Pieper CF, Cornoni-Huntley JC, et al. Nutrition and function: is there a relationship between body mass index and the functional capabilities of community-dwelling elderly? J Am Geriatr Soc. 1994;42:368–373 [DOI] [PubMed] [Google Scholar]
- 60. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680 [DOI] [PubMed] [Google Scholar]
- 61. Langlois JA, Keyl PM, Guralnik JM, et al. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fjeldstad C, Fieldstad AS, Acree LS, et al. The influence of obesity on falls and quality of life. Dyn Med. 2008;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42:1110–1117 [DOI] [PubMed] [Google Scholar]
- 65. Ochi M, Tabara Y, Kido T, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010;10:233–243 [DOI] [PubMed] [Google Scholar]
- 66. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 67. Koster A, Penninx BW, Newman AB, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity (Silver Spring). 2007;15:3122–3132 [DOI] [PubMed] [Google Scholar]
- 68. Brach JS, Van Swearingen JM, FitzGerald SJ, et al. The relationship among physical activity, obesity, and physical function in community-dwelling older women. Prev Med. 2004;39:74–80 [DOI] [PubMed] [Google Scholar]
- 69. Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017 [DOI] [PubMed] [Google Scholar]
- 70. Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9:459–463 [DOI] [PubMed] [Google Scholar]
- 71. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062–1072 [DOI] [PubMed] [Google Scholar]
- 72. Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66:433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510 [DOI] [PubMed] [Google Scholar]
- 74. Borkan GA, Hults DE, Gerzof SG, et al. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38:673–677 [DOI] [PubMed] [Google Scholar]
- 75. Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond). 2005;29:1011–1029 [DOI] [PubMed] [Google Scholar]
- 76. Storti KL, Brach JS, FitzGerald SJ, et al. Relationships among body composition measures in community-dwelling older women. Obesity (Silver Spring). 2006;14:244–251 [DOI] [PubMed] [Google Scholar]