Non-technical summary
Signals generated by the brain are thought to create the sensation of heaviness when we lift an object. We show here that signals arising from the body underlie this sensation. We fatigued the thumb muscles to half strength and had subjects lift a weight. Two subjects without sensation from the body said it felt twice as heavy, as expected by the central-signal theory. However, normal subjects felt that the weight was lighter, inconsistent with the central-signal theory. When we paralysed the muscles with curare and let them recover to half-strength, the weights felt lighter despite the greater central signal. This is explained by smaller signals returning from muscle spindles because they have also been paralysed by the curare. Thus, peripheral signals with a major contribution from muscle spindles normally create the sense of heaviness. It also shows that muscle spindles provide more than information about limb position and movement.
Abstract
Abstract
Signals associated with the command the brain sends to muscles are thought to create the sensation of heaviness when we lift an object. Thus, as a muscle is weakened by fatigue or partial paralysis (neuromuscular blockade), the increase in the motor command needed to lift a weight is thought to explain the increasing subjective heaviness of the lifted object. With different fatiguing contractions we approximately halved the force output of the thumb flexor muscles, which were then used to lift an object. For two deafferented subjects the perceived heaviness of the lifted object approximately doubled, in keeping with the central-signal theory. However, for normal subjects this resulted in objects feeling the same or lighter, inconsistent with the central-signal theory but consistent with the expected effects of the conditioning contractions on the sensitivity of peripheral receptors. In separate experiments we subjected the forearm muscles to complete paralysis with a non-depolarising neuromuscular blocking agent and then allowed them to recover to approximately half-force output. This also resulted in objects feeling lighter when lifted by the semi-paralysed thumb, even though the motor command to the motoneurons must have been greater. This is readily explained by reduced lift-related reafference caused by the prolonged paralysis of muscle spindle intrafusal fibres. We conclude that peripheral signals, including a major contribution from muscle spindles, normally give rise to the sense of exerted force. In concept, however, reafference from peripheral receptors may also be considered a centrally generated signal that traverses efferent and then afferent pathways to feed perceptual centres rather than one confined entirely to the central nervous system. These results therefore challenge the distinction between central- and peripheral-based perception, and the concept that muscle spindles provide only information about limb position and movement.
Introduction
Two sources of neural information could give rise to the sense of heaviness or force exerted by our muscles when we lift an object. Sensory receptors in the periphery could respond directly to applied forces and a signal of effort could be generated centrally as a corollary discharge of the motor command sent to the muscles. It is generally considered that the central signal provides the sensation of the load when we lift an object (McCloskey, 1981; Jones, 1986). This view comes from studies showing that when muscles are weakened by fatigue or partial curarisation, lifted objects feel heavier (McCloskey et al. 1974; Gandevia & McCloskey, 1976, 1977a; Jones & Hunter, 1983). The increase in motoneuron drive required to generate the same force from the weakened muscle is considered to result in a corollary discharge signal that makes the same weight feel heavier (Gandevia & McCloskey, 1977a).
There is, however, a problem with the central command being the exclusive determinant of perceived heaviness. Simply put, a weight lifted with a muscle rendered half as strong feels considerably less than twice as heavy. Of course this discrepancy could be explained if the central signal were highly non-linear but the alternative hypothesis, which we examine here, is that peripheral sensory receptors provide the dominant signal for perception. Indeed, anaesthesia of the thumb makes a weight lifted by it feel heavier (Gandevia & McCloskey, 1977a; Gandevia et al. 1980), indicating that contact forces detected by cutaneous and perhaps joint afferents contribute to this sense.
Golgi tendon organs, with their discharge that reflects the tension in muscles and tendons and projection to the cortex (McIntyre et al. 1984), should be a natural source for information about muscle tension and the heaviness of a load. This could be tested by blocking their sensory signals with anaesthesia but this necessarily blocks muscle contraction. Another approach is to look for correlations between known tendon organ properties and perceptions of force. For example, during constant imposed tension, tendon organ sensitivity and firing rates decline (Gregory & Proske, 1975), which can explain errors in force matching after brief forceful contractions (Thompson et al. 1990). This adaptation varies with the level of tension developed with higher tensions producing proportionally larger declines in firing rates (Gregory & Proske, 1979). Thus, a high-force contraction over time should render tendon organs relatively unresponsive compared with a low-force contraction. Similar adaptation also occurs in the discharge of slowly adapting cutaneous afferents (Iggo & Muir, 1969) and muscle spindle primary afferents during intrafusal contraction (Cheney & Preston, 1976; Macefield et al. 1991), although this is a complex matter for spindles as they have different forms of static and dynamic fusimotor drive.
Curare compounds and their synthetic analogues are competitive antagonists at the neuromuscular junction and block muscle excitation. With this block, a central motor command can be issued but force output from the muscle is reduced. Sensory receptors and afferent neurons are unaffected and so will reliably transmit the true signal of reduced force output. The only exception to this comes from the neuromuscular junction block of muscle spindle intrafusal fibres as they share the same acetylcholine receptor. This indirectly affects the firing of spindle primary and secondary sensory endings during muscle activation in a time- and dose-dependent manner.
Beginning with these observations of the differential effects of muscle tension history and neuromuscular blockade (curarisation) on muscle contractile performance and afferent sensitivity, the present experiments explore the origin of the senses of force, heaviness and effort with a series of weight-matching experiments. In normal subjects, fatiguing contractions of high and low force are made to affect peripheral receptors differently but create the same reduction in maximal force output. That is, the same loss of force output can be achieved by the two contractions but the high-force contraction will produce a greater degree of desensitisation of Golgi tendon organ and cutaneous receptors. Two subjects with large-fibre peripheral sensory deafferentation are studied in this same way to determine how heaviness is perceived when peripheral signals are unavailable and only centrally generated signals can contribute. In normal subjects, perceptions of heaviness are determined after partial recovery from prolonged complete neuromuscular blockade that paralysed both the extrafusal force-generating muscle and the intrafusal muscle spindle fibres but preserved other afferent sources.
Methods
The experiments described here involve contralateral weight-matching tasks in which subjects use the unaffected indicator limb to match a weight lifted by the reference limb, before and after conditioning by fatigue, vibration or neuromuscular blockade with curare. Another experiment involves a contralateral isometric force-matching task in which subjects intermittently match with the indicator limb the perceived force exerted by the reference limb in a sustained fatiguing contraction.
The experiments were conducted in accordance with the Declaration of Helsinki (1964) and approved by either the Human Research Ethics Committee of the University of New South Wales or the University College London Research Ethics Committee. All participants gave informed consent in writing.
Subjects
A total of 16 healthy adults aged 22–59 years (9F, 7M) with no history of neurological disorder, and two with peripheral sensory loss participated as subjects. Subject IW has a large-fibre sensory neuropathy (Sterman et al. 1980) with absent cutaneous and proprioceptive sensibility below the neck (Cole & Sedgwick, 1992). Since onset 26 years previously, he has regained good motor control but relies on vision (Cole, 1995). Subject K has a large sensory-fibre peripheral neuropathy of 6 years duration affecting the limbs with a relative sparing of the trunk. He shows rather coarse movement control with sensory ataxia although manages well when only a single limb is used. He has lost limb movement sense and position sense for the hands and wrists with better function at the shoulders. In the upper limbs, light cutaneous touch and vibration sense are absent with some sharp touch preserved.
Set-up
To undertake the contralateral weight-matching task, subjects sat with both forearms resting on a table and stabilised the hands by loosely wrapping the fingers around metal rods fixed to the table (Fig. 1A). The thumbs were free to press down on a low-friction lever with no mechanical advantage and lift weights suspended out of sight below the table. Adjustable stops set an appropriate lever height for each subject and a movement range of 2 cm. To measure maximal contraction force, the weight was disconnected and a load cell was attached between the lever and the floor. This arrangement with the isometric load cells was also used for an experiment in which subjects matched isometric force during fatigue of the flexors of one thumb.
Figure 1. Set-up and design.
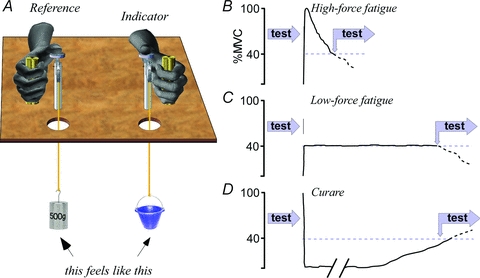
A, subjects flexed the thumbs to press down on a lever and lift weights suspended out of sight. A constant 500 g lifted by the reference hand was matched by adjusting the weight lifted by the indicator hand. Weight-matching tests were performed before and after conditioning the lifting muscle of the reference limb, with minimal delay between testing and conditioning. B–D, five conditioning protocols were tested: B, high-force fatigue in which the subject made a maximum isometric contraction until force had fallen to 40% of the initial maximum; C, low-force fatigue with the 40% contraction maintained until it could not be sustained; D, after a prolonged period of complete paralysis with rocuronium and then allowing the muscle to recover maximal force output to 40% of the initial level. Test times differ between subjects and between conditions B, C and D.
Protocol
Subjects simultaneously lifted a 500 g reference weight with one thumb and an indicator weight with the other. While lifting, normal subjects shut their eyes to exclude any visual cues that might signal a weight difference. The reference side was randomised between subjects and kept throughout the experiment. Initial indicator weights varied randomly at half to double the reference weight, differences that were clearly detected on the first lift. After each lift, if they nominated that the indicator weight felt lighter or heavier than the reference, the weight was adjusted according to the subject's instructions to increase or decrease. The increment or decrement was varied randomly by the experimenter (minimum 25 g) and could overshoot the target reference weight. When the weights were judged equal, the indicator weight was recorded as the subject's perception of the reference weight. Twenty control matches were made prior to each experimental intervention, fatigue or paralysis, to establish baseline.
Perception of heaviness after a fatiguing contraction
Two methods were used to fatigue the thumb flexors of the reference hand: a high-force fatigue by maintaining a maximal voluntary contraction, and a low-force fatigue by maintaining a steady 40% of maximum force, which had been measured at another time. Both were isometric contractions against a strain gauge that was connected between the thumb lever and the floor. Both fatigue protocols ended when force output was 40% of maximum. For the high-force fatigue, subjects were vigorously encouraged to maintain maximum force until it had fallen to the 40% target (Fig. 1B). For the low-force fatigue, the steady 40% maximal force was maintained by visual feedback and stopped when it fell below the target and could not be restored despite our best verbal encouragement (Fig. 1C). At the fatigue end point, subjects immediately relaxed and then began a series of 20 weight matches with the same protocol of the pre-fatigue matches. After completion, maximum contraction force was measured again to determine the extent of recovery. The different trials were performed at least 2 days apart and in randomised order between subjects (n = 16, 10 for the vibration trials).
In another trial, the low-force fatiguing contraction was accompanied by intense focal vibration over flexor pollicis longus (∼2 mm at 50 Hz) using a tattoo machine with an 8 mm spherical bead, the aim being greater desensitisation of Golgi tendon organs as vibration further increases their discharge if they have not saturated (Brown et al. 1967; Burke et al. 1976; Fallon & Macefield, 2007). At the fatigue end point, subjects immediately relaxed and the vibration was stopped before commencing the series of 20 weight matches.
The deafferented subjects could only be studied on a single occasion. Subject IW performed the high-force fatigue protocol and K performed the low-force protocol. The degree of fatigue was reduced so that the target was 50% maximum rather than the 40% target of the normal subjects. Both lifted with the eyes open, necessary to know that they had lifted the weight off the rest and not hit the stop. Subject K required the pads of the thumbs to be stuck to the levers with double-sided tape to keep the thumbs in position when he relaxed between lifts. The maximum contraction forces used to determine the target levels were performed before testing (4 h for IW, 1 h for K). Testing these subjects took longer and only eight matches were made.
Perception of force exerted during a fatiguing contraction
For the normal but not the deafferented subjects (n = 12), force-matching experiments were undertaken to measure perceptions of force exerted by the thumb flexors during the course of the isometric high-force (maximal force) or low-force (40% initial maximum) fatiguing contractions. The subject made a brief isometric contraction with the indicator thumb every 30 s to match the perception of force exerted with the reference thumb, which was making the isometric fatiguing contraction. A key difference in this protocol was that subjects continued with the low-force fatiguing contraction, and matching it, after the break point (40% initial maximum force) when force output was declining. The two trials were performed at least 2 days apart and in randomised order between subjects.
Paralysis by neuromuscular block (curarisation)
A non-depolarising neuromuscular blocker, rocuronium, was used to paralyse all muscles distal to the cuff (Wierda et al. 1994). The rocuronium (5–7 mg; 0.1 mg.kg−1) was in 50 ml of Plasma-Lyte (Baxter) with 2 ml of 8.4% NaHCO3 solution to balance pH. After the control experiments, an intravenous cannula was inserted proximal to the wrist. Blood was removed from the arm vasculature by elevation and compression by a rubber toroid rolled proximally before a dual-chamber cuff around the upper arm was inflated rapidly to 300 mmHg by regulated pressurised air. A retrograde infusion of the rocuronium solution over 60 s began within 30 s. Paralysis was complete within 10 min, verified by no detectable flexor or extensor force or EMG in the forearm muscles. The cuff remained on for 13–18 min and subjects generally developed some transient dizziness and diplopia on deflating the cuff. These symptoms were short-lived (5–10 min) and the subject rested with the arm in a sling until partial recovery to 40% of initial maximum force. This recovery took 1–2 h, during which brief maximal efforts of thumb flexion were made. When the target 40% force recovery was reached, subjects made twenty weight matches and after completion, maximal contraction force was measured to determine the extent of recovery.
Measurement and analysis
Matches were not synchronised and subjects had idiosyncratic rates of matching. Thus, for purposes of averaging, matched weights of all subjects were pooled and responses divided into 100 s bins backwards from the end of the pre-fatigue matches and forward from the start of the post-fatigue matches. The differences between the responses of the normal and deafferented subjects and force matching during and after fatigue are described quantitatively. Physiologically important effects of conditioning are apparent by visual inspection but ANOVA by epoch (before and after conditioning) and post hoc Student's t tests of bin data were applied to confirm statistical significance at Pα < 0.05. Mean data, generally as percentage changes after conditioning, are presented with 95% confidence intervals in the format: mean [low 95% limit, high 95% limit] (Curran-Everett & Benos, 2007).
Results
Perception of heaviness after a fatiguing contraction
Subjects (n = 16) matched the 500 g reference weight with reasonable accuracy (coefficient of variation 0.088) that did not vary with repeated tests. With the muscle fatigued to 40% of the initial maximum force, subjects had no difficulty lifting the reference weight. Immediately after the high-force fatiguing contraction of the muscle lifting the reference weight, the mean indicated weight was 15.2%[6.8, 23.6] less. That is, the weight lifted by the fatigued muscle felt lighter (Fig. 2A). Over the following 5 min, the indicated weight recovered towards the pre-fatigued levels. In contrast, after low-force fatigue to the same level of 40% maximal force, there was no difference in the reported weight immediately afterwards. Note that there was a small upward drift in matched forces over the 500 s. When vibration was applied to the tendon during the low-force fatiguing contraction (10 subjects), the mean indicated weight immediately afterwards was 14.0%[3, 25] less. We also applied vibration with the muscle relaxed or making a non-fatiguing weak 5% maximal contraction and this had no effect on perceived heaviness afterwards.
Figure 2. Effects of muscle fatigue on weight perception.
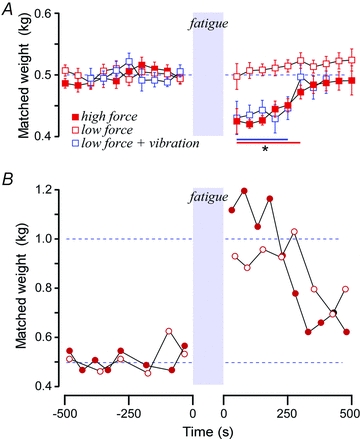
A, weights selected to match 500 g lifted with the reference thumb before (left) and after (right) fatiguing the reference muscle until its force was reduced to 40% of the initial MVC level. Data are means ±SEM (n = 16, and n = 10 for the vibration data). After the high-force fatigue (filled red squares) subjects indicated that the reference weight felt lighter and this recovered over several minutes. After low-force fatigue (open red squares) to the same level of 40% maximal force output, they indicated that the weight felt the same as that lifted by the non-fatigued arm. After the same low-force fatigue but with strong vibration of the muscle tendon (open blue squares), they indicated that the reference weight felt lighter as it had with the high-force fatigue. *P < 0.05 from baseline by ANOVA. B, weights selected by two deafferented subjects after fatiguing the reference side to 50% of the maximal force level. Subject IW (filled circles) made a high-force fatiguing contraction of the thumb flexors and subject K (open circles) made a sustained 50% maximal contraction until he could not hold it. For these subjects, halving strength caused the reference weight to feel approximately twice as heavy.
An entirely different picture is seen in the subjects with large-diameter afferent sensory loss. Before fatigue, matching accuracy was not significantly different from the normal (coefficient of variation 0.102). When the muscle was fatigued to 50% of initial force output, the lifted weight was initially reported approximately twice as heavy (112% increase for IW with high-force fatigue; 92% increase for K with low-force fatigue; 102% increase combined), before recovery towards baseline.
Perception of force exerted during a fatiguing contraction
Figure 3 shows forces made by isometric contraction of the indicator thumb to match the perceived force exerted by the reference thumb during the isometric fatiguing contractions. For the sustained maximal high-force contraction, the indicated perceived force declined immediately, following the actual decline in force output. However, indicated force was always greater than perceived force, declining at only 50–60% of the actual decline in force output (Fig. 3, left). During the sustained low-force isometric contraction, the perception of force exerted rose steadily, reaching 53%[23, 83] higher than actual at the point of 40% maximal force output. After that time, perceived force fell at the same rate as actual force exerted. For both high-force and low-force contractions the perceived force did not correspond to that predicted by the central command signal (orange dashed lines, Fig. 3B).
Figure 3. Perceived force exerted during an isometric fatiguing contraction.

A, on the left, the black trace shows the declining force output of a continued maximal isometric contraction made with the reference thumb by one subject (i.e. the high-force fatiguing contraction of the prior study: see Fig. 1B). The blue traces show intermittent isometric contractions made with the indicator thumb to match the reference force. On the right, the reference contraction is at 40% of a pre-test maximal force until it cannot be held (i.e. the low-force fatiguing contraction: Fig. 1C). At that time (break), force output begins to decline. The forces indicated by the contralateral indicator thumb increase during the period of steady force output but decline steeply when the force output has become maximal. B, in the format of A are the group means (±SEM, n = 12) of the actual and perceived (indicated) force for each fatigue protocol. To allow for different times to reach the prescribed level of fatigue, time is scaled to the break point with relative equal-width bins averaged across subjects. The orange lines represent the prediction of perceived force exerted if perception is based on a central command with a gain of unity; i.e. halving maximal force output by muscle fatigue requires doubling the central command to maintain force output, as indicated by the results of subjects IW and K in Fig. 2.
Paralysis by neuromuscular block (curarisation)
Paralysis by neuromuscular junction blockade (rocuronium) will leave Golgi tendon organs and slowly adapting cutaneous receptors unaffected by the desensitisation that is produced by the force applied during a fatiguing contraction. Thus, any resulting muscle weakness should bias perception to make lifted objects feel heavier if perception is influenced by the magnitude of the central command or corollary discharge, which would be greater during curarisation. The opposite observation was made. After complete and prolonged paralysis with rocuronium and recovery of maximal force output to 40% of the pre-paralysis maximum, subjects (n = 5) chose lighter weights with the unaffected indicator thumb to match the 500 g lifted with the weakened reference thumb (Fig. 4A). The first post-paralysis match with the unaffected thumb was 31%[17, 45] less than the 500 g reference weight lifted with the thumb that had been weakened by 60%. Recovery over time brought the matched weights back towards the reference but after 10 min a residual shortfall remained. Individual match data for one subject who made fast weight-matching decisions shows a steady recovery of perceived force that parallels the muscle recovery (Fig. 4B).
Figure 4. Effects of paralysis by neuromuscular block on force perception.

A, weights selected to match 500 g lifted by the reference hand, before and after paralysis with rocuronium. During the period ‘curare’ the reference arm was completely paralysed and allowed to recover to 40% of initial maximal force before testing. Afterwards, lighter weights were chosen to indicate the heaviness of the weight lifted by the paralysed reference hand. Individual responses were binned into 100 s epochs before averaging. B, individual matches (blue circles) for one subject superimposed on the maximal contraction force before and after paralysis (red bars relative to the initial force on arbitrary scale). The reduction in matched weight is proportionally less than the reduction in maximal contraction force but recovery follows the force recovery. *P < 0.05 from baseline by ANOVA.
Discussion
Von Helmholtz (1867) proposed that sensations of innervation are generated within the brain in parallel with the process of activating the muscles. It is generally accepted that this internal signal or corollary discharge (Sperry, 1950) gives rise to the sensation of force exerted by muscular contraction (McCloskey, 1981; Jones, 1986). Sherrington held a contrary view: ‘that during a willed movement the outgoing current of impulses from brain to muscle is accompanied by a sensation for innervation … remains unproven’ (Sherrington, 1900; see Matthews, 1982 for historical review).
The heavier weights chosen by the deafferented subjects to match the weight lifted by the fatigued limb might suggest that a central signal can be quantified internally and used for the task. However, it should be recognised that movement feedback based on visual information was still available to them and necessary to perform the task. Without feedback there can be no knowledge that a weight had been successfully lifted. With feedback, quantitative knowledge of the motor command is not the only means by which deafferented subjects could match the two sides. For example, they could issue a similar command to each side in turn, observe which limb moved earlier and deduce that the weight on that side is the smaller, as suggested by observations of deafferented weight matching (Rothwell et al. 1982; Fleury et al. 1995).
Whatever the mechanism used by deafferented subjects, it is clear from their entirely different behaviour that healthy subjects do not normally match weights in the same way. Presumably, the greater richness of sensory information available to them makes the difference. However, the dichotomous view that sensory information is exclusively used to perform the matching task in healthy subjects cannot easily be upheld. There are many instances of deviations from this simple model.
Here we propose a unifying hypothesis in which the weight-matching task is achieved using both efferent and afferent information. The hypothesis relies on the concept that with every volitional motor command there is an associated expectation of reafference arising from the consequences of the motor output. This expectation could come from a memory of past actions or from operation of an internal model. Deviations from the expected reafference may be interpreted as indicating that the load being acted on is not as predicted. With this, subjects are able to gauge whether the load acting against one limb is the same as that acting against the other. In the following we interpret our results in the light of this model and attempt to reconcile the findings of others using the same hypothesis. Figure 5 summarises the results and interpretations of the different studies.
Figure 5. Interpretation: a model of force perception based on fusimotor reafference.

In all panels (A–D), the same weight (500 g) is lifted by equal amounts of muscle shortening. The key point is that the central command signal (Action) was increased by similar amounts in the paralysis (B), fatigue (C) and deafferented (D) experiments. The thickness of each pathway indicates the relative strength of the signal. A, implicated in the perceptions of force and weight are central corollary discharge (cd) and/or efference copy (ec) signals, and peripheral signals largely from tendon organs (Ib). α–γ linkage is indicated at supraspinal and spinal levels but the site is not critical for this interpretation. The results of these studies show that spindle afference (Ia and II) contribute to the perception of force. B, during the early phase of neuromuscular blockade, as tested by Gandevia & McCloskey (1977a), relative extrafusal paresis (left) sees a greater spindle receptor stretch through intrafusal shortening as the γ drive is linked to the increased α drive required to offset extrafusal weakness. Increased spindle reafference creates a perception of greater force exerted relative to the unparalysed side. During recovery from complete paralysis, relative intrafusal paresis results in spindle unloading so that reduced spindle reafference creates a perception of less force exerted. C, after high-force fatigue, greater receptor desensitisation reduces spindle and tendon organ reafference (left) so that weights feel lighter than on the unfatigued side and after low-force fatigue (right). D, deafferented subjects have a quality of force perception that is different from normal and based entirely on the central signals. These double in size after fatigue so that weights are reported twice as heavy compared with the unfatigued side.
Paralysis
Roland & Ladegaard-Pedersen (1977) weakened the muscles of one arm by partial neuromuscular blockade using gallamine. When asked to match forces between limbs, subjects did not indicate that they applied more force with the weakened limb although this would have generated a greater corollary signal of effort. This was taken to indicate that a peripheral signal, probably from Golgi tendon organs, gives rise to a sensation of force. However, when asked to match effort with the unaffected side, subjects generated a force inversely proportional to the residual strength of the weakened muscle, showing that the central effort signal gives rise to a distinctive sensation. At the same time, Gandevia & McCloskey (1977a) reported that weakening the lifting muscle by partial neuromuscular blockade with a curare compound (d-tubocurarine) makes objects lifted by that arm feel heavier. They concluded that this was evidence for the corollary or efference copy of the central motor command dominating the sense of the heaviness of lifted objects.
How can these results be resolved with the observations here that objects feel lighter after paralysis? After recovery from total paralysis to 40% maximal force output, the motor command sent to the muscle would more than double if an efference copy signal was used to judge heaviness centrally, yet objects were perceived one-third lighter. Being quaternary amines, curare drugs (rocuronium in this study) do not cross the blood–brain barrier and subjects reported no sensory or motor changes beyond the paralysed forearm so we can exclude a direct central effect.
Only two subjects experienced weak and transient effects on releasing the tourniquet through effects on the extraocular muscles, which are the muscles most sensitive to curare. Any spill-over of the drug that might have created a transient small weakness of the reporting hand would have resolved by the time of testing more than an hour later, the time taken for recovery on the paralysed side to reach 40% maximal force. However, according to the central signal hypothesis, any weakness of the reporting hand should lead to relatively lighter matches of objects lifted on the reference paralysed side and therefore the result we show here would underestimate the true effect.
Complete muscle paralysis by neuromuscular junction block with curare drugs is a dynamic process that affects both extrafusal and intrafusal fibres. Block of intrafusal fibres requires higher doses of curare and the effect lags the block of extrafusal neuromuscular junctions (Carli et al. 1967; Smith & Albuquerque, 1967; Emonet-Dénand & Houk, 1968). The elegant data of Smith & Albuquerque (1967) show that repeated ventral root stimulation during curare infusion results in an increasing discharge from muscle spindle primary afferents (Ia) during the period when twitch force declines. Some time after zero force output had been reached, Ia discharge stops rising and begins to decline until eventually it does not respond to the contraction. The explanation for this two-phase response is that the extrafusal fibres, being blocked first, do not unload the spindle during contraction and the effect of γ-mediated intrafusal contraction on the primary afferents is revealed. After the extrafusal fibres have been blocked completely, intrafusal paralysis catches up and afferent discharge then declines.
As with the onset of paralysis, intrafusal recovery from paralysis also lags extrafusal recovery (Carli et al. 1967; Emonet-Dénand & Houk, 1968) consistent with the proposal that the spindle capsule acts as a diffusion barrier (Matthews, 1972). Gallamine is a synthetic non-depolarising neuromuscular blocker with the triple quaternary amine structure of the curare compounds and similar transport rate constant (ke0) between plasma and effect (rocuronium 0.14; gallamine 0.16; d-tubocurarine 0.17) and requires similar effective plasma concentrations (Proost & Wright, 2006). Yamamoto et al. (1994) demonstrate this delayed fusimotor paralysis and recovery with gallamine and show that a differential block in addition to a diffusion-lag are the mechanisms responsible. In addition, at lower doses, the pharmacokinetics of these competitive antagonists causes a rapid uptake and concentration at the neuromuscular junction and the rapid recovery from paralysis is due to drug redistribution (Goodman & Gilman, 1996).
These pharmacokinetic differences between the extrafusal and intrafusal effects of curare drugs underpin our explanation of the perceptions of heaviness after the neuromuscular junction block. During induction and with low doses, partial paralysis of extrafusal fibres (Fig. 5B.I) results in a loss of spindle unloading during voluntary contraction because the muscle does not shorten at the normal rate. Thus, the uncompensated γ-mediated intrafusal shortening increases primary and probably secondary spindle reafference. This means that the subject receives a signal reafference from the spindles greater than expected for the motor command that was issued. It is therefore likely that the results of Gandevia & McCloskey (1977b) reflect these pharmacodynamics of curare. With their transient partial paralysis, the extrafusal effect would dominate initially leading to a greater than expected reafference from muscle spindles. This would be equivalent to a heavier than expected weight causing a smaller than expected rate of muscle shortening and therefore gives rise to a perception of increased exerted force. After complete extrafusal paralysis, the delayed intrafusal paralysis results in a declining afferent response because the extrafusal fibres fail to shorten while the spindle intrafusal fibres are failing to respond to γ-drive. Eventually there is no response and spindle afferent firing rates during contraction do not rise above resting rates. During recovery from paralysis, the reverse happens. The pharmacokinetic time lag on the intrafusal fibres means that extrafusal shortening will exceed the intrafusal and by unloading the spindles prevent discharge rising above resting levels and perhaps even cause it to fall (Fig. 5Bb). This means that the subject receives a less than expected reafference back from the spindles relative to the motor command that was issued.
By this argument, the straightforward explanation for our finding that a lifted weight feels lighter during recovery from complete paralysis is that reafference from muscle spindles contributes significantly to the sense of heaviness and force exerted. The rocuronium has paralysed the intrafusal muscle fibres so that fusimotor co-activation accompanying the volitional extrafusal contraction creates at most a small positive reafference from spindle afferents, with negative reafference (reduced firing) a possibility. The lagged effect on the intrafusal fibres could also explain the rapid disappearance of the increased heaviness observed by Gandevia & McCloskey (1977b). Their results show that by the time subjects’ perceptions of heaviness had returned to baseline their force output had only recovered by one-third.
Fatigue
Numerous studies have shown that muscular fatigue induced by sustained contraction increases the perception of force exerted and the heaviness of lifted objects (McCloskey et al. 1974; Gandevia & McCloskey, 1976, 1977a; Jones & Hunter, 1983). As muscle contractility declines, motoneuronal drive must increase to maintain force output. The central corollary discharge hypothesis proposes that the greater descending corticospinal drive to support the contraction gives rise to the greater sensation of force exerted. However, the results here need a different explanation. After the high-force fatiguing contraction or low-force fatigue with vibration, the effect is the opposite with weights actually feeling lighter. After the low-force fatiguing contraction to the same level, weights felt the same but certainly not heavier. We propose that this is because the reafferent signals associated with these motor commands have diminished with the high-force contraction (Fig. 5Ca) or are relatively unchanged with the low-force contraction (Fig. 5Cb).
In the deafferented subjects in the absence of large fibre afferent inflow, lifted objects feel twice as heavy when lifted with a fatigued muscle that can only produce half of its original force (Fig. 2B). We can probably assume that in these subjects we see the central corollary discharge sense in the absence of an afferent contribution and that this is the increased heaviness predicted by the central corollary discharge hypothesis (Fig. 5D). This suggests that the gain of this central process, overall, is approximately unity. That is, half the strength requires double the motor command to generate the same force output. It is worth noting that subject IW in particular, who after many years has good insight to his condition (see Cole, 1995), described a different and less overt or vivid sense that he had learned to equate with heaviness and force. It should also be noted that these subjects retain small diameter group III and IV afferents that could contribute to their sensation during contraction. However, if so, the results here suggest that this afference reflects the motor command rather than actual force as they delivered a bigger motor command to generate the same force output. For example, these small afferents could sense the metabolic state of the muscles during lifting rather than actual force applied. Other evidence suggests that these small afferents do not play a significant role. After the low-force slow fatigue of normal subjects, there was no appreciable effect on weight perception whereas the effect of vibration on large-fibre afferents had a strong effect, suggesting that they and not small fibres underpin force perception.
Despite uncertainties about the processes underlying central perception, central fatigue and central adaptation during a sustained contraction, this unity gain means that we can hazard a guess at the strength of this centrally generated sense during fatiguing contractions. A basic model indicates that a pure centrally generated force perception should remain unchanged as muscle force output is lost during a sustained maximal effort, and should increase steadily to a maximum as fatigue requires a greater central drive during a sustained contraction at constant force (Fig. 3B). On the other hand, a basic model of sensation produced by afferent inflow alone should follow actual force output with an allowance for time-varying effects of sensor transduction. Using a constant- force protocol similar to the contraction made in the right panel in Fig. 3B, McCloskey et al. (1974) also showed that the perception of exerted force rose during the period in which constant force could be maintained and this was taken as evidence that perception was based on a central corollary discharge signal. We have demonstrated this same result here (Fig. 3B before break point) but we also obtained perceptions of force beyond the point at which the target force could not be maintained. From this point onwards, perceived force fell dramatically in parallel with the fall in actual force output. This is inconsistent with the central-signal model and indicates a dominant input from afferent signals.
The firing rates of Golgi tendon organs decline through desensitisation during sustained force and this effect is greater at high force levels (Vallbo, 1974; Gregory & Proske, 1975, 1979). The same applies to muscle spindle primary afferents with applied stretch (Vallbo, 1974). Following high-force fatigue to a level of 40% maximal force output, objects actually felt lighter whereas perceived heaviness was unchanged after the low-force fatigue. Tendon vibration during a low-force fatiguing contraction had an effect similar to high-force fatigue in that objects felt lighter afterwards (i.e. when the vibration and fatiguing contraction had ceased). Obviously, vibration could have multiple and complex effects. A segmental tonic vibration reflex could mean that a smaller central command was required during the fatigue conditioning but greater desensitisation of spindle receptors and the loss of afferent support for the contraction could require a greater central command. Vibration during contraction drives Golgi tendon organs’ discharge (Brown et al. 1967; Fallon & Macefield, 2007) and could increase their desensitisation to resemble that during a high-force contraction. Thus, the different effects of high-force and low-force fatigue as well as the effect of vibration during the fatiguing contraction are again consistent with the dominance of afferent inflow for the sense of exerted force.
The afferent signals
The results of the paralysis study specifically implicate reafference from muscle spindles as a major contributor to the sense of exerted force and the heaviness of lifted objects. The results of the fatigue study are also in keeping with a significant spindle contribution. With progressive fatigue, the increasing motor command to maintain force output results in a greater fusimotor drive. However, through different processes that include receptor and mechanical adaptations, muscle spindles become less responsive to fusimotor drive so that the discharge frequency of spindle primary afferents declines progressively during a sustained isometric contraction, roughly halving after a minute during a 20% MVC contraction but more for higher initial firing rates (Macefield et al. 1991).
Although much less than predicted by the central corollary discharge theory, matched force did rise during the sustained isometric contraction (Fig. 3), but perceived heaviness of the lifted weight immediately after stopping the contraction had not risen (Fig. 2, open squares). This cannot be explained by central adaptation or a difference in spindle or other receptor desensitisation as the sustained conditioning contractions were identical, indicating that a peripheral factor related to the different test contractions (isometric vs. isotonic) was responsible. Thixotropy and muscle contraction history could explain this observation (Gregory et al. 1986; Proske et al. 1993) as the spindles will remain under tension through their fusimotor drive during the sustained isometric contraction so that intermittent matched contractions with the contralateral muscle will reflect the resulting spindle discharge, albeit a declining one through receptor desensitisation. When discontinuing the isometric conditioning contraction to begin the weight matching, the muscle was relaxed and also would have shortened somewhat, leaving the spindle slack with the release of intrafusal tension. In addition, the extrafusal shortening during the weight matching is likely to introduce some unloading of the spindle not present during the isometric contraction. The slow upward drift in matched weights (Fig. 2A, open squares) could reflect mechanical recovery of spindle sensitivity.
With firing rates largely proportional to muscle contraction force until saturation, Golgi tendon organs appear to be the natural candidate for signalling exerted muscle force (Gregory & Proske, 1979). Cutaneous slowly adapting receptors also provide a signal proportional to the contact force and contribute to the sense of heaviness of a lifted object (Gandevia & McCloskey, 1977a; Gandevia et al. 1980). When both arms are in the same state and the external loads are identical, the motor outflow and the sensory reafference for all modalities (cutaneous, Golgi, spindle) will be similar on both sides (Fig. 6A). The experience is perfectly matched in all dimensions and it doesn't matter if the experimenter asks the subject to concentrate on effort, tension, force exerted or heaviness – the subject will always get it right although the experimenter will never know what the subject attended. What is being matched when the state of the reference arm is altered by neuromuscular block? The behavioural results indicate that not one of the afferent or efferent signals is equivalent on the two sides when a subject declares a match. When a heavier weight is matched, cutaneous and Golgi signals are greater from the indicator arm than the reference arm (Fig. 6B: extrafusal > intrafusal paralysis) and when a lighter weight is matched these signals are smaller on the indicator side (Fig. 6C: intrafusal > extrafusal paralysis). We therefore suggest that the match is a compromise experience arising from total reafference rather than based entirely on any single afferent class. The contribution of a central efferent perceptual process, as used by deafferented subjects, clearly makes a minor contribution under normal conditions.
Figure 6. Central and peripheral signals during contralateral weight matching.

In all panels (A–C), the strength of signals at the time that matches are declared are represented by the number of arrows (mc, motor command; c, cutaneous receptors; g, Golgi tendon organs; s, muscle spindles). The reference limb is on the left and the indicator on the right. A is the normal condition. B is the expected signals during early partial paralysis when the extrafusal effect dominates and a heavier weight is chosen to match 500 g. C shows the expected signals during recovery from total paralysis when the intrafusal effect dominates and a lighter weight is chosen to match 500 g. In these altered conditions no single afferent class provides the same signal side to side.
Applying the concept and nomenclature of von Holst & Mittelstaedt's (1950)reafference principle, each of these receptor systems will create reafference related to the force of the muscular contraction but each has a different relationship with externally imposed perturbations. Exafference from muscle spindles largely reflects imposed changes in muscle length and its time derivatives, exafference from Golgi tendon organs largely reflects imposed forces along the axis of the active muscle, whereas cutaneous exafference reflects the location, strength and direction of imposed forces. The studies here show a strong contribution by muscle spindle reafference of fusimotor action to the perception of force exerted during muscular activity. This suggests that the corollary discharge or efference-copy signal that is thought to give rise to these sensations may not be exclusive to the central nervous system but also can significantly involve the peripheral nervous system. Although shown here for the muscle spindle system, all receptor classes could provide this ‘reafference corollary discharge’. Thus, the apparently conflicting views of von Helmholtz (1867) and Sherrington (1900) can be resolved if we consider the efference-copy or corollary discharge pathways to include passage through peripheral as well as central pathways.
Conclusions
The key conclusion of these experiments is that the sense of exerted force and heaviness are largely derived from peripheral afferent inflow rather than central signals, and this takes the form of reafference related to the command sent to the muscles. Muscle spindles, through the fusimotor loop, contribute significantly to this reafference although other sensory receptors responding to muscle contraction are undoubtedly involved. These results challenge the distinction between the central and peripheral origins of perception and the view that muscle spindles provide only signals of limb position and movement.
Acknowledgments
Authors received research funding from the National Health and Medical Research Council of Australia, the UK Medical Research Council and The Wellcome Trust.
Author contributions
Experiments were conducted at Neuroscience Research Australia and University College London. B.L.L. conducted most of the experiments, analysed the data and prepared the initial draft. All authors contributed to conception and design of the studies, interpretation of data, and critically revising the manuscript, before approving the final version.
References
- Brown M, Engberg I, Matthews P. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G, Diete-Spiff K, Pompeiano O. Mechanisms of muscle spindle ex-citation. Archiv Ital Biol. 1967;105:273. [PubMed] [Google Scholar]
- Cheney PD, Preston JB. Classification of fusimotor fibers in the primate. J Neurophysiol. 1976;39:9–19. doi: 10.1152/jn.1976.39.1.9. [DOI] [PubMed] [Google Scholar]
- Cole J. Pride and a Daily Marathon. Boston, MA: The MIT Press; 1995. [Google Scholar]
- Cole JD, Sedgwick EM. The perceptions of force and of movement in a man without large myelinated sensory afferents below the neck. J Physiol. 1992;449:503–515. doi: 10.1113/jphysiol.1992.sp019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society: the sequel. Adv Physiol Educ. 2007;31:295–298. doi: 10.1152/advan.00022.2007. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F, Houk J. Etude comparative de la curarisation des synapses neuromusculaires des fibres fusimotrices gamma dynamiques et statiques, chez le chat. J Physiol (Paris) 1968;60:367–372. [PubMed] [Google Scholar]
- Fallon J, Macefield V. Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle Nerve. 2007;36:21–29. doi: 10.1002/mus.20796. [DOI] [PubMed] [Google Scholar]
- Fleury M, Bard C, Teasdale N, Paillard J, Cole J, Lajoie Y, Lamarre Y. Weight judgment. The discrimination capacity of a deafferented subject. Brain. 1995;118:1149–1156. doi: 10.1093/brain/118.5.1149. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Perceived heaviness of lifted objects and effects of sensory inputs from related, non-lifting parts. Brain Res. 1976;109:399–401. doi: 10.1016/0006-8993(76)90542-4. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anaesthesia in man. J Physiol. 1977a;272:673–689. doi: 10.1113/jphysiol.1977.sp012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Sensations of heaviness. Brain. 1977b;100:345–354. doi: 10.1093/brain/100.2.345. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Potter EK. Alterations in perceived heaviness during digital anaesthesia. J Physiol. 1980;306:365–375. doi: 10.1113/jphysiol.1980.sp013402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L, Gilman A. The Pharmacological Basis of Therapeutics. New York: Mcgraw-Hill; 1996. [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol. 1986;56:451–461. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Proske U. Responses of tendon organs in a lizard. J Physiol. 1975;248:519–529. doi: 10.1113/jphysiol.1975.sp010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Proske U. The responses of Golgi tendon organs to stimulation of different combinations of motor units. J Physiol. 1979;295:251–262. doi: 10.1113/jphysiol.1979.sp012966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA. Perception of force and weight: Theory and research. Psychol Bull. 1986;100:29–42. [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Effect of fatigue on force sensation. Exp Neurol. 1983;81:640–650. doi: 10.1016/0014-4886(83)90332-1. [DOI] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. Mammalian Muscle Receptors and their Central Actions. London: Edward Arnold; 1972. [Google Scholar]
- Matthews PBC. Where does Sherrington's 'Muscular Sense' originate? Muscles, joints, corollary discharges? Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- McCloskey D. Corollary discharges: Motor commands and perception. In: Brookhart J, Mountcastle V, Brooks V, Geiger S, editors. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda, MD: American Physiological Society; 1981. pp. 1415–1447. [Google Scholar]
- McCloskey DI, Ebeling P, Goodwin GM. Estimation of weights and tensions and apparent involvement of a 'sense of effort'. Exp Neurol. 1974;42:220–232. doi: 10.1016/0014-4886(74)90019-3. [DOI] [PubMed] [Google Scholar]
- McIntyre AK, Proske U, Rawson JA. Cortical projection of afferent information from tendon organs in the cat. J Physiol. 1984;354:395–406. doi: 10.1113/jphysiol.1984.sp015383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost J, Wright P. A pharmacokinetic-dynamic explanation of the rapid onsetñoffset of rapacuronium. Eur J Anaesthesiol. 2006;18:83–89. [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Roland P, Ladegaard-Pedersen H. A quantitative analysis of sensations of tension and of kinaesthesia in man: Evidence for a peripherally originating muscular sense and for a sense of effort. Brain. 1977;100:671–692. doi: 10.1093/brain/100.4.671. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain. 1982;105:515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The muscular sense. In: Schafer EA, editor. Text-book of Physiology. Vol. 2. Pentland, Edinburgh: 1900. [Google Scholar]
- Smith CM, Albuquerque EX. Differences in the tubocurarine antagonism of the activation of muscle spindle afferents by succinylcholine, acetylcholine and nicotine. J Pharmacol Exp Ther. 1967;156:573–584. [PubMed] [Google Scholar]
- Sperry R. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Sterman A, Schaumburg H, Asbury A. The acute sensory neuronopathy syndrome: a distinct clinical entity. Ann Neurol. 1980;7:354–358. doi: 10.1002/ana.410070413. [DOI] [PubMed] [Google Scholar]
- Thompson S, Gregory JE, Proske U. Errors in force estimation can be explained by tendon organ desensitization. Exp Brain Res. 1990;79:365–372. doi: 10.1007/BF00608246. [DOI] [PubMed] [Google Scholar]
- Vallbo AB. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974;90:303–318. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. Treatise on Physiological Optics, vol. III. English edition translated from the 3rd German edition by J. P. C. Southall, 1962. Dover, New York: 1867. [Google Scholar]
- von Holst E, Mittelstaedt H. Das reafferenzprinzip. Naturwissenschaften. 1950;37:1109–1116. [Google Scholar]
- Wierda JM, Proost JH, Schiere S, Hommes FD. Pharmacokinetics and pharmacokinetic/dynamic relationship of rocuronium bromide in humans. Eur J Anaesthesiol. 1994;9:66–74. [PubMed] [Google Scholar]
- Yamamoto T, Morgan D, Gregory J, Proske U. Blockade of intrafusal neuromuscular junctions of cat muscle spindles with gallamine. Exp Physiol. 1994;79:365–376. doi: 10.1113/expphysiol.1994.sp003771. [DOI] [PubMed] [Google Scholar]


