Abstract
Background. Patients with symptomatic kidney stones are characterized by older age, male gender, white race, hypertension, obesity, metabolic syndrome and chronic kidney disease. Whether these characteristics differ in patients with asymptomatic kidney stones is unknown.
Methods. All potential kidney donors who underwent protocol computed tomography angiograms/urograms (2000–08) at the Mayo Clinic were identified. Renal abnormalities, including kidney stones, were assessed radiographically. Comorbidities, including past symptomatic kidney stones, were abstracted from the medical record. Characteristics of persons with and without radiographic stones were compared. Stone burden among persons with and without past symptomatic stones was compared.
Results. Among 1957 potential kidney donors, 3% had past symptomatic stones and 11% had radiographic stones (10% had only asymptomatic radiographic stones). Asymptomatic stone formers were more likely to be of white race, have low urine volumes and have radiographic findings of renal parenchymal thinning, focal renal scarring, medullary sponge kidney and polycystic kidney disease. Asymptomatic stone formers were not characterized by older age, male gender, hypertension, obesity, metabolic syndrome, abnormal kidney function, hyperuricemia, hypercalcemia or hypophosphatemia. Among persons with radiographic stones, those with past symptomatic stones had a slightly higher number of stones (mean 2.7 versus 2.4; P = 0.04), but a much greater diameter for the largest stone (mean 4.8 versus 1.6 mm; P < 0.001).
Conclusions. Asymptomatic kidney stone formers have different demographic characteristics and many lack the comorbidities that have been described in persons with symptomatic kidney stones. These findings suggest that different pathophysiologic mechanisms could be involved in asymptomatic stone formation versus symptomatic stone passage.
Keywords: chronic kidney disease, kidney anatomy, kidney stone, nephrolithiasis
Introduction
Up to 12% of men and 5% of women will develop a symptomatic kidney stone during their lifetime [1], and almost all of what we know about risk factors for kidney stones are derived from this symptomatic subset of stone formers [1–3]. Symptomatic stones are more prevalent in men, white individuals and older adults [1, 4] and have been linked to many systemic conditions including hyperparathyroidism [5, 6], diabetes mellitus [7], obesity [8, 9], metabolic syndrome [10–12], hypertension [13–15], gastric bypass [16] and chronic kidney disease [17–21]. Symptomatic kidney stones have also been associated with polycystic kidneys [22], upper urinary tract dilatation [23] and medullary sponge kidney [24, 25]. However, it is unknown whether asymptomatic stone disease has similar risk factor and comorbidity associations, and the stone burden among persons with asymptomatic versus symptomatic kidney stones has not been adequately characterized. This is an important issue to resolve since shared risk factors would imply that asymptomatic kidney stones are simply the precursors of later symptomatic ones, while a different risk factor profile would suggest instead that pathophysiological mechanisms may differ between asymptomatic and symptomatic stones formers. Unfortunately, the lack of systematic data collection on asymptomatic kidney stone patients has hampered efforts to answer this question. We took advantage of the opportunity to study potential kidney donors, who undergo high resolution computed tomography (CT) scans of the kidneys that are not available in existing general population studies. Recognizing that potential kidney donors are selected on health, the primary objective of this study was to determine if asymptomatic kidney stone formers are characterized by any of the comorbidities described in symptomatic kidney stone formers. A secondary objective was to characterize the radiographic stone burden between potential kidney donors with and without a past history of symptomatic kidney stones.
Materials and methods
Study population
Per standard protocol, all potential living kidney donors seen at the Mayo Clinic between 30 March 2000 and 23 July 2008, underwent CT angiography/urography to evaluate anatomy and identify any abnormalities of the renal arteries and kidneys [26]. Prior to their initial clinic visit, all potential donors were pre-screened. Based on a telephone interview with a transplant nurse, potential donors were excluded from further evaluation if they reported diabetes mellitus, use of more than one antihypertensive medication (two were permitted if one was a thiazide diuretic), illegal drug use, psychiatric disorders, hepatitis, human immunodeficiency virus, significant cardiovascular disease or a history of urologic procedures that would preclude donation. Some potential donors who reported a history of symptomatic kidney stones on the telephone interview were excluded from further evaluation, particularly if frequent symptomatic stone passage was present. The remaining potential donors underwent further evaluation at a clinic visit that included pre-scheduled CT angiography/urography of the kidneys. The medical records of the potential kidney donors who authorized the use of their medical records for research in accordance with Minnesota State law (97%) were reviewed for clinical, laboratory and radiographic findings [27].
CT scans
CT angiograms/urograms were interpreted by radiologists with specialized interest in genitourinary imaging and included precontrast scans for kidney stones. In 2000–05, all exams were acquired on a 4-channel multidetector CT scanner (Qxi; GE Medical Systems, Milwaukee, WI). Beginning in September of 2005, all renal donor CT evaluations were obtained on a 64-channel multidetector CT scanner (Sensation 64; Siemens Medical Solutions, Forchheim, Germany). All CT scan reports were manually reviewed (enhanced chemiluminescence), and stones identified by the radiologist as being ‘tiny’ were labeled as 0.5 mm in diameter. If the size of the largest stone or the number of stones was not specified in the report, the CT images were reviewed to capture this data. Reported parenchymal calcifications were not considered stones. Any mention of caliectasis, pyelectasis or a generous dilated or prominent collecting system was identified as upper urinary tract dilatation. Polycystic kidney disease was identified if the radiologist described findings consistent with polycystic kidney disease or if it was diagnosed in the medical record.
Other donor characteristics
Potential kidney donors undergo a detailed clinical evaluation by a nephrologist to determine suitability for donation. All charts were manually reviewed for race, medications (calcium supplement, multivitamin, vitamin C supplement, allopurinol and thiazide diuretic), blood pressure, body mass index (BMI), past symptomatic kidney stones, comorbidities [diabetes, hypertension, history of urinary tract infection, gastric bypass, chronic diarrhea and history of cardiovascular events (stroke, myocardial infarction, heart failure and symptomatic peripheral arterial disease)] and habits (alcohol use and cigarette smoking). Protocol-based laboratory studies included glomerular filtration rate (iothalamate clearance), 24-h urine volume, 24-h urine albumin and protein, as well as serum total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, fasting glucose, uric acid, calcium and phosphorus. Urine volume was dichotomized at 1000 mL to assess the association between very low urine volumes and kidney stones. Urine albumin was dichotomized at 15 mg and 30 mg/24 h since 23% of potential donors had urine albumin levels below the threshold of detection. Metabolic syndrome was defined by a BMI > 30 kg/m2 and any two of the following: (i) triglycerides ≥ 150 mg/dL, (ii) HDL cholesterol < 40 mg/dL in men and <50 mg/dL in women, (iii) fasting glucose ≥ 100 mg/dL or (iv) 18-h mean systolic blood pressure ≥ 130 or 18-h mean diastolic blood pressure ≥ 85 or antihypertensive therapy [28].
Statistical analysis
For the primary analysis, we assessed associations with asymptomatic kidney stones by comparing persons with radiographic stones to persons without radiographic stones, after excluding potential kidney donors with past symptomatic kidney stones. To be comparable to prior studies on kidney stones, we also compared persons with and without a history of past symptomatic stones. Demographic characteristics, comorbidities, medications, laboratory studies and radiographic findings were compared between groups by Rank sums tests (continuous) and chi-square tests (categorical). Among potential donors with radiographic kidney stones, the number of stones, the diameter of the largest stone and the proportion with bilateral stones were compared between persons with and without past symptomatic kidney stones. A multivariable logistic regression model assessed the likelihood of past symptomatic kidney stones with the radiographic number of stones, diameter of largest stone and presence of bilateral stones. A P-value <0.05 was considered statistically significant. All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC).
Results
The study sample consisted of 1957 potential living kidney donors who were 58% female, 92% white and had a mean age of 43 years (range, 18–76 years). As expected, potential living kidney donors were characterized as a population with a low burden of diabetes mellitus (0.2%) and past cardiovascular events (0.4%), and these comorbidities were not further evaluated. However, other comorbidities known to associate with kidney stones, particularly, hypertension (15%), obesity (30%) and metabolic syndrome (28%) were common. Overall, 2.7% (n = 53) of the potential donors had past symptomatic kidney stones, whereas 11% (n = 210) had radiographic kidney stones on CT scan. The 53 subjects with past symptomatic stones were more likely (P < 0.0001) to have radiographic stones [47% (95% CI: 34–60%)] than persons without past symptomatic stones [9.7% (95% CI: 8.5–11%)].
Many of the associations reported with symptomatic kidney stones were not reproduced in the subjects with asymptomatic stones (Tables 1 and 2). The prevalence of asymptomatic stones did not vary by age (>45 years: 9.8% versus <45 years: 9.8%; P = 0.93) or sex (men: 9.9% versus women: 9.4%; P = 0.68) but differed by race (whites: 10% versus non-whites: 5.2%; P = 0.045). The prevalence of past symptomatic stones did differ by age (>45 years: 3.9% versus <45 years: 1.7%; P = 0.003) but was not statistically significant for gender (men: 3.4% versus women: 2.2%; P = 0.12) or race (whites: 2.5% versus non-whites: 2.5%; P = 0.89). Potential donors with asymptomatic radiographic kidney stones were more likely than the 1719 unaffected subjects to have 24-h urine volume <1000 mL , renal parenchymal thinning, renal focal scarring, medullary sponge kidney and polycystic kidney disease but were not more likely to have hypertension, obesity, metabolic syndrome, albuminuria, reduced glomerular filtration rate, hyperuricemia, hypercalcemia or hypophosphatemia. In comparison, potential donors with past symptomatic kidney stones were more likely to have hypertension, albuminuria and hypophosphatemia. With age-adjustment, the association with hypertension was not statistically significant (P = 0.25), but the associations with 24-h urine albumin >15 mg (P = 0.002) and lower serum phosphorus (P = 0.004) remained.
Table 1.
Demographics, comorbidities and medications by kidney stone group among 1957 potential kidney donors evaluated at the Mayo Clinic between 2000 and 2008
| Group 1: no radiographic or past symptomatic stones (N = 1719) | Group 2: asymptomatic radiographic stones only (N = 185) | Group 3: past symptomatic stones (N = 53)a | P-value | ||
| Characteristic | Mean ± SD or n (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | Group 1 versus 2 | Groups 1 and 2 versus 3 |
| Age, years | 43 ± 12 | 44 ± 12 | 48 ± 11 | 0.83 | 0.007 |
| Male gender | 724 (42) | 75 (41) | 728 (53) | 0.68 | 0.12 |
| White race | 1514 (87) | 172 (96) | 747 (92) | 0.045 | 0.89 |
| Hypertension | 247 (14) | 26 (14) | 13 (25) | 0.91 | 0.038 |
| History of urinary tract infection | 109 (6.3) | 16 (8.6) | 6 (11) | 0.23 | 0.17 |
| Daily alcohol use | 108 (6.3) | 8 (4.3) | 3 (5.7) | 0.29 | 0.90 |
| Current smoker | 370 (22) | 33 (18) | 12 (23) | 0.24 | 0.80 |
| Chronic diarrhea | 15 (0.9) | 0 (0) | 1 (1.9) | 0.20 | 0.38 |
| Gastric bypass | 8 (0.5) | 1 (0.5) | 1 (1.9) | 0.89 | 0.15 |
| Body mass index > 30 kg/m2 | 602 (35) | 52 (28) | 17 (32) | 0.06 | 0.73 |
| Metabolic syndrome | 480 (28) | 41 (22) | 14 (26) | 0.10 | 0.85 |
| Calcium supplement | 191 (11) | 23 (12) | 9 (17) | 0.59 | 0.20 |
| Multivitamin | 444 (26) | 44 (24) | 16 (30) | 0.55 | 0.45 |
| Vitamin C supplement | 108 (6.3) | 8 (4.3) | 5 (9.4) | 0.29 | 0.32 |
| Thiazide diuretic | 65 (3.8) | 6 (3.2) | 3 (5.7) | 0.71 | 0.47 |
Subjects included those with and without current radiographic stones.
Table 2.
Laboratory and radiographic findings by kidney stone group among 1957 potential kidney donors evaluated at the Mayo Clinic between 2000 and 2008
| Group 1: no radiographic or past symptomatic stones (N = 1719) | Group 2: asymptomatic radiographic stones only (N = 185) | Group 3: past symptomatic stones (N = 53)a | P-value | ||
| Characteristic | Mean ± SD or n (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | Group 1 versus 2 | Groups 1 and 2 versus 3 |
| GLomerualr filtration rate, mL/min/1.73 m2 | 101 ± 19 | 102 ± 21 | 102 ± 19 | 0.29 | 0.60 |
| 24-h urine volume, mL | 2045 ± 904 | 1960 ± 868 | 1807 ± 884 | 0.34 | 0.06 |
| 24-h urine volume < 1000 mL | 166 (10) | 27 (15) | 9 (17) | 0.028 | 0.12 |
| 24-h urine protein, mg | 48 ± 41 | 55 ± 49 | 55 ± 43 | 0.071 | 0.32 |
| 24-h urine albumin > 15 mg | 144 (9.0) | 18 (11) | 12 (23) | 0.49 | 0.0007 |
| 24-h urine albumin > 30 mg | 56 (3.5) | 6 (3.6) | 7 (13) | 0.96 | 0.0002 |
| Total cholesterol, mg/dL | 197 ± 38 | 199 ± 41 | 203 ± 49 | 0.90 | 0.75 |
| Fasting glucose, mg/dL | 96 ± 9.5 | 96 ± 9.8 | 97 ± 11 | 0.29 | 0.48 |
| Serum uric acid, mg/dL | 5.4 ± 1.4 | 5.2 ± 1.4 | 5.2 ± 1.3 | 0.12 | 0.55 |
| Serum calcium, mg/dL | 9.6 ± 0.3 | 9.6 ± 0.4 | 9.6 ± 0.3 | 0.64 | 0.69 |
| Serum phosphorus, mg/dL | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.3 ± 0.5 | 0.06 | 0.002 |
| Any upper tract dilatation | 38 (2.2) | 8 (4.3) | 3 (5.7) | 0.08 | 0.14 |
| Medullary sponge kidney | 17 (1) | 19 (10) | 4 (7.6) | <0.0001 | <0.0001 |
| Focal scarring | 49 (2.9) | 15 (8.1) | 6 (11) | 0.0002 | 0.002 |
| Parenchymal thinning/atrophy | 11 (0.6) | 5 (2.7) | 1 (1.9) | 0.004 | 0.42 |
| Polycystic kidney disease | 1 (0.1) | 5 (2.7) | 0 (0) | <0.0001 | 0.68 |
Subjects included those with and without current radiographic stones.
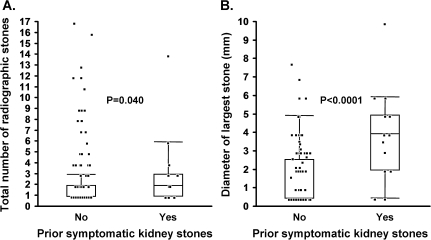
Among persons with radiographic stones, those who also had past symptomatic stones had a higher number of stones (mean: 2.7 versus 2.4; P = 0.04) and a greater diameter (mean: 4.8 versus 1.6 mm; P < 0.0001) of the largest stone (Figure 1). The prevalence of bilateral kidney stones was also higher in persons with past symptomatic kidney stones (48% versus 23%; P = 0.010). In the multivariable model, past symptomatic stones had a statistically significant association with the diameter of the largest stone (P < 0.0001) but not with the total number of stones (P = 0.083) or with bilateral stones (P = 0.056).
Fig. 1.
Among 210 potential kidney donors with kidney stones on their CT scan, the association of prior symptomatic kidney stones with (A) total number of radiographic stones and with (B) diameter of largest radiographic stone. Points representing each person were jittered with superimposed box plots. P-value by rank sum test.
Discussion
Our study shows that asymptomatic kidney stone formers may not share the same burden of comorbidities that has been described in symptomatic stone formers. Whereas previous studies have shown that symptomatic stones to be more prevalent among older adults and men [1, 4] and to be associated with systemic conditions such as hypertension [13–15], obesity [8, 9], metabolic syndrome [10–12] and chronic kidney disease [17–21, 29], we found that the prevalence of asymptomatic radiographic stones did not differ by age or gender or with hypertension, obesity, metabolic syndrome, urine albumin or glomerular filtration rate. The differing characteristics among persons with asymptomatic compared to symptomatic stones suggest that there could be different underlying mechanisms and sequelae for stone formation versus stone growth and passage.
In our cohort of potential kidney donors, asymptomatic stones were common and found in 9.7%. Asymptomatic stones were associated with a different set of risk factors compared to symptomatic stones. Potential donors with asymptomatic stones were more likely to be of white race but were not characterized by older age or male gender as is associated with symptomatic stones [1, 4]. Donors with asymptomatic stones were more likely to have 24-h urine volume < 1000 mL, but no significant difference in mean urine volumes, suggesting that only very low urine volumes contribute to stone formation in this population. Among our total cohort of potential donors, 25% had at least one anatomical abnormality of the renal arteries or kidneys on the CT scan [26]. The association of asymptomatic stones with renal parenchymal thinning/atrophy and focal scarring suggests presence of either occult reflux or parenchymal injury from the stones in these donors. The association of stones with medullary sponge kidney [24, 25] and polycystic kidney disease [22] has been previously reported. Interestingly, many cardiovascular risk factors known to associate with symptomatic stones, including hypertension, obesity, metabolic syndrome and urine albumin, were not associated with asymptomatic stones.
Findings for the potential donors with past symptomatic kidney stones were more consistent with the published literature insofar as they had larger radiographic stones and were more likely to be older and have hypertension. Symptomatic stone formers were also more likely to have albuminuria, as other investigators have noted [30]. This finding is important because most prior studies associating kidney stones with chronic kidney disease have relied on serum creatinine or diagnostic codes and lacked urine albumin measures [17–21, 29]. In addition, we confirmed an association between symptomatic kidney stones and lower serum phosphorus levels that others have reported [31]. Lower serum phosphorus levels in stone formers may be a manifestation of occult hyperparathyroidism [32] or other genetic or acquired causes of renal phosphate wasting that promote hypercalciuria [33].
Among persons with radiographic stones, past symptomatic stone formers had much larger stones but only slightly more stones than asymptomatic stone formers. Thus stone size, more than stone number and bilateral stones, was the most informative predictor of past symptomatic stone passage. This relationship is biologically plausible as a single large stone will obstruct with passage, leading to pain, whereas many small stones can pass through the urinary tract without obstruction or pain. These findings support the use of stone growth as a surrogate for symptomatic passage. It appears that low urine volume (<1000 mL in 24 h) may be a modifiable risk factor for preventing stone formation. Whether other dietary interventions that are commonly used in symptomatic stone formers [34] are of clinical benefit in patients with small asymptomatic stones is unclear.
Our study has several potential limitations that may explain why we could not detect several previously reported associations with symptomatic kidney stones. Given that our study population consisted of potential kidney donors, the spectrum and severity of symptomatic stone disease and comorbidities was limited compared to the general population unselected on health status. In particular, many patients with a history of past symptomatic stones, diabetes and cardiovascular disease were excluded. Some potential kidney donors are also related to recipients with kidney failure from kidney stones (0.2%) or conditions that cause kidney stones (polycystic kidney disease in 5% and congenital obstruction or reflux in 3%), but these conditions were not associated with either radiographic or past symptomatic stones in the donors (P > 0.05 for all). Nonetheless, these limitations must be balanced with the lack of systematically collected CT scans in less select populations. Furthermore, asymptomatic kidney stones were still common (10%) among potential kidney donors despite selection on health. Another limitation of our study was the absence of information on stone composition or urine chemistries. Finally, the CT scan technology changed over the 9-year study period, but this did not lead to changes in prevalence of radiographic findings including kidney stones [26].
In conclusion, our study suggests that asymptomatic stones are not only common but may be relatively benign given the lack of association with many of the comorbidities that associate with symptomatic kidney stones. The pathophysiology of asymptomatic stones appears to involve different genetic and environmental factors than those governing stone growth and symptomatic passage. Asymptomatic stones do not appear to always be precursors for symptomatic stones. In fact, the mean age difference between the two groups of stone formers in our study was only 4 years. Future research is needed to assess the long-term outcome of radiographic stones, particularly in persons who only have small stones and never develop symptomatic disease.
Acknowledgments
This study was presented at the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
Confilict of interest statement. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P50 DK083007, R01 DK090358, K23 DK078229), U.S. Public Health Service.
References
- 1.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Bansal AD, Hui J, Goldfarb DS. Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol. 2009;4:680–684. doi: 10.2215/CJN.05181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CM, Wilson DM, O'Fallon WM, et al. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int. 1979;16:624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- 4.Soucie JM, Coates RJ, McClellan W, et al. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol. 1996;143:487–495. doi: 10.1093/oxfordjournals.aje.a008769. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo A, Calo L, Cantaro S, et al. Calciotropic hormones and nephrolithiasis. Miner Electrolyte Metab. 1997;23:269–272. [PubMed] [Google Scholar]
- 6.Parks J, Coe F, Favus M. Hyperparathyroidism in nephrolithiasis. Arch Intern Med. 1980;140:1479–1481. [PubMed] [Google Scholar]
- 7.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 8.Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645–1652. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 9.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 10.Abate N, Chandalia M, Cabo-Chan AV, Jr., et al. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 11.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 12.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Cappuccio FP, Strazzullo P, Mancini M. Kidney stones and hypertension: population based study of an independent clinical association. BMJ. 1990;300:1234–1236. doi: 10.1136/bmj.300.6734.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 15.Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 16.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–7. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 17.Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67:685–690. doi: 10.1111/j.1523-1755.2005.67128.x. [DOI] [PubMed] [Google Scholar]
- 18.Jungers P, Joly D, Barbey F, et al. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44:799–805. [PubMed] [Google Scholar]
- 19.Rule AD, Bergstralh EJ, Melton LJ, 3rd, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vupputuri S, Soucie JM, McClellan W, et al. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004;14:222–228. doi: 10.1016/S1047-2797(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 21.Worcester EM, Parks JH, Evan AP, et al. Renal function in patients with nephrolithiasis. J Urol. 2006;176:600–603. doi: 10.1016/j.juro.2006.03.095. discussion 3. [DOI] [PubMed] [Google Scholar]
- 22.Torres VE, Wilson DM, Hattery RR, et al. Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22:513–519. doi: 10.1016/s0272-6386(12)80922-x. [DOI] [PubMed] [Google Scholar]
- 23.Varanelli MJ, Coll DM, Levine JA, et al. Relationship between duration of pain and secondary signs of obstruction of the urinary tract on unenhanced helical CT. AJR Am J Roentgenol. 2001;177:325–330. doi: 10.2214/ajr.177.2.1770325. [DOI] [PubMed] [Google Scholar]
- 24.Parks JH, Coe FL, Strauss AL. Calcium nephrolithiasis and medullary sponge kidney in women. N Engl J Med. 1982;306:1088–1091. doi: 10.1056/NEJM198205063061805. [DOI] [PubMed] [Google Scholar]
- 25.Yagisawa T, Kobayashi C, Hayashi T, et al. Contributory metabolic factors in the development of nephrolithiasis in patients with medullary sponge kidney. Am J Kidney Dis. 2001;37:1140–1143. doi: 10.1053/ajkd.2001.24515. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5:431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 28.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 29.Saucier NA, Sinha MK, Liang KV, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2009;55:61–68. doi: 10.1053/j.ajkd.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao KC, Wu TL, Chang PY, et al. Multiple risk markers for atherogenesis associated with chronic inflammation are detectable in patients with renal stones. J Clin Lab Anal. 2007;21:426–431. doi: 10.1002/jcla.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parks JH, Coe FL, Evan AP, et al. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. BJU Int. 2009;103:670–678. doi: 10.1111/j.1464-410X.2008.08064.x. [DOI] [PubMed] [Google Scholar]
- 32.Younes NA, Shafagoj Y, Khatib F, et al. Laboratory screening for hyperparathyroidism. Clin Chim Acta. 2005;353:1–12. doi: 10.1016/j.cccn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Prie D, Huart V, Bakouh N, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 34.Taylor EN, Curhan GC. Diet and fluid prescription in stone disease. Kidney Int. 2006;70:835–839. doi: 10.1038/sj.ki.5001656. [DOI] [PubMed] [Google Scholar]