Abstract
Accumulating evidence suggests that endogenous dopamine may act as a neurotoxin and thereby participate in the pathophysiology of Parkinson’s disease (PD). Cyclooxygenase-2 (COX-2) has been implicated in the pathogenesis of PD due to its ability to generate reactive oxygen species (ROS). Inhibition of COX-2 leads to neuroprotection by preventing the formation of dopamine-quinone. In this study, we examined whether dopamine mediates 1-methyl-4-phenylpyridinium (MPP+)-induced toxicity in primary ventral mesencephalic (VM) neurons, an in vitro model of PD, and if so, whether the protective effects of COX-2 inhibitors on dopamine mediated MPP+-induced VM neurotoxicity and VM dopaminergic cell apoptosis result from the reduction of ROS. Reserpine, a dopamine-depleting agent, significantly reduced VM neurotoxicity induced by MPP+, whereas dopamine had an additive effect on MPP+-induced VM neurotoxicity and VM dopaminergic cell apoptosis. However, inhibition of COX-2 by a selective COX-2 inhibitor (DFU) or ibuprofen significantly attenuated MPP+-induced VM cell toxicity and VM dopaminergic cell apoptosis, which was accompanied by a decrease in ROS production in VM dopaminergic neurons. These results suggest that dopamine itself mediates MPP+-induced VM neurotoxicity and VM dopaminergic cell apoptosis in the presence of COX-2.
Keywords: Parkinson’s disease, Ventral mesencephalon, MPP+, Apoptosis, Neuroinflammation
Introduction
The primary neuropathological hallmark of Parkinson’s disease (PD) is the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) that project into the striatum (Fahn and Przedborski 2000). There is increasing evidence that endogenous dopamine may contribute to neurodegeneration of dopaminergic neurons in PD (Xu et al. 2002). As a consequence of the degeneration of nigrostriatal dopaminergic neurons in the SNpc of PD patients the remaining dopaminergic neurons become hyperactive in an attempt to restore dopaminergic neurotransmission and function (Yu et al. 1996). This then leads to an increased dopamine turnover (Yu et al. 1996) consequently resulting in an elevation of hydrogen peroxide generation as well as neuronal apoptosis (Chu et al. 2006). Additionally it has been shown that injection of dopamine into rat striatum causes selective death of dopaminergic neurons, which can be significantly attenuated by antioxidants (Hastings et al. 1996; Hattori et al. 1998).
It has been suggested that oxidative stress might be a key contributor to the degeneration of nigrostriatal dopaminergic neurons in PD (Andersen 2004; Jenner 2003), and that cyclooxygenase-2 (COX-2) can act synergistically to induce neurodegeneration of dopaminergic neurons (Teismann et al. 2003a, b). COX-2, a pro-inflammatory enzyme which generates prostaglandins, has been reported to be a key culprit in mediating 1-methyl-4-phenyl-1,2,3,6-tetrahydroperidine (MPTP)-induced neurotoxicity, a mouse model of PD (Teismann et al. 2003a). Moreover, 1-methyl-4-phenylpyridinium (MPP+), which is extensively used to induce dopaminergic cell loss in primary mesencephalic cell cultures or cell lines (Leonardi and Mytilineou 1998), can induce COX-2 expression and activity, and application of a COX-2 inhibitor leads to reduced levels of MPP+-induced prostaglandin E2 (PGE2) production and subsequent decreased dopaminergic neurotoxicity in mixed neuron–microglia cultures (Wang et al. 2005).
Additionally it has been shown, that COX-2 can oxidize dopamine to reactive dopamine-quinone which has been implicated in the pathogenesis of PD (Hastings 1995; Teismann et al. 2003a). Indeed, our previous study has found that blocking COX-2 leads to a decrease in dopamine-quinone levels and an attenuation of MPTP-induced neurodegeneration (Teismann et al. 2003a). Thus, the neuroprotective effect of COX-2 inhibition appears to be related to the blockade of COX-2-mediated dopamine oxidation in PD. A further deleterious consequence of dopamine-quinone is the accumulation of toxic α-synuclein protofibrils which are thought to lead to the formation of Lewy bodies, a pathological hallmark of PD (Periquet et al. 2007; Wilson et al. 2004).
In view of this, we hypothesized that dopamine itself mediates MPP+-induced dopaminergic neurotoxicity and that COX-2 modulates dopamine-mediated MPP+-induced neurotoxicity through generation of ROS. To test this hypothesis, we used an in vitro model of PD by culturing primary ventral mesencephalic (VM) neurons treated with MPP+. We examined the effects of reserpine (a dopamine-depleting agent) and dopamine on MPP+-treated VM toxicity and apoptosis in tyrosine hydroxylase (TH)-positive neurons. Moreover, dopamine and MPP+-cotreated cells were further incubated with COX-2 inhibitors in order to determine whether the protective effects of COX-2 inhibitors on dopamine-mediated MPP+-induced neurotoxicity were mediated via reduction of reactive oxygen species (ROS).
Materials and methods
Primary midbrain neuron cultures
Mesencephalic dissociated neurons were prepared from the ventral mesencephalon of E14 rat (Sprague–Dawley) fetus according to the procedure described (Krieglstein et al. 1995) with minor modifications. Experimental protocols were in accordance with Home Office and institutional guidelines. The ventral mesencephalons from 15 embryos were collected in calcium–magnesium free Hank’s balanced salt solution (Invitrogen) containing 5 mM sodium bicarbonate (pH 7.0–7.2). Cells were dissociated with 0.25% trypsin in Hank’s balanced salt solution. Dissociation was stopped by addition of an equal volume of fetal calf serum and 1 mg/ml DNAse (Roche). Thereafter, tissue was triturated three times with a wide pore, siliconized Pasteur pipette. Cells were plated on polyornithine and laminin-coated coverslips at a density of 2.5 × 105 cells/cm2. Culture medium consisting of Dulbecco’s modified eagle medium with F12 nutrient mixture (Sigma) plus 1% N1 mix (Sigma), 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin/streptomycin (Invitrogen), and 1 μg/ml insulin (Sigma) was supplied at 500 μl/well. Cells were maintained at 37°C, 5% CO2 for 6 days. The culture medium was changed after 24 h and then changed every second day. MPP+ (20 μM; Sigma), dopamine (100 and 250 μM; Sigma), DFU [5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone] (10 and 100 nM; Merck), and ibuprofen (25 and 250 μM; Sigma) were added on day in vitro (DIV) 4 for 48 h. Cells were pretreated with reserpine (50 and 500 nM; Sigma) before MPP+.
These preparations yield 95% neurons, with the rest being primarily astrocytes and a few contaminating microglia cells (Liu and Hong 2003; Takeshima et al. 1994). Thus, the effects observed from the addition of COX-2 inhibitors are derived mainly from COX-2 expressed by dopaminergic neurons, as COX-2 is induced in dopaminergic neurons by MPP+ (Wang et al. 2005).
Immunocytochemistry
Cultures were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 20 min. After fixation, they were washed with PBS and incubated with 10% goat serum in PBS for 10 min at room temperature. Cells were washed and incubated over night at 4°C with a rabbit antibody against tyrosine hydroxylase (TH, 1:200; Chemicon). Immunostaining was visualised with a cyanine 3 (Cy3, red)-labeled goat anti-rabbit antibody (1:200; Jackson Immunoresearch) and an Alexa Fluor 488 goat anti-mouse antibody (1:200; Invitrogen). Negative controls were performed by substitution of non-immune sera for the primary or secondary antisera. Coverslips were mounted on glass slides and observed with a Zeiss Axioplan microscope.
MTT assay
Cell viability was determined by the method of modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma) assay. Cells were plated on 96-well microtiter plates, and treatment occurred 48 h after plating. Treatment was then left for another 48 h after which the MTT solution (5 mg/ml in PBS) was added, and 4 h were allowed for incubation. Then, cells were solubilised and mixed thoroughly in 100 μl of dimethyl sulfoxide (DMSO) per well. The absorption was measured at 570 nm with reference at 670 nm. Results were expressed as the percentage of the absorbance of the vehicle-treated control culture wells.
LDH assay
Lactate dehydrogenase (LDH) enzymatic activity in the medium was measured using the LDH Cytotoxicity assay kit (Cayman) according to the manufacturer’s instructions. LDH is released into the medium upon cell lysis, and the activity measured in the medium is therefore proportional to the number of lysed cells. The amount of cell death was calculated as released LDH/total LDH (value obtained by lysing all cells with 1% Triton-X100 in the untreated wells). Results were expressed as the percentage of the vehicle-treated control culture wells.
Apoptosis assay
Apoptosis was detected by Hoechst 33258 staining (Molecular Probes). After immunocytochemistry staining, cells were incubated for 20 min with Hoechst 33258 (2 μg/ml). Healthy cells were identified from their evenly and uniformly stained nuclei. Apoptotic cells showed cell nuclear condensation and/or fragmentation. Apoptotic nuclei were counted as a percentage of total TH-positive staining cells.
Reactive oxygen species production
ROS production was detected using the fluorescence probe carboxy-H2DCFDA (6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate). Cells were loaded with H2DCFDA (10 μM) 30 min before the end of the incubation period (48 h). Cells were washed twice with PBS and fixed with 4% paraformaldehyde. Fluorescence (FITC filter) images of cells were taken immediately using a fluorescence microscope equipped with a digital camera (DP70, Sony). DCFDA cells were counted as a percentage of total TH-positive staining cells.
Cell counting
Cell counting of positive stained cells was carried out using stereology (West et al. 1991). For each well counted, a similar area was delineated and systematically sampled at high magnification (×63) from a random start position to verify cell types and observe apoptosis state in duplicate fashion. Only cells with distinct immunoreactivity, clear neuronal shape with a round soma and a nucleus were counted as TH, or H2DCFDA-positive neurons. To obtain unbiased counting, slides were coded and cells were scored blindly without previous knowledge of treatment.
Statistical analysis
All data are presented as means ± SD. One-way analysis of variance (ANOVA) and Newman–Keuls test were employed for the comparison among groups, and differences were considered significant at p < 0.05.
Results
Effects of reserpine on MPP+-induced neurotoxicity and cell apoptosis
The cultures were preincubated with reserpine (50 and 500 nM), a dopamine-depleting agent, for 4 h and then exposed to MPP+ (20 μM) for 48 h. Cell toxicity was assessed by LDH release and MTT reduction. As shown in Fig. 1a, a significant loss of cell viability, as indicated by LDH release (200% compared to basic levels set at 100%), was observed at 20 μM MPP+ compared to untreated control cells. However, when the cells were pre-incubated with reserpine (50 and 500 nM), MPP+-induced VM cell toxicity was attenuated significantly to 143% and 115%, respectively. Reserpine (500 nM) was even capable of abolishing apoptosis by MPP+ and restoring cell viability back to normal levels in the presence of MPP+ (Fig. 1b and c). No cell toxicity or apoptosis was observed in cells treated with reserpine alone.
Fig. 1.
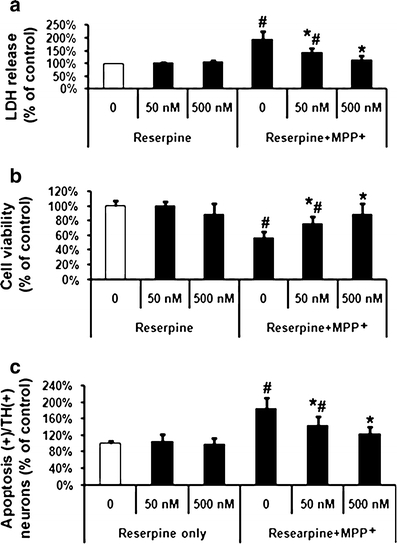
Effects of reserpine on MPP+-induced ventral mesencephalic (VM) dopaminergic neurotoxicity and cell apoptosis. MPP+ (20 μM) treated cells were pre-incubated with two concentrations (50 and 500 nM) of reserpine. a LDH release, b MTT assay, and c quantification of apoptosis in TH-positive cells were performed at 48 h after treatment with MPP+. Data are expressed as percent of values of untreated control cells and are means ± SD of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. # p < 0.05 vs. control cells treated with saline, and 50 and 500 nM reserpine, *p < 0.05 vs. MPP+ alone. There was no significant difference between cells treated with saline and 500 nM reserpine and those treated with MPP+ and 500 nM reserpine
Effects of dopamine on MPP+-induced neurotoxicity and cell apoptosis
Cells were co-treated with MPP+ and dopamine (100 and 250 μM) or dopamine alone for 48 h. Dopamine at 100 μM did not significantly affect VM cell toxicity or apoptosis in TH-positive neurons, whilst at a concentration of 250 μM led to an increase in cell toxicity and apoptosis in TH-positive neurons compared to untreated control cells (Fig. 2). Dopamine (250 μM) leads to a decrease in cell viability to 24% as assessed by MTT assay, while cell apoptosis was increased to 200% at the same time. Moreover, MPP+-induced cell toxicity and apoptosis was increased in the presence of dopamine (Fig. 2b and c), with apoptosis levels at 309% and cell survival being reduced to 24% in the presence of MPP+ and dopamine (250 μM).
Fig. 2.
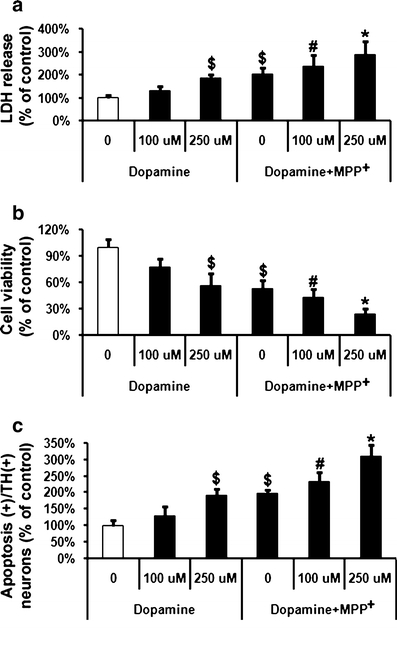
Effects of dopamine on MPP+-induced VM dopaminergic neurotoxicity and cell apoptosis. MPP+ (20 μM)-treated cells were incubated with two concentrations (100 and 250 μM) of dopamine. a LDH release, b MTT assay, and c quantification of apoptosis in TH-positive neurons were performed 48 h after treatment with MPP+. Data are expressed as percent of values in untreated control cells and are means ± S.D. of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. $Significant difference to the first column (p < 0.05 vs. control cells treated with saline), #significant difference to the first and second column (p < 0.05 vs. control cells treated with saline and vs. saline + dopamine 100 µM), *significant difference to the first, second, third and fourth columns (p < 0.05 vs. control cells treated with saline, vs. control + dopamine 100 µM, vs. control + 250 µM dopamine and vs. MPP+ alone)
Effects of COX-2 inhibitors on MPP+-induced neurotoxicity and cell apoptosis
Cells were treated with MPP+ (20 μM), the specific COX-2 inhibitor DFU (10 and 100 nM), or the non-specific COX inhibitor ibuprofen (25 and 250 μM) for 48 h. As shown in Fig. 3, DFU attenuated MPP+-induced cell toxicity as demonstrated by changes in LDH release. DFU (10 nM) reduced MPP+ induced LDH release from 196% (MPP+-treated controls) to 149% and MTT measurement was increased by DFU (10 nM) to 64% compared to 44% (MPP+ treated controls). Apoptosis in dopaminergic neurons was lower when cells were treated with DFU (10 nM), down to 152%, compared to cells which received MPP+ alone. Ibuprofen treatment resulted in similar results as those observed with DFU (Fig. 4).
Fig. 3.
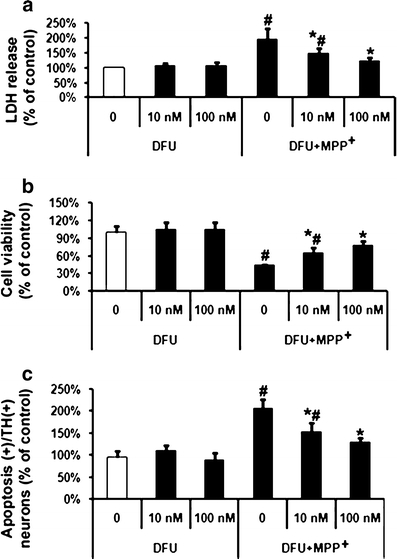
Effects of specific COX-2 inhibitor DFU on MPP+-induced VM dopaminergic neurotoxicity and cell apoptosis. MPP+ (20 μM)-treated cells were incubated with two concentrations (10 and 100 nM) of DFU. a LDH release, b MTT assay, and c quantification of apoptosis among TH-positive cells were performed at 48 h after treatment with MPP+. Data are expressed as percent of values of untreated control cells and are means ± S.D. of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. # p < 0.05 vs. control cells treated with saline, and 10 and 100 nM DFU; *p < 0.05 vs. MPP+ alone
Fig. 4.
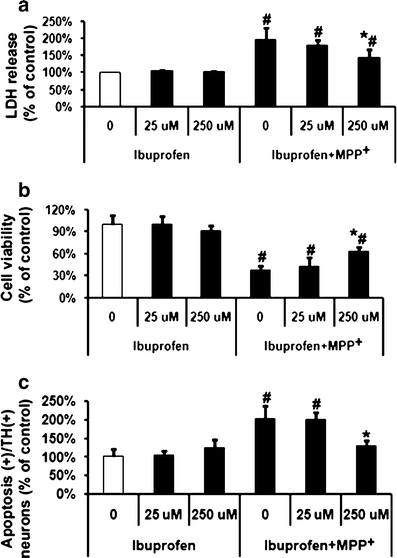
Effects of non-specific COX inhibitor ibuprofen on MPP+-induced VM dopaminergic neurotoxicity and cell apoptosis. MPP+ (20 μM)-treated cells were incubated with two concentrations (25 and 250 μM) of ibuprofen. a LDH release, b MTT assay, and c quantification of apoptosis among TH-positive cells were performed at 48 h after treatment with MPP+. Data are expressed as percent of values of untreated control cells and are means ± S.D. of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. # p < 0.05 vs. control cells treated with saline, 25 and 250 μM ibuprofen; *p < 0.05 vs. MPP+ alone
Effects of COX-2 inhibitors on dopamine-exacerbated MPP+-induced neurotoxicity and cell apoptosis
To examine whether dopamine-exacerbated MPP+-induced cell death is caused by COX-2, either DFU (10 nM) or ibuprofen (250 μM) was added after treatment with dopamine (250 μM) and/or MPP+ (20 μM). The combined treatment of VM cells with MPP+ (20 μM) and dopamine (250 μM) treatment led to an increase in VM cell toxicity (LDH release at 318%) (Fig. 5a) and apoptosis among TH-positive neurons (330%) compared to untreated control cells set at 100% (Fig. 5c). Also cell viability was reduced to 23% in the presence of dopamine (250 μM) and MPP+ (20 μM) as measured via MTT assay (Fig. 5b). DFU (100 nM) had an even more pronounced effect on MPP+-induced toxicity. DFU (100 nM) reduced MPP+-induced LDH release from 196% (MPP+-treated controls) to 124% (Fig. 3a) and MTT measurement was increased by DFU (100 nM) to 77% compared to 44% (MPP+-treated controls) (Fig. 3b). Apoptosis in dopaminergic neurons was lower when cells were treated with DFU (100 nM), down to 128% compared to cells which received MPP+ alone. (Fig. 3c) Ibuprofen treatment resulted in similar results as those observed with DFU (Fig. 4), but the lower dose of ibuprofen (25 μM) failed to have any effect on MPP+-induced apoptosis (Fig. 4c).
Fig. 5.
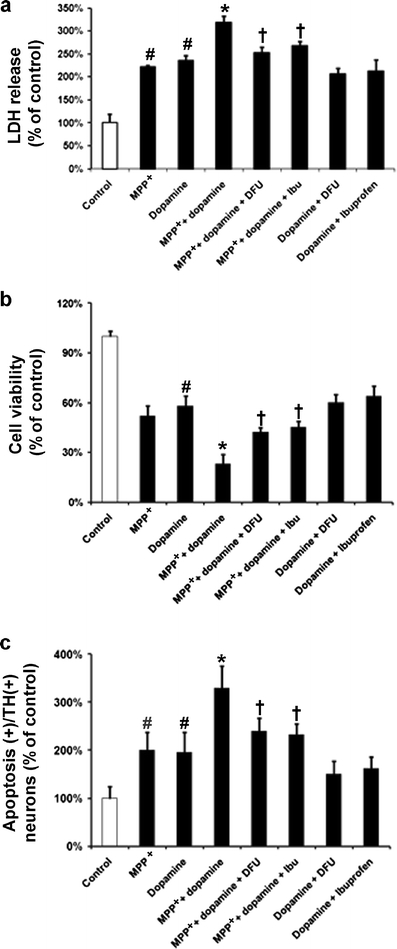
Effects of COX-2 inhibitors on MPP+-induced VM dopaminergic neurotoxicity and cell apoptosis in combination with dopamine. Cells treated with MPP+ (20 μM) or/and dopamine (250 μM) were incubated with either DFU (10 nM) or ibuprofen (250 μM). a LDH release, b MTT assay, and c quantification of apoptosis in TH-positive cells were performed 48 h after treatment with MPP+. Data are expressed as percent of values of untreated control cells and are means ± SD of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. # p < 0.05 vs. control cells; *p < 0.05 vs. MPP+ alone; † p < 0.05 vs. MPP+ + dopamine
MPP+-induced cell toxicity and apoptosis in TH-positive neurons were substantially increased when dopamine was added compared to MPP+ alone; however, this was significantly attenuated by DFU (10 nM) or ibuprofen (250 μM). LDH release due to the combined treatment of VM cells with MPP+ and dopamine was reduced to 253% in the presence of DFU (10 nM) or 268% when co-treated with ibuprofen (250 μM) (Fig. 5a). Addition of DFU (10 nM) led to a significant increase of cell viability to 42%, whereas ibuprofen (250 μM) increased cell viability to 45% (Fig. 5b). Apoptosis, as observed by counting apoptotic nuclei due to VM cells treated with MPP+ and dopamine, was reduced to 240% in the presence of DFU (10 nM) or 233% in the presence of ibuprofen (250 μM) (Fig. 5c).
Treatment with DFU (10 nM) or ibuprofen (250 μM) did not lead to any significant changes in dopamine-induced VM cell toxicity, cell viability or apoptosis (Fig. 5a, b and c). Photomicrographs demonstrating the morphology of apoptotic cells in TH-immunoreactive neurons in untreated control and treated cells are shown in Fig. 6. Cells exposed to MPP+ or/and dopamine exhibited a marked increase in nuclear fragmentation in TH-positive neurons, whereas those cells treated with DFU displayed a reduction in nuclear fragmentation. In addition, after treatment with MPP+, the numbers of TH-positive cells were decreased compared to control cells (data not shown); however, the numbers of TH-positive “apoptotic” cells in MPP+-treated cells were still increased compared to TH-positive “normal” cells. Thus, the increased apoptotic cell death observed after treatment with MPP+ is not due to the decreased number of TH-positive cells.
Fig. 6.
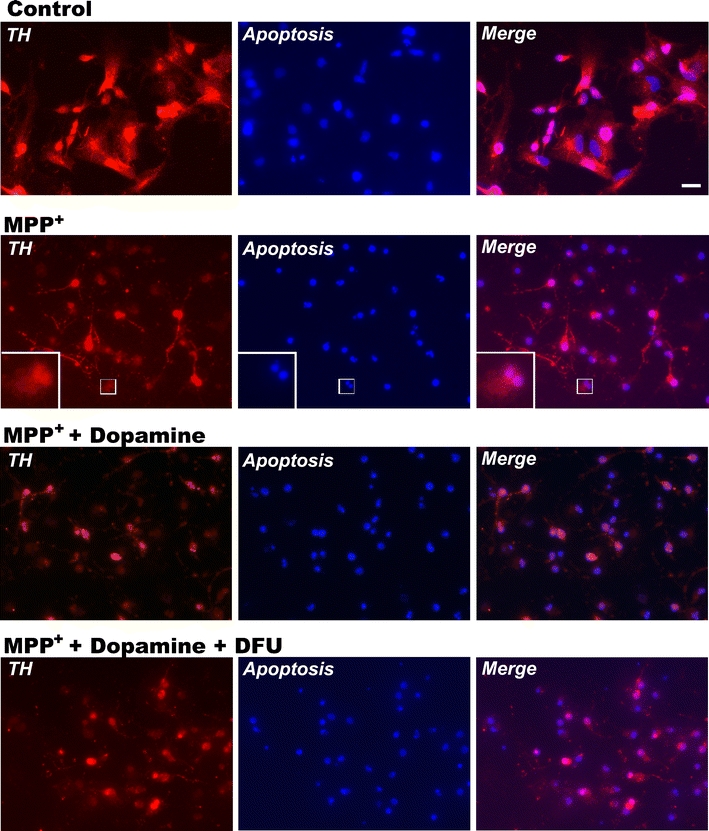
Imaging of apoptotic/TH staining. Imaging was performed 48 h after treatment with MPP+. Hoechst 33342 staining was carried out to visualize the apoptotic cells. Condensed or fragmented nuclei that were brightly stained were considered to be from apoptotic cells. Apoptosis: blue fluorescence; TH: red fluorescence. Co-localization of apoptosis and TH was observed by merging red and blue fluorescence signals. Scale bar, 10 μm
A total of 301 cells were counted for the control treatment, with an average of 16.7 ± 1.5 cells per area counted. Out of these, 20 (or 1.1 ± 0.4 per area) showed apoptosis (6.6%). There was a significant increase of apoptotic cells in the presence of dopamine, 289 cells were counted, out of which 38 showed apoptosis (13.1%); and in the case of MPP+, 232 cells were counted, with 31 showing apoptosis (13.4%). The combined treatment of dopamine and MPP+ led to apoptosis in 47 cells out of 214 counted (22%). Addition of DFU (10 nM) to the combined treatment with dopamine and MPP+ led to a significant reduction in apoptosis — 39 cells out of 247 counted (15.8%). Also ibuprofen (250 μM) was able to significantly reduce apoptosis with 34 cells out of 221 counted showing apoptosis (15.4%). Addition of DFU (10 nM) to dopamine led to a reduction in the number of apoptotic cells to 27 cells out of 266 counted (10.2%) and, in the case of ibuprofen (250 μM) 26 cells out of 243 counted showed apoptosis (10.7%). Both values showed no significant difference when compared to the control treatment where 6.6% or 20 out of 301 cells showed apoptosis.
Effects of COX-2 inhibitors on dopamine augmented MPP+-induced ROS production in VM dopaminergic neurons
To investigate the possible implication of increased ROS involvment in COX-2 toxicity, DCFDA, an indicator for the formation of hydroxyl radical (OH•), peroxynitrite, and hydrogen peroxide was used. Cells treated with dopamine (250 μM) and MPP+ (20 μM) were incubated either with DFU (10 nM) or ibuprofen (250 μM). The result showed that MPP+-induced ROS levels in TH-positive cells were markedly augmented when cells were treated with dopamine compared to cells which received only dopamine treatment without MPP+.
Numbers of cells with sufficient ROS to produce a signal were increased from 186% in the presence of MPP+ to 402% in the presence of MPP+ and dopamine. However, cells co-treated with dopamine and MPP+ incubated in the presence of either DFU or ibuprofen showed a reduction in the levels of ROS formation in TH-positive neurons to 308% and 330%, respectively (Fig. 7). No significant difference was observed in the ROS levels in TH-positive neurons after co-treatment with dopamine and DFU/ibuprofen. Photomicrographs demonstrating the DCFDA staining in TH-immunoreactive neurons in untreated control and treated cells are shown in Fig. 8. Cells exposed to MPP+ or/and dopamine displayed an increase in TH-positive neuronal staining with DCFDA, whereas those cells treated with DFU in combination with MPP+ and dopamine were able to reduce measureable ROS generation.
Fig. 7.
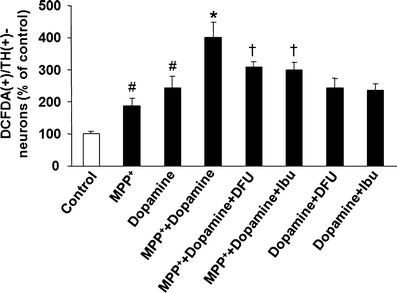
Effects of COX-2 inhibitors on dopamine augmented MPP+-induced ROS production in VM dopaminergic neurons. Cells treated with MPP+ (20 μM) and/or dopamine (250 μM) were incubated with either DFU (10 nM) or ibuprofen (250 μM). Quantification of ROS formation in TH-positive cells was performed at 48 h after treatment with MPP+. Data are expressed as percent of values in untreated control cells and are means ± SD of three independent experiments. All treatments were performed in duplicate and data expressed were averaged values of all cells counted in each condition. The results were compared by one-way ANOVA and Newman–Keuls test. # p < 0.05 vs. control cells; *p < 0.05 vs. MPP+ alone; † p < 0.05 vs. MPP+ + dopamine. Scale bar, 20 μm
Fig. 8.
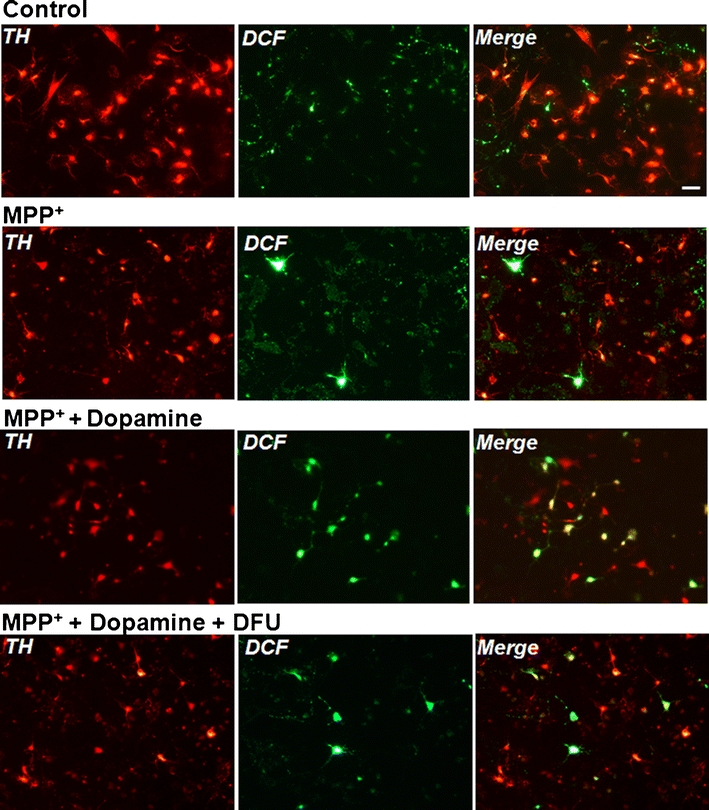
Imaging of MPP+-induced ROS production in VM dopaminergic neurons. Imaging of DCFDA/TH staining were performed at 48 h after treatment with MPP+. ROS production was detected by DCFDA staining. DCFDA: green fluorescence; TH: red fluorescence. Co-localization of DCFDA and TH was observed by merging red and green fluorescence signals. DCFDA 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; Scale bar, 20 μm
Discussion
The findings of this study reveal that reserpine significantly reduced VM dopaminergic neurotoxicity induced by MPP+, whereas dopamine increased the MPP+-induced VM cell toxicity and apoptosis in TH-positive neurons. Despite our own hypothesis we were only able to demonstrate an additive effect of dopamine on MPP+-induced toxicity instead of any synergism.
Herein we demonstrate that dopamine added to a neurotoxin increases cellular apoptosis in an additive manner and ROS formation can be attenuated by the addition of a COX-2 inhibitor. Although the exact mechanism is unknown, three possible mechanisms of action can be attributed to the observed effects: the ability of COX-2 to generate ROS (Smith et al. 2000), the ability of COX-2 generated ROS, with dopamine itself inside the dopaminergic neurons to generate dopamine-quinone (Hastings 1995; Teismann et al. 2003a), and, finally, the ability of COX-2 to produce neurotoxic PGE2 (O’Banion 1999).
Inhibition of COX-2 by DFU or ibuprofen significantly attenuated the effect obtained with dopamine given in addition to MPP+ with respect to cell toxicity, apoptosis and ROS production. Inhibition of COX-2 appears to be through inhibition of ROS production. It also has been suggested that dopamine plays a key role in the demise of nigrostriatal neurons since dopamine containing neurons die in PD.
In this study, we demonstrated that dopamine depletion by treatment with reserpine protected against MPP+-induced cell toxicity and apoptosis in VM dopaminergic neurons. This indicates that dopamine plays a role in the toxicity of MPP+ in VM dopaminergic neurons. Moreover, the mechanism of dopamine neurotoxicity is highly linked to increased production of oxidizing species, which has been implicated in the pathogenesis of PD (Liang et al. 2005; Teismann et al. 2003a). Several reports have shown that dopamine can be oxidized to dopamine-quinone, which is toxic to cells (Blum et al. 2001; Dryhurst 2001). A study has also shown that treatment with dopamine of HEK293 cells or rat striatal neuronal cultures induces apoptosis through a mechanism dependent on ROS (Luo et al. 1998).
Thus, identification of the cellular factor that could facilitate or induce oxidation of dopamine would provide an attractive strategy in the understanding of the pathogenesis of dopaminergic degeneration in PD. MPP+ possesses two opposing effects, on one hand it leads to an extensive release of dopamine and on the other hand MPP+ inhibits monamine oxidase (MAO)-A (Feuerstein et al. 1988), with MAO-B only slightly inhibited (Fritz et al. 1985), thereby counteracting the oxidation of dopamine. A process by which dopamine oxidation still could occur is via COX-2, as COX-2 itself can lead to the generation of ROS (Smith et al. 1991) and has been shown to react with dopamine to form dopamine-quinone (Teismann et al. 2003a).
l-Dihydroxyphenylalanine (l-DOPA) which is used to relieve parkinsonian symptoms is converted by neuronal aromatic l-amino acid decarboxylase into dopamine after administration. This could lead to increased ROS formation as systemic administration of l-DOPA has been shown to significantly increase nigral hydroxyl radical production in the freely moving rat (Spencer Smith et al. 1994). Additionally, rats lesioned with 6-hydroxy-dopamine (6-OHDA) showed less generation of ROS and lesion volume when subjected to malonate treatment using microdialysis (Ferger et al. 1999). Intrastriatal malonate injections generate selective neuronal cell death similar to that seen in transient ischemia or Huntington’s disease. Herein malonate was applied via the probe to study synaptic dopamine release and the generation of hydroxyl (•OH) radicals by microdialysis.
On the other hand several studies have demonstrated that neither genetic nor pharmacologic dopamine depletion protects the nigrostriatal pathway from acute MPTP toxicity in mice (Hasbani et al. 2005). There are several lines of evidence in vivo as well as in vitro, supporting this hypothesis (Hastings et al. 1996; Rabinovic et al. 2000). Moreover, l-DOPA taken by healthy patients did not cause any reduction in numbers of nigral neuronal cells nor did it promote PD (Quinn et al. 1986; Rajput et al. 1997). l-DOPA is then converted by neuronal aromatic l-amino acid decarboxylase into dopamine, thereby restoring dopamine levels in surviving neurons. However these cell populations continue to die despite the l-DOPA treatment (Basma et al. 1995).
Studies have shown that a COX-1 inhibitor failed to antagonize the MPTP-induced striatal dopamine depletion in mice (Aubin et al. 1998). Ablation of the COX-1 gene in knockout mice failed to produce neuroprotection on MPTP-elicited toxicity in contrast to COX-2 knockout mice (Feng et al. 2002; Teismann et al. 2003a). Therefore, COX-2 plays a major role in mediating MPP+-induced VM neurotoxicity and VM dopaminergic cell apoptosis. Additionally, in the present study, COX-2 inhibitors do not prevent toxicity of dopamine alone, and consistently inhibit only the “MPP+ portion” of toxicity in the co-stress condition, leaving intact the same degree of cell death observed with dopamine alone.
This suggests that COX-2 may only be relevant when MPP+ is applied and COX-2 inhibition does not affect normal ROS formation due to dopamine turnover. This also suggests that dopamine itself leads to radical formation, but a healthy system is capable of dealing with these low levels of ROS. In the case of a dysregulation, as seen in PD, dopamine becomes a substrate for activated COX-2 and therefore a source of ROS, leading to cell death.
In our hands DFU showed a major effect already at very low concentrations (10 nM), whereas similar results could only be obtained with a higher dose of ibuprofen (250 μM). DFU alone is a very potent COX-2 inhibitor, with the highest specificity for COX-2 inhibition (1,000-fold selectivity for COX-2 vs. COX-1). Also, the ED50 of DFU vs. COX-2 is higher than that of ibuprofen (Riendeau et al. 1997), which very likely resulted in the low effective dose of DFU as seen in this study.
It is widely accepted that inflammation and oxidative stress are interrelated. Oxidative stress can increase inflammatory activity and, conversely, inflammation is known to cause oxidative stress (Liao et al. 2007; Madrigal et al. 2003; Teismann et al. 2003a). It is also evident that COX-2 plays a detrimental role in stimulation of an inflammatory process following neuronal death. PGE2, a key product of COX-2, is the principal pro-inflammatory prostanoid and PGE2 levels were shown to increase in MPTP-treated mice as well as after neurotoxic insult in mixed and enriched mesencephalic cultures (Teismann et al. 2003a; Wang et al. 2005). Future studies are needed to clarify whether PGE2, generation of dopamine-quinone or a combination of both effects are responsible for the selective vulnerability of dopaminergic neurons to COX-2-induced oxidative stress.
In summary, dopamine appears to be a component of MPP+-induced toxicity in VM dopaminergic neurons. The protective effects of COX-2 inhibitors on MPP+-induced neurotoxicity in the presence of dopamine and cell apoptosis may be mediated via reduction of ROS. Dopamine toxicity seems to be driven mainly by conversion through COX-2 in the presence of MPP+ and thus might be one factor responsible for the specific cell death of dopaminergic neurons as seen in PD. Our observations confirm that inhibition of COX-2 activity is a valid strategy to delay or prevent the loss of dopaminergic neurons in PD.
Acknowledgements
This work was supported by NHS Endowment Fund 06/09 and Wellcome Trust WT080782MF. DFU was provided by Merck & Co. Inc. (Rahway, NJ, USA). We thank Professor Peter McCaffery for his assistance and help in the preparation of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998;71:1635–1642. doi: 10.1046/j.1471-4159.1998.71041635.x. [DOI] [PubMed] [Google Scholar]
- Basma AN, Morris EJ, Nicklas WJ, Geller HM. l-DOPA cytotoxicity to PC12 cells in culture is via its autoxidation. J Neurochem. 1995;64:825–832. doi: 10.1046/j.1471-4159.1995.64020825.x. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/S0301-0082(01)00003-X. [DOI] [PubMed] [Google Scholar]
- Chu CY, Liu YL, Chiu HC, Jee SH. Dopamine-induced apoptosis in human melanocytes involves generation of reactive oxygen species. Br J Dermatol. 2006;154:1071–1079. doi: 10.1111/j.1365-2133.2006.07293.x. [DOI] [PubMed] [Google Scholar]
- Dryhurst G. Are dopamine, norepinephrine, and serotonin precursors of biologically reactive intermediates involved in the pathogenesis of neurodegenerative brain disorders? Adv Exp Med Biol. 2001;500:373–396. doi: 10.1007/978-1-4615-0667-6_61. [DOI] [PubMed] [Google Scholar]
- Fahn S, Przedborski S. Parkinsonism. In: Rowland LP, editor. Merritt’s neurology. New York: Lippincott Williams & Wilkins; 2000. pp. 679–693. [Google Scholar]
- Feng Z, Wang T, Li D, Fung P, Wilson B, Liu B, Ali S, Langenbach R, Hong J. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354. doi: 10.1016/S0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- Ferger B, Eberhardt O, Teismann P, de Groote C, Schulz JB. Malonate-induced generation of reactive oxygen species in rat striatum depends on dopamine release but not on NMDA receptor activation. J Neurochem. 1999;73:1329–1332. doi: 10.1046/j.1471-4159.1999.0731329.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ, Hedler L, Jackisch R, Hertting G. An in vitro model of 1-methyl-4-phenyl-pyridinium (MPP+) toxicity: incubation of rabbit caudate nucleus slices with MPP + followed by biochemical and functional analysis. Br J Pharmacol. 1988;95:449–458. doi: 10.1111/j.1476-5381.1988.tb11665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz RR, Abell CW, Patel NT, Gessner W, Brossi A. Metabolism of the neurotoxin in MPTP by human liver monoamine oxidase B. FEBS Lett. 1985;186:224–228. doi: 10.1016/0014-5793(85)80713-4. [DOI] [PubMed] [Google Scholar]
- Hasbani DM, Perez FA, Palmiter RD, O’Malley KL. Dopamine depletion does not protect against acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in vivo. J Neurosci. 2005;25:9428–9433. doi: 10.1523/JNEUROSCI.0130-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem. 1995;64:919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Luo Y, Umegaki H, Munoz J, Roth GS. Intrastriatal injection of dopamine results in DNA damage and apoptosis in rats. Neuroreport. 1998;9:2569–2572. doi: 10.1097/00001756-199808030-00026. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K. TGF-β superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 1995;14:736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi ET, Mytilineou C. Cell culture models of neuronal degeneration and neuroprotection. Implications for Parkinson’s disease. Adv Exp Med Biol. 1998;446:203–222. doi: 10.1007/978-1-4615-4869-0_12. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem Int. 2007;50:905–915. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Primary rat mesencephalic neuron-glia, neuron-enriched, microglia-enriched, and astroglia-enriched cultures. Methods Mol Med. 2003;79:387–395. doi: 10.1385/1-59259-358-5:387. [DOI] [PubMed] [Google Scholar]
- Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, Bosca L, Leza JC. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn N, Parkes D, Janota I, Marsden CD. Preservation of the substantia nigra and locus coeruleus in a patient receiving levodopa (2 kg) plus decarboxylase inhibitor over a four-year period. Mov Disord. 1986;1:65–68. doi: 10.1002/mds.870010109. [DOI] [PubMed] [Google Scholar]
- Rabinovic AD, Lewis DA, Hastings TG. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience. 2000;101:67–76. doi: 10.1016/S0306-4522(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Rajput A, Kishore A, Snow B, Bolton CF, Rajput AH. Dopa-responsive, nonprogressive juvenile parkinsonism: report of a case. Mov Disord. 1997;12:453–456. doi: 10.1002/mds.870120332. [DOI] [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret JP, Ford-Hutchinson AW, Gordon R, Greig G, Gresser M, Guay J, Kargman S, Leger S, Mancini JA, O’Neill G, Ouellet M, Rodger IW, Therien M, Wang Z, Webb JK, Wong E, Chan CC. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-P. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Spencer Smith T, Parker WD, Jr, Bennett JP., Jr L-DOPA increases nigral production of hydroxyl radicals in vivo: Potential L-DOPA toxicity. Neuroreport. 1994;5:1009–1011. doi: 10.1097/00001756-199404000-00039. [DOI] [PubMed] [Google Scholar]
- Takeshima T, Shimoda K, Sauve Y, Commissiong JW. Astrocyte-dependent and -independent phases of the development and survival of rat embryonic day 14 mesencephalic, dopaminergic neurons in culture. Neuroscience. 1994;60:809–823. doi: 10.1016/0306-4522(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jacksonlewis V, Przedborski S. COX-2 and Neurodegeneration in Parkinson’s Disease. Ann N Y Acad Sci. 2003;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS. MPP + −induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005;19:1134–1136. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Murphy DD, Giasson BI, Zhang B, Trojanowski JQ, Lee VM. Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons. J Cell Biol. 2004;165:335–346. doi: 10.1083/jcb.200403061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- Yu PH, Lai CT, Boulton AA. Effect of adding selegeline to levodopa in early, mild Parkinson’s disease. Selegeline may be toxic in presence of increased dopamine concentrations. BMJ. 1996;312:703–704. doi: 10.1136/bmj.312.7032.703b. [DOI] [PMC free article] [PubMed] [Google Scholar]


