Non-technical summary
Cardiac arrhythmias occur when the heart is unable to beat rhythmically, and can be induced if the heart is forced to work in excess of its capacity. Here we examine how cells isolated from the atrial chamber of the human heart respond when their beating rate is increased. Our results show that there are differences in the ability of individual heart cells to respond rhythmically when they are forced to beat fast. Moreover, we find that mechanisms that regulate the level of calcium ions in the heart cells determine their ability to respond rhythmically. These results show that pharmacological control of calcium levels in heart cells might be useful in the treatment of people who suffer cardiac arrhythmias during excessive exercise.
Abstract
Abstract
Irregularities in intracellular calcium on a beat-to-beat basis can precede cardiac arrhythmia, but the mechanisms inducing such irregularities remain elusive. This study tested the hypothesis that sarcoplasmic reticulum (SR) and L-type calcium channel activity determine the beat-to-beat response and its rate dependency. For this purpose, patch-clamp technique and confocal calcium imaging was used to record L-type calcium current (ICa) and visualize calcium in human atrial myocytes subjected to increasing stimulation frequencies (from 0.2 to 2 Hz). The beat-to-beat response was heterogeneous among a population of 133 myocytes, with 30 myocytes responding uniformly at all frequencies, while alternating and irregular responses were induced in 78 and 25 myocytes, respectively. Myocytes with uniform responses had the lowest frequency of calcium wave-induced transient inward currents (ITI; 0.4 ± 0.2 min−1), ICa density (1.8 ± 0.3 pA pF−1) and caffeine-releasable calcium load (6.2 ± 0.5 amol pF−1), while those with alternating responses had the highest ITI frequency (1.8 ± 0.3 min−1, P = 0.003) and ICa density (2.4 ± 0.2 pA pF−1, P = 0.04). In contrast, the calcium load was highest in myocytes with irregular responses (8.5 ± 0.7 amol pF−1, P = 0.01). Accordingly, partial ICa inhibition reduced the incidence (from 78 to 44%, P < 0.05) and increased the threshold frequency for beat-to-beat alternation (from 1.3 ± 0.2 to 1.9 ± 0.2 Hz, P < 0.05). Partial inhibition of SR calcium release reduced the ITI frequency, increased calcium loading and favoured induction of irregular responses, while complete inhibition abolished beat-to-beat alternation at all frequencies. In conclusion, the beat-to-beat response was heterogeneous among human atrial myocytes subjected to increasing stimulation frequencies, and the nature and stability of the response were determined by the SR and L-type calcium channel activities, suggesting that these mechanisms are key to controlling cardiac beat-to-beat stability.
Introduction
Sequential beat-to-beat changes in the morphology of the ST segment and T wave (electrical alternans) and in cardiac contraction (mechanical alternans) is a phenomenon that has been observed in several pathological settings (cardiomyopathy, ischaemia, heart failure and atrial fibrillation; Navarro-Lopez et al. 1978b; Pastore et al. 1999; Qu et al. 2000; Hiromoto et al. 2005), heralding the occurrence of atrial fibrillation (Hiromoto et al. 2005; Gong et al. 2007) and severe ventricular arrhythmias (Rosenbaum et al. 1994; Kodama et al. 2001; Shusterman et al. 2006). Alternation in the amplitude and duration of the action potential is commonly the underlying mechanism of electrical alternans (Pastore et al. 1999), and experimentally, alternation of the action potential can be induced by artificially increasing the heart rate (Cinca et al. 1978; Narayan et al. 2002; Hiromoto et al. 2005).
Experimental models have revealed that the development of electrical and mechanical alternans is associated with the plasma calcium concentration, because alternans can be induced by lowering the plasma calcium concentration with calcium chelating agents, such as EDTA (Cinca et al. 1978; Navarro-Lopez et al. 1978a). Moreover, electrical and mechanical alternans have been reversed by calcium administration in humans (Pastore et al. 1999). Likewise, at the cellular level, it has been shown in isolated rat ventricular myocytes that reduction of calcium entry through L-type calcium channels induces alternating calcium transients (calcium alternans) caused by alternation in the calcium released from the sarcoplasmic reticulum (SR; Diaz et al. 2004; Li et al. 2009). Calcium alternans has also been induced in mammalian cardiomyocytes by metabolic inhibition (Huser et al. 2000; Kockskamper et al. 2005) or by increasing the stimulation frequency (Aistrup et al. 2006; Picht et al. 2006) and has been ascribed to inter- and/or intracellular inhomogeneity in calcium handling (Kockskamper & Blatter, 2002; Diaz et al. 2004; Mackenzie et al. 2004; Aistrup et al. 2006; Picht et al. 2006; Shiferaw & Karma, 2006; Li et al. 2009).
In the human atrium, it has been shown that electromechanical alternans may precede and induce atrial fibrillation (Hiromoto et al. 2005). Although it has been shown that reduction of the opening of L-type calcium channels (Li et al. 2009) or ryanodine receptors (RyR2; Diaz et al. 2002) can promote calcium alternans in rat ventricular myocytes paced at a constant slow frequency (0.5 Hz), there is no information on how the activity of key calcium handling proteins affects the nature of the beat-to-beat response (uniform, alternating or irregular) or the stimulation frequency, where calcium handling becomes non-uniform on a beat-to-beat basis when cardiomyocytes are subjected to increasing stimulation frequencies. Therefore, the purpose of the present study was to test the hypothesis that the nature and stability of the beat-to-beat response of human atrial myocytes is determined by the activity of SR and L-type calcium channels. To do this, we first used fast two-dimensional confocal calcium imaging and the patch-clamp technique to characterize the rate-dependent beat-to-beat response in human atrial myocytes, and we then determined how SR calcium handling and the L-type calcium current (ICa) density modify calcium handling on a beat-to-beat basis and its rate dependency. Our results show that the nature of the beat-to-beat response was modulated by the ICa density, by the SR calcium load and by the rate of spontaneous SR calcium release at rest. Moreover, partial inhibition of ICa reduced the incidence of beat-to-beat alternations, and inhibition of SR calcium release abolished irregularities in the beat-to-beat response at all stimulation frequencies. Thus, our data afford a physiological rationale for using pharmacological control of ICa or SR calcium handling as means to enhance the beat-to-beat stability of intracellular calcium handling in human atrial myocytes.
Methods
Ethical approval
Although the atrial tissue samples consisted of tissue that would normally be discarded during surgery, permission to use it in this study was obtained from each patient. The study was approved by the Ethical Committee of our institution, and was conducted in accordance with the principles of the Declaration of Helsinki.
Human atrial myocytes
Atrial myocytes were isolated as previously described (Hove-Madsen et al. 2004). A total of 177 myocytes from 102 patients without atrial fibrillation were used in this study. Patients treated with Ca2+ antagonists were excluded from the study. One hundred and thirty-three of the myocytes were subjected to the patch-clamp technique only, while patch clamp and calcium imaging were performed simultaneously in 44 myocytes.
Confocal calcium imaging and membrane staining
To visualize changes in the intracellular calcium concentration, myocytes were loaded with 2.5 μm fluo-4 AM for 15 min, followed by at least 30 min wash and de-esterification. Confocal calcium images were recorded at a frame rate of 100 Hz with a resonance-scanning confocal microscope (Leica SP5 AOBS, Leica Microsystems, Mannheim, Germany). Fluo-4 was excited at 488 nm with the laser power set to 20% of maximum and attenuation to 4%, and fluorescence emission was collected between 500 and 650 nm. Synchronization of confocal images and ionic current recordings was achieved using a Leica DAQ box and HEKA patch-master software (HEKA Elektronik, Lambrecht/Pfalz, Germany). Patch-master was used to design electrophysiological protocols and to generate triggers for confocal image acquisition and event marking in the confocal time-lapse protocols. Global calcium transients were quantified using a region of interest covering the whole cell or specific subcellular regions when appropriate. The calcium signal measured in each region of interest represents the average fluorescence within the region of interest. When applicable, calcium fluorescence was normalized to the baseline fluorescence (F0) recorded with the membrane potential clamped to –80 mV.
The sarcolemma was stained with 4-(2-(6- (Dibutylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl) pyridinium hydroxide (di-4-ANEPPS) in order to visualize t-tubules or invaginations of the sarcolemma in isolated atrial myocytes. To ensure that t-tubules could be appropriately visualized, a combination of different di-4-ANEPPS concentrations (5 and 10 μm) and incubation times (5, 15 or 25 min) were employed. The dye was excited at 488 nm with the laser power set to 20% of maximum and subsequently attenuated to 15 or 20%. Fluorescence emission was measured between 590 and 670 nm.
Patch-clamp technique
The L-type calcium current (ICa), the tail current elicited upon repolarization of the membrane potential to –80 mV (Itail), spontaneous transient inward currents (ITI) and caffeine-induced inward currents were measured with the perforated patch-clamp technique using an EPC-10 patch-clamp amplifier (HEKA) as previously described in human atrial myocytes (Hove-Madsen et al. 2004). Experiments were carried out at room temperature and began when the access resistance was stable and had decreased to less than five times the pipette resistance. Only elongated myocytes with clear striations were used, and those presenting spontaneous calcium waves and/or ITI at a holding potential of –80 mV were only included if the spontaneous wave/ITI frequency was stable for at least 5 min. The extracellular solution contained (mm): NaCl, 127; TEA, 5; Hepes, 10; NaHCO3, 4; NaH2PO4, 0.33; glucose, 10; pyruvic acid, 5; CaCl2, 2; and MgCl2, 1.8 (pH 7.4). The standard pipette solution contained (mm): aspartic acid, 109; CsCl, 47; Mg2ATP, 3; MgCl2, 1; sodium phosphocreatine, 5; Li2GTP, 0.42; Hepes, 10 (pH adjusted to 7.2 with CsOH); and 250 μg ml−1 amphotericin B. The time integral of the current elicited by rapid application of 10 mm caffeine was used as a measure of SR calcium loading. The time integral of the caffeine-induced current was converted to amoles (10−18 mol) of calcium released from the SR, assuming a stoichiometry of 3 Na+:1 Ca2+ for the Na+–Ca2+ exchanger (Hove-Madsen et al. 2006a). Beat-to-beat variations in intracellular calcium transients, and ionic currents were induced by a stepwise increase in the frequency of repetitive 200 ms depolarizations from 0.2 to 2 Hz. Sodium currents were eliminated with a 50 ms prepulse to –50 mV. To estimate sarcolemmal calcium fluxes elicited by 200 ms depolarizations to 0 mV, calcium entry was calculated from the time integral of ICa. The time integral of ICa was obtained by integrating the difference between ICa and the current at the end of the depolarization pulse. Calcium efflux was calculated from the time integral of the Itail activated upon repolarization to –80 mV, assuming that Itail was primarily due to forward-mode Na+–Ca2+ exchange. The time integral of the tail current was obtained by subtracting the current at the end of the repolarization to –80 mV (dotted lines in Figs 2B and 3B) from the tail current and integrating the resulting net tail current. As the current during the first 2–4 ms after repolarization to –80 mV was largely capacitive, this segment was excluded from the time integral of the tail current. Stock solutions of ryanodine (100 mM), nifedipine (10 mM) and S(–)Bay K 8644 (1 mM) were prepared by dissolving the compounds in DMSO.
Figure 2. The alternating beat-to-beat response.
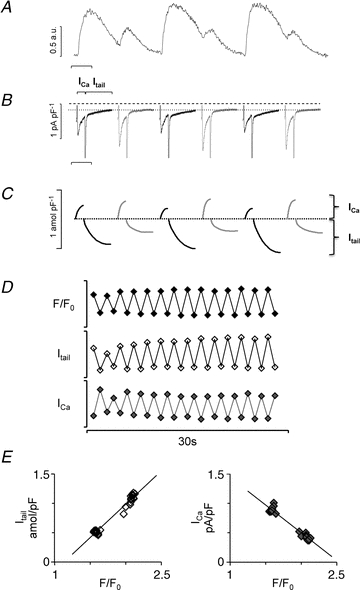
A, six consecutive current traces from a myocyte with alternating calcium transients. B, corresponding membrane currents, ICa and Itail. To appreciate the alternation in Itail, a dotted line is drawn through the holding current at –80 mV, and the dashed line indicates 0 pA pF−1. Time scale bar is 0.5 s. C, time integrals of the membrane currents shown in B. D, parallel changes in the peak calcium transient (F/F0), the calcium carried by the tail current (Itail) and the L-type calcium current amplitude (ICa) during 28 consecutive depolarization pulses at a stimulation frequency of 1 Hz. Scale bars are 2.5 for F/F0, 0.8 amol pF−1 for Itail and 1 pA pF−1 for ICa. E, relationship between the peak calcium transient and Itail (left-hand panel) or ICa (right-hand panel).
Figure 3. The irregular beat-to-beat response.
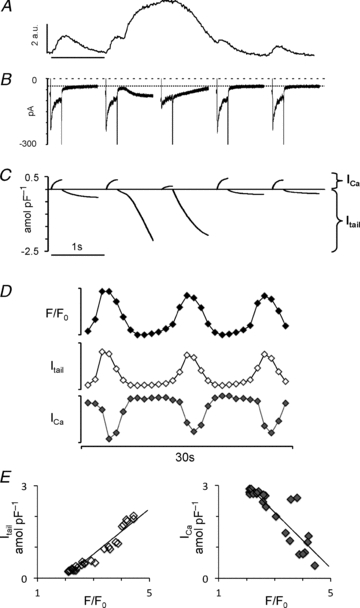
A, five consecutive current traces from a myocyte with irregular calcium transients. B, corresponding membrane currents ICa and Itail. Notice that the peak ICa was inversely proportional to the amplitude of the calcium transient. Time scale bar is 1 s. C, time integrals of the membrane currents shown in B. D, parallel changes in the peak calcium transient (F/F0), the calcium carried by the tail current (Itail) and the L-type calcium current amplitude (ICa) during 28 consecutive depolarization pulses at a stimulation frequency of 1 Hz. Scale bars are 2.5 for F/F0, 0.8 amol pF−1 for Itail and 1 pA pF−1 for ICa. E, relationship between the peak calcium transient and Itail (left-hand panel) or ICa (right-hand panel).
Data analysis and statistics
Values used for statistical analysis are expressed as means ± SEM. Data sets were tested for normality. Two-tailed Student's t test for paired samples was used to assess significant differences when testing a specific effect. The threshold for statistical significance was P = 0.05. ANOVA was used for comparison of multiple effects, and Student–Newman–Keuls post hoc test was used to evaluate the significance of specific effects.
Results
Rate dependency of the beat-to-beat response in human atrial myocytes
In order to characterize the rate-dependent beat-to-beat response in human atrial myocytes, the effects of a stepwise increase in the stimulation frequency from 0.2 to 2.0 Hz was examined in a separate set of 44 myocytes subjected simultaneously to the patch-clamp technique and confocal calcium imaging. The stability of the beat-to-beat response varied from myocyte to myocyte, and three distinct classes of beat-to-beat responses (uniform, alternating and irregular responses) were identified when the stimulation frequency was increased from 0.2 to 2 Hz.
The uniform response was normally observed at low stimulation frequencies, where myocytes were at steady state. Figure 1A shows consecutive recordings of intracellular calcium transients (top panel) and membrane currents (bottom panel) in a myocyte presenting a uniform beat-to-beat response. The combination of the patch-clamp technique with fast confocal frame scanning also allowed cross-sectional analysis of calcium transients, and revealed a temporal delay between the calcium transient recorded at the sarcolemma and in the central region of myocytes with uniform beat-to-beat responses (Fig. 1B). In accordance with the observed temporal delay, di-4-ANEPPS staining revealed a sparsely developed t-tubular system in human atrial myocytes (Fig. 1C). Along the longitudinal axis, the calcium transient was homogeneous in cells with a uniform beat-to-beat response.
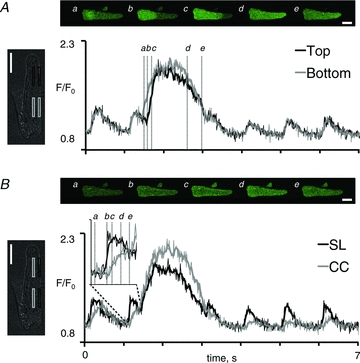
Figure 1. The uniform beat-to-beat response.
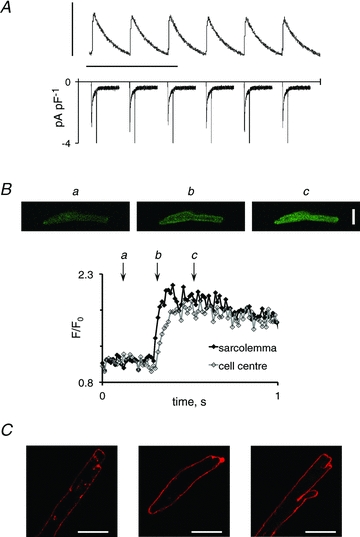
A, uniform calcium transients elicited by consecutive membrane depolarizations at 0.25 Hz (top) and the corresponding membrane currents (bottom). Vertical and horizontal scale bars represent 0.5 a.u. and 10 s, respectively. B, consecutive images recorded before, 20 and 120 ms after depolarization of a myocyte with a uniform beat-to-beat pattern are shown in the top panel. Quantification of calcium at the sarcolemma and in the cell centre is shown in the superimposed traces in the bottom panel. Letters indicate the time when the images in the top panel were recorded. C, representative examples of di-4-ANEPPS-stained human atrial myocytes. Staining was performed in myocytes isolated from six patients. Scale bars represent 20 μm.
The alternating beat-to-beat response (shown in Fig. 2) consisted of repetitive alternations in the calcium transient amplitude (Fig. 2A), in the ICa amplitude (Fig. 2B) and in the time integral of Itail (Fig. 2C). The time integral of Itail was used as an estimate of calcium efflux. Alternations in calcium entry (ICa) were out of phase with alternations in the peak calcium transient and in the calcium efflux (Itail), suggesting that alternation in the ICa amplitude is not the cause of calcium alternans. Furthermore, analysis of the fast time constant (τfast) for ICa inactivation in myocytes with alternating responses showed that inactivation was faster on large (16 ± 2 ms) than on small calcium transients (24 ± 4 ms, P < 0.01), suggesting a negative feedback of calcium released from the SR on ICa. Analysis of myocytes with alternating beat-to-beat responses showed that fluctuations in the time integral of the tail current were proportional to fluctuations in the peak calcium transient, while the ICa amplitude was inversely proportional to the peak calcium transient (Fig. 2D and E). Moreover, myocytes with alternating responses could be divided into cells that had either synchronized concordant (Fig. 2) or discordant subcellular calcium alternans (see Fig. 4C and D).
Figure 4. Subcellular calcium handling during alternating beat-to-beat responses.
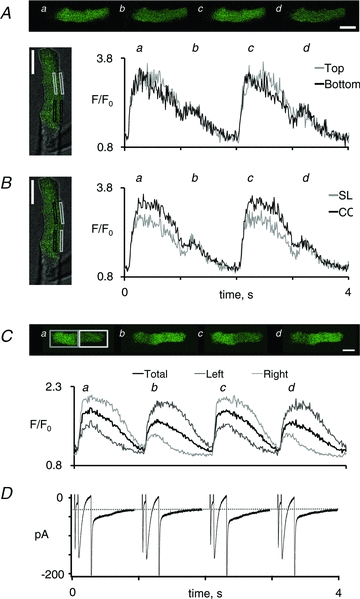
A, four consecutive images showing alternation in the peak calcium transient induced by elevation of the stimulation frequency from 0.5 to 1 Hz. A combined transmission and confocal calcium image of the myocyte is shown on the left, with indication of the regions used for measurement of the calcium signal. Average signals from the top (grey rectangles and traces) and bottom part of the myoyte (black rectangles and traces) are shown on the right. B, average calcium transients in the subsarcolemmal region (SL; grey rectangles and trace) and in the central region of the myocyte (CC; black rectangles and trace). C, four consecutive images showing discordant alternations in the peak calcium transient recorded in the left and the right half of a myocyte. The resulting calcium transients in the left half (dark grey trace), the right half (light grey trace) and the entire myocyte (black trace) are shown below. D, simultaneously recorded ionic currents. The dotted line indicates the holding current at –80 mV. Notice the concordant alternation in the tail current and the global calcium transient. White scale bars represent 20 μm. The frame rate was 100 Hz, and images shown are the average of four consecutive images.
The irregular beat-to-beat pattern was ascribed to myocytes where only irregular beat-to-beat responses (Fig. 3) were observed when the stimulation frequency was increased. This response consisted in a number of small calcium transients interspaced with large calcium transients spanning several stimulation pulses (Fig. 3A and 5). The corresponding beat-to-beat responses of ICa and Itail are shown in Fig. 3B, and the sarcolemmal calcium movements during each pulse are shown in Fig. 3C. Figure 3D shows parallel fluctuations in peak calcium, Itail integral and ICa amplitude during the full length of the stimulation protocol. Notice that the dramatic increase in the cytosolic calcium level produced during large calcium transients was associated with a concomitant ICa depression. Indeed, as illustrated in Fig. 3E, the severity of the ICa depression was proportional to the amplitude of the corresponding calcium transient in all myocytes examined. On average, the slope was −1.35 ± 0.41 amol pF-1 in 12 myocytes showing calcium alternans or waves when paced at 1 Hz. Moreover, changes in the time integral of Itail were proportional to the changes in the peak cytosolic calcium level (Fig. 3E, left-hand panel), with an average slope of 1.82 ± 0.39 amol pF-1 (n = 12) for myocytes with calcium alternans or calcium waves, confirming that Itail is a faithful reporter of fluctuations in the peak cytosolic calcium level in myocytes subjected simultaneously to patch-clamp and calcium imaging. This was true even in myocytes with discordant subcellular calcium transients (Fig. 4C and D). Therefore, changes in the time integral of Itail on a beat-to-beat basis was used to classify the beat-to-beat response in myocytes that were subjected to patch-clamp technique only (Figs 6–10).
Figure 5. Subcellular calcium handling during irregular beat-to-beat responses.
A, image sequence illustrating the propagation of a calcium wave along the longitudinal cell axis. The regions used to measure calcium signals in the myocyte are shown on the left, and average signals from the grey and black rectangles are shown on the right. B, five consecutive images showing the small calcium transient elicited immediately before the calcium wave. The regions used to measure calcium transients signals in the subsarcolemmal and central region are indicated on the myocyte shown on the left, and the corresponding subsarcolemmal (SL; black trace) and central calcium transients (CC; grey trace) are shown on the right. White scale bars represent 20 μm. The inset illustrates the subsarcolemmal and central calcium transients recorded immediately before the onset of the calcium wave. Letters indicate the time where images in the top panel were recorded. The thin lines represent the calcium signal recorded at a frame rate of 100 Hz. The thick lines and the image sequences in A and B were obtained by averaging three consecutive images.
Figure 6. Distribution and frequency dependency of the beat-to-beat response in a myocyte population.
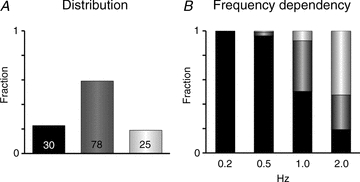
A, fraction of 133 myocytes presenting uniform, alternating or irregular beat-to-beat responses when the stimulation frequency was increased from 0.2 to 2 Hz. Numbers indicate the number of myocytes with the corresponding pattern. B, distribution of uniform, alternating and irregular beat-to-beat responses at stimulation frequencies of 0.2, 0.5, 1.0 and 2.0 Hz (indicated below columns).
Figure 10. Partial RyR2 inhibition alters the relationship between SR load and ICa inactivation.
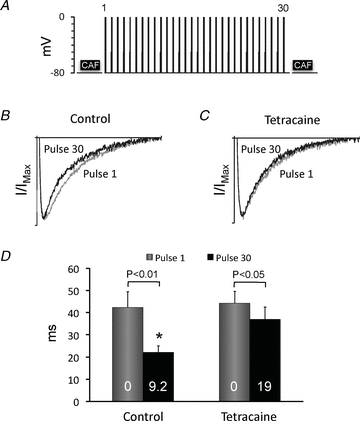
A, schematic representation of the electrophysiological protocol used to determine how SR calcium loading affects calcium-dependent ICa inactivation. The SR calcium content was released by application of 10 mm caffeine (CAF) for 5 s. Subsequently, ICa was elicited by 30 depolarizations from −80 to 0 mV applied every 2 s, and the calcium accumulated in the SR during the 30 pulses was released with a second caffeine pulse. B and C, average traces of ICa normalized to the peak ICa amplitude (I/IMax) for the first and the 30th stimulation pulse in control conditions (B) and with tetracaine (C). The horizontal scale is 200 ms. D, representation of the fast time constant (τfast) obtained by fitting of the decaying phase of ICa in B and C with a bi-exponential decay function. Data are from nine experiments, and P values for statistically significant differences in τfast for the first and the 30th pulse are given above the bars. * Significant difference (P < 0.05) between τfast for the 30th pulse before (control) and after exposure to tetracaine. White numbers indicate the SR calcium load (in amol pF−1).
Analysis of local calcium transients in myocytes with calcium alternans showed that calcium release was always synchronized by the stimulation pulses (Fig. 4A), even in myocytes with discordant subcellular calcium alternans (Fig. 4C). Moreover, subsarcolemmal calcium levels on large calcium transients returned closer to baseline than calcium levels in the cell centre, and the small calcium transient was clearly distinguishable from the large calcium transient in the subsarcolemmal domain (Fig. 4B). Figure 4C and D shows concurrent and proportional changes in the charge carried by the tail current and the corresponding global calcium transient, confirming that the tail current can be used as a reporter of the global calcium transient amplitude even in myocytes with discordant subcellular calcium transients.
Analysis of subcellular calcium handling in myocytes with irregular beat-to-beat patterns revealed that the large calcium transients lasting several depolarization pulses were calcium overload-induced calcium waves propagating through the myocyte (Fig. 5A). In contrast, the small transients recorded during irregular beat-to-beat patterns were uniform along the longitudinal axis (Fig. 5A) and opposite to the calcium waves the time delay between sarcolemma and cell centre was preserved in the small transients (Fig. 5B).
Mechanisms underlying the induction of non-uniform beat-to-beat responses
In the subsequent experiments, we attempted to determine whether uniform, alternating or irregular beat-to-beat responses were associated with a specific set of calcium-handling characteristics. For this purpose, myocytes were only subjected to the perforated patch-clamp technique because calcium-sensitive dyes such as fluo-4 constitute an exogenous calcium buffer that could potentially interfere with the calcium handling on a beat-to-beat basis (Hove-Madsen & Bers, 1992). Indeed, we found that the incidence of alternating responses was lower in fluo-4-loaded myocytes (19% in fluo-4-loaded cells vs. 58% in unloaded cells), while the opposite was true for the incidence of irregular responses (45% in fluo-4-loaded cells vs. 19% in unloaded cells). Moreover, as described above, the beat-to-beat response could easily be identified from the time integral of Itail, and the subsequent analysis was therefore performed in 133 myocytes subjected to the patch-clamp technique only. With this approach, a uniform response was observed at all stimulation frequencies in 30 of 133 cells, and this is referred to as a uniform beat-to-beat pattern. The alternating beat-to-beat response was observed first in 78 of the 133 cells (referred to as an alternating pattern) and in the remaining 25 of the 133 cells examined only irregular responses were observed when the stimulation frequency was increased (referred to as an irregular pattern). Figure 6A shows the abundance of each of the three beat-to-beat patterns among the 133 myocytes examined, and Fig. 6B shows the distribution of the three beat-to-beat responses at different stimulation frequencies. On average, the threshold for the induction of a non-uniform (alternating or irregular) response was 1.49 ± 0.07 Hz.
To determine whether uniform, alternating and irregular patterns were each associated with a specific cellular calcium-handling signature, we measured the activity of three key mechanisms (ICa density, SR calcium release and SR calcium load) controlling intracellular calcium levels in each of the 133 myocytes, and then pooled values for each of the three beat-to-beat patterns. First, the ICa density was measured in each myocyte in conditions where calcium influx and efflux were at a steady state (0.5 Hz), and values were averaged for each of the three beat-to-beat patterns. Figure 7A shows that the ICa density was significantly larger (P < 0.01) in cells with alternating patterns than in cells with a uniform pattern. In contrast, myocytes with irregular patterns had an ICa density comparable to myocytes with a uniform beat-to-beat pattern, suggesting that myocytes with large ICa amplitudes are more prone to present alternating beat-to-beat patterns. To test whether the ICa density does regulate the beat-to-beat response, ICa was partly inhibited with the selective L-type calcium channel antagonist nifedipine (Fig. 7B) or stimulated with the agonist Bay K 8644 in a subset of nine cells. Figure 7C shows that reduction of the ICa (by 35 ± 6%) with 50 nM nifedipine increased the number of cells with a uniform pattern (from one of nine cells to five of nine cells, P < 0.05). Moreover, the threshold for the induction of beat-to-beat alternation was significantly increased from 1.30 ± 0.21 to 1.94 ± 0.22 Hz by nifedipine (P < 0.05, n = 9). Subsequent stimulation of ICa by replacing nifedipine with 100 nm Bay K 8644 (from 2.7 ± 0.5 pA pF−1 in control conditions to 5.6 ± 0.9 pA pF−1) induced non-uniform beat-to-beat responses in all cells and lowered the threshold for their induction from 1.30 ± 0.21 to 0.87 ± 0.10 Hz (P < 0.05).
Figure 7. Modulation of the beat-to-beat response by the ICa density.
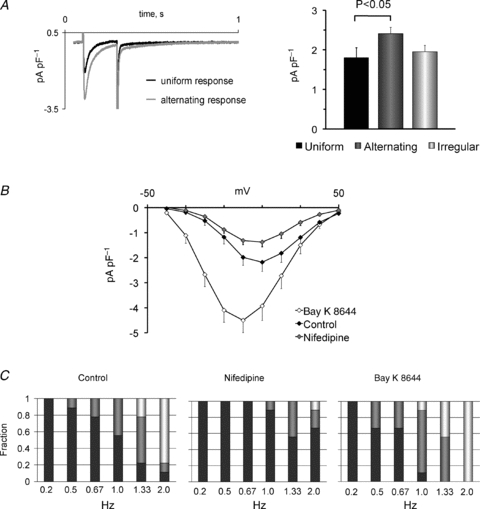
A, representative L-type calcium currents elicited by a 200 ms depolarization in myocytes with a uniform beat-to-beat pattern (black trace) and an alternating beat-to-beat pattern (grey trace). The right-hand panel shows that ICa density is higher in cells with alternating than cells with uniform or irregular beat-to-beat responses. Significant P values are given above the bars. B, current–voltage relationship for ICa before and after exposure to 50 nm nifedipine or 50 nm Bay K 8644. C, frequency-dependent distribution of the beat-to-beat responses in control conditions (left-hand panel) and in the presence of 50 nm nifedipine (middle panel) or 50 nm Bay K 8644 (right-hand panel). Stimulation frequencies are given below columns.
To determine how the lability of the SR calcium affects the beat-to-beat pattern, we first used the spontaneous ITI frequency as an indicator of SR calcium lability. As shown in Fig. 8A, the spontaneous ITI frequency was highest in myocytes with alternating patterns, intermediate in myocytes with irregular patterns and lowest in those with a uniform patterns (P < 0.01 uniform vs. alternating patterns). Next, we blocked calcium release from the SR with 100–400 μm ryanodine (n = 10) or 0.25–1 mm tetracaine (n = 9). Both treatments completely blocked non-uniform beat-to-beat responses at all stimulation frequencies examined (Fig. 8B and C), showing that SR calcium release is necessary for the induction of non-uniform beat-to-beat responses. Ryanodine also prolonged τfast for ICa inactivation from 16 ± 3 to 33 ± 8 ms (P < 0.01).
Figure 8. Effect of the rate of spontaneous calcium release on the beat-to-beat response.
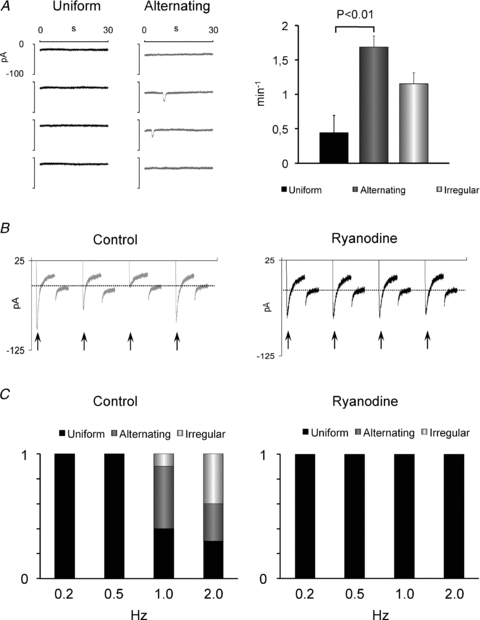
A, spontaneous ITI recordings in myocytes with uniform (black trace) and alternating beat-to-beat patterns (grey trace). The spontaneous ITI frequency is shown on the right for myocytes with uniform, alternating or irregular beat-to-beat responses. Significant P values are given above bars. B, membrane currents recorded from a myocyte with an irregular beat-to-beat response before (control) and a uniform response after inhibition of SR Ca2+ release with 100 μm ryanodine. Arrows indicate the peak ICa for each depolarization pulse. Dashed lines indicate the holding current. C, frequency-dependent distribution of the beat-to-beat responses in control conditions (on the left) and in the presence of 100 μm ryanodine (on the right). Stimulation frequencies are given below columns.
Finally, we investigated the relationship between SR calcium loading and the rate-dependent beat-to-beat pattern. Figure 9A shows that the time integral of the caffeine-induced current was smallest in myocytes with uniform patterns and significantly larger in myocytes with irregular patterns (P < 0.01). This supports the notion that the calcium waves observed during irregular beat-to-beat responses (see Figs 3 and 5) are calcium overload-induced calcium waves. To test whether SR calcium overload favours irregular beat-to-beat patterns, calcium release through RyR2 was partly blocked with 50 or 100 μm tetracaine. As expected, this reduced the ITI frequency by 81% and increased the SR calcium load by 141% (Fig. 9B), and it did induce irregular beat-to-beat responses in place of the alternating responses observed before exposure to tetracaine (Fig. 9C). Moreover, induction of irregular responses occurred at significantly lower stimulation frequencies (1.79 ± 0.16 Hz before and 1.28 ± 0.25 Hz after exposure to tetracaine, P < 0.05).
Figure 9. Dependency of the beat-to-beat response on SR calcium loading.
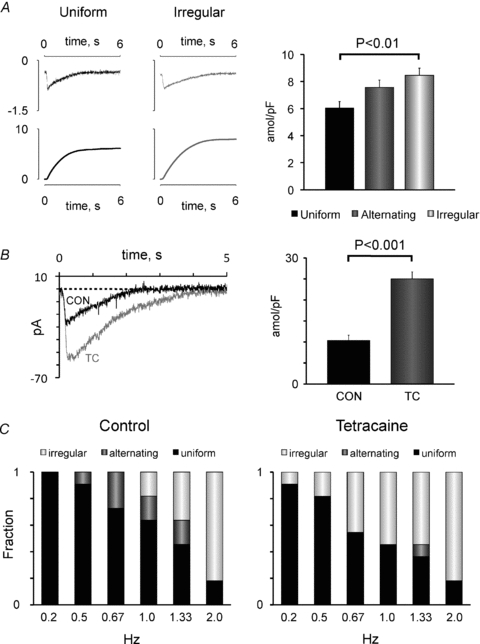
A, representative current traces elicited by rapid caffeine application (top panel) and their time integral (bottom panel) recorded in myocytes with uniform (black trace) and irregular beat-to-beat patterns (grey trace). The right panel shows the caffeine-releasable calcium load in myocytes with uniform, alternating or irregular beat-to-beat responses. Significant P values are given above bars. B, superimposed currents induced by rapid caffeine application before (CON) and after exposure of a human atrial myocyte to 50 μm tetracaine (TC). The average caffeine-releasable calcium load (n = 11) before and after exposure to tetracaine is shown on the right. The P value is given above the bars. C, frequency-dependent distribution of the beat-to-beat responses in control conditions (on the left) and in the presence of 50 μm tetracaine (on the right). Stimulation frequencies are given below columns.
To determine whether tetracaine impairs efficient triggering of calcium release from the SR in spite of the increased SR calcium loading, we examined how it affects the SR calcium release-induced ICa inactivation. To address this issue, a 5 s caffeine pulse was first used to clear the SR calcium content. Next, 30 depolarization pulses were applied to elicit ICa and reload the SR. Finally, a second caffeine pulse was applied to release the calcium reloaded into the SR during the 30 pulses. This protocol, outlined schematically in Fig. 10A, was repeated before and after exposure of the myocyte to tetracaine. As shown in Fig. 10B, clearance of SR calcium loading with the caffeine pulse significantly slowed down the kinetics of ICa inactivation. Specifically, τfast gradually decreased from 42 ± 7 ms on the first pulse after clearance of the SR calcium content to 22 ± 3 ms (P < 0.01, n = 9) on the 30th pulse. The decrease in τfast on pulse 30 occurred in parallel with a small decrease in the ICa density (93 ± 3% of the density on pulse 1), suggesting that ICa triggers release of increasingly larger amounts of calcium from the SR as it reloads, and that the ratio (τfast:τfast on pulse 1) may be a useful indicator of the amount of calcium released from the SR. Figure 10C shows that τfast only decreased minimally from the first to the 30th pulse when the same myocytes were exposed to tetracaine. Figure 10D compares τfast before and after exposure of nine myocytes to tetracaine. Considering that tetracaine increased the SR calcium load after 30 depolarizations 2.2-fold and without significantly changing the the ICa density on pulse 30 (90 ± 11% of control), the results in Fig. 10C and D show that tetracaine reduces the ability of ICa to efficiently trigger calcium release from the SR in spite of the strong increase in SR calcium loading.
Discussion
Irregularities in the beat-to-beat response are known to precede cardiac arrhythmias (Pastore et al. 1999), and in particular, discordantly alternating beat-to-beat responses have been proposed to contribute to arrhythmogenesis (Qu et al. 2000). It has also been documented that alternations in the intracellular calcium transient are intimately linked to fluctuations in the amount of calcium released from the SR (Kockskamper & Blatter, 2002; Diaz et al. 2004; Mackenzie et al. 2004; Picht et al. 2006). Yet, the cellular mechanisms that determine when a cardiac myocyte subjected to rapid pacing becomes unable to maintain a stable beat-to-beat response have not been clearly identified. Here, we show that the stability and nature of the beat-to-beat response is heterogeneous among a population of human atrial myocytes when subjected to elevations of the beating frequency. Moreover, we show that the response depends on the combination of ICa amplitude, SR calcium loading and ITI frequency, which determines calcium release from the SR on each beat and the ability of the myocyte to maintain equilibrium between SR calcium uptake and release.
Frequency dependency of the beat-to-beat response
It has previously been reported that elevation of the beating rate can induce electrical alternans and arrhythmogenesis in the human heart (Goldreyer et al. 1976; Rosenbaum et al. 1994). In accordance with this, we were able to induce alternating or irregular beat-to-beat patterns in human atrial myocytes by increasing the stimulation frequency. Noticeably, alternations in the calcium transient were out of phase with alternations in ICa, and the ICa amplitude was inversely proportional to the Ca2+ transient (see Fig. 2), suggesting that ICa alternans is not the cause of calcium alternans. In contrast, there was always temporal concordance between the amplitude of the calcium transient and the Itail integral. Moreover, inhibition of SR calcium release completely abolished rate-dependent irregularities in the beat-to-beat response in all myocytes examined (see Fig. 8), affirming that fluctuations in SR calcium release are the underlying cause of non-uniform beat-to-beat responses. These results agree with animal studies (Diaz et al. 2002, 2004; Mackenzie et al. 2004; Aistrup et al. 2006; Picht et al. 2006), but these reports did not investigate whether variability in the beat-to-beat response exists in a population of isolated myocytes. Here, we show that there is a considerable variation in the stability and rate dependency of the beat-to-beat response in a population of isolated human atrial myocytes, which in itself may be a substrate for the generation of discordant beat-to-beat responses and arrhythmia upon elevation of the beating frequency. Moreover, this heterogeneity in the beat-to-beat response became conspicuous only at stimulation frequencies higher than the beating rates encountered in patients with normal heart rhythm. Thus, assuming that a stimulation frequency of 0.5 Hz at room temperature is equivalent to a stimulation frequency of 1.2 Hz at 37°C (Bolter & Atkinson, 1988), 96% of all myocytes had a uniform beat-to-beat response when stimulated at a normal frequency. At 1 Hz (equivalent to 2.4 Hz at 37°C), 92% of all myocytes still responded to each stimulation pulse, although a significant number of myocytes had alternating responses. However, at stimulation frequencies that myocytes are expected to experience only during episodes of atrial arrhythmia, the fraction of myocytes presenting irregular beat-to-beat responses increased dramatically. At 2 Hz (equivalent to 4.8 Hz at 37°C), more than 50% of all myocytes had irregular responses and only 19% had a uniform response. If such variability in the beat-to-beat response is representative of the variability encountered among myocytes in atrial tissue, this probably hampers a synchronized propagation of the calcium signal between adjacent myocytes. In particular, irregular patterns with spontaneous calcium waves (Figs 3 and 5A and B) are expected to block calcium conductance intermittently. Indeed, variability in the beat-to-beat response among individual myocytes could be an underlying cause of the discordant beat-to-beat responses observed in a line of connected cardiomyocytes in perfused rat hearts subjected to high pacing rates (Aistrup et al. 2006).
Calcium-dependent regulation of the nature of the beat-to-beat response
The variability in the beat-to-beat response among individual myocytes also suggests that identification of cellular mechanisms underlying such variability might provide specific molecular targets for pharmacological stabilization of intracellular calcium handling on a beat-to-beat basis.
In this context, cardiac arrhythmias have been associated with abnormalities in several key calcium-handling mechanisms (Lai et al. 1999; Van Wagoner et al. 1999; Jiang et al. 2002, 2004; Hove-Madsen et al. 2004), and our data show that the nature of the beat-to-beat response depends critically on the activity of the L-type calcium channel. Thus, the ICa density was significantly larger in myocytes with alternating beat-to-beat patterns. Moreover, partial ICa inhibition reduced the incidence of beat-to-beat alternation by increasing the threshold for its induction, demonstrating that a large ICa amplitude favours induction of alternating responses. In agreement with these results, a three-dimensional mathematical anatomical model of the human atria has recently shown that a premise for the development of electrical alternans and atrial fibrillation in this model is an increased L-type calcium channel conduction (Gong et al. 2007). Also in accordance with our data, acute administration of the ICa antagonist verapamil suppresses discordant repolarization alternans-induced atrial fibrillation (Hiromoto et al. 2005), and patients with large ICa densities are more prone to suffer postoperative atrial fibrillation (Van Wagoner et al. 1999). Originally, the latter finding was proposed to cause calcium overload, but our findings show that a large ICa density also promotes beat-to-beat alternation rather than calcium overload-induced calcium waves (see Figs 8 and 9).
Calcium handling by the SR appears to be equally important in shaping the beat-to-beat response. Thus, myocytes with alternating patterns had a fourfold higher incidence of spontaneous calcium release (ITI) than those with a uniform pattern, and inhibition of SR calcium release completely abolished rate-dependent beat-to-beat alternation. This suggests that the association of atrial fibrillation, heart failure and polymorphic ventricular tachycardia with increased spontaneous SR calcium release (Lee, 1986; Reiken et al. 2003a; Vest et al. 2005) may be arrhythmogenic not only through promotion of after-depolarizations, as previously suggested (Schlotthauer & Bers, 2000; Burashnikov & Antzelevitch, 2003), but also by facilitating induction of calcium alternans. The latter is in line with observations in a transgenic mouse model with a RyR2 mutation causing abnormally high SR calcium release, in which elevation of the stimulation frequency induced non-uniform beat-to-beat responses (Fernandez-Velasco et al. 2009). Apparently at variance with this, reducing SR calcium release with 50 μm tetracaine (Diaz et al. 2002) or by using small depolarization pulses in high extracellular calcium (Diaz et al. 2004; Li et al. 2009) induced calcium alternans in isolated rat ventricular myocytes. It is, however, important to stress that these interventions are expected to reduce the efficacy of ICa in triggering calcium release from the SR and to induce SR calcium overload (see Figs 9 and 10), and the reported response did in fact consist of large calcium waves alternating with tiny calcium transients. This is more closely related to the characteristics of the myocytes with irregular beat-to-beat patterns in the present study, which had low ICa density and high SR calcium load and showed periodic calcium overload-induced calcium waves interspaced with small calcium transients.
Calcium-dependent regulation of the stability of the beat-to-beat response
Previous reports have documented that the fraction of calcium released from the SR, i.e. the amount of calcium released relative to the total calcium content, increases with the amplitude of the calcium trigger source, SR calcium loading and opening of the SR calcium release channels (Bassani et al. 1995; Ginsburg & Bers, 2004). This suggests that uniform beat-to-beat patterns will predominate in myocytes with moderate ICa density, moderate SR calcium load and infrequent SR calcium release, because this favours release of a moderate fraction of the SR calcium content, and equilibrium between SR calcium uptake and release can be maintained even at relatively high stimulation frequencies. Opposite to this, myocytes with high ICa densities and frequent SR calcium release are expected to release more calcium from the SR on each beat. This will favour incomplete SR calcium reuptake and/or recovery of the RyR2 from inactivation between successive beats when the stimulation frequency is increased and cause alternation between a fully and an incompletely recovered state. In agreement with this, τfast for ICa inactivation, which is proportional to the amount of calcium released from the SR (see Fig. 10), was faster on the strong beats (16 ms) in myocytes with beat-to-beat alternation. However, even on weak beats the ICa inactivation was considerably faster (24 ms) than when SR calcium release was prevented with 100 μm ryanodine (33 ms) or immediately after clearing the SR calcium content with caffeine (40 ms), suggesting that SR calcium release alternates between a full and an intermediate level rather than alternating between a calcium-overloaded and a calcium-deficient state. In agreement with this notion, calcium release was synchronized in myocytes with either concordant or discordant subcellular calcium alternans (see Fig. 4).
Irregular beat-to-beat patterns are likely to be induced in myocytes with small ICa and high SR calcium load because the small ICa elicits a moderate SR calcium release. However, opposite to myocytes with uniform responses, an imbalance in SR calcium handling on a beat-to-beat basis in myocytes with irregular patterns leads to a gradual build up of SR calcium loading on each pulse until a calcium overload-induced calcium wave is triggered (see Fig. 5). This notion is supported by the observation that stimulation of SR calcium overloading and reduction of ICa-triggered calcium release with tetracaine strongly favoured induction of irregular beat-to-beat responses. The particular set of experimental conditions used by Diaz et al. (2004) and Li et al. (2009), i.e. small depolarization pulses that reduce the trigger for SR calcium release and 5 mm external calcium, would also be expected to favour moderate SR calcium release and a gradual build up of SR calcium overload. Indeed, the alternating calcium waves observed in these conditions disappeared when the calcium trigger (ICa) was increased to normal levels (Li et al. 2009). Thus, the mechanism proposed by Li et al. (2009) is not likely to underlie beat-to-beat alternation induced by elevation of the beating rate, but rather a mechanism relevant to specific experimental conditions that favour calcium overload-induced calcium waves on every second beat at low stimulation frequencies where the pulse interval is about twice the periodicity of spontaneous calcium waves.
From a therapeutic point of view, the present data suggest that reduction of abnormal ICa, sarco-endoplasmic reticulum calcium ATPase (SERCA) or RyR2 activity would stabilize the beat-to-beat response, and receptor-mediated control of the phosphorylation status of these calcium-handling proteins may be of particular relevance to this issue. Indeed, it has been shown that administration of β-adrenergic antagonists to patients with heart failure reduces hyperphosphorylation of the RyR2 (Reiken et al. 2003b). However, subdivision of our human atrial myocyte population into two groups corresponding to patients with and without β-blocker treatment did not show any difference in the beat-to-beat stability between the two groups (data not shown). One possible reason for this apparent divergence between human atrial and ventricular myocardium may be found in the higher SERCA level and lower phospholamban:SERCA ratio in the atrium (Boknik et al. 1999). In contrast, stimulation of adenosine A2A receptors selectively stimulates spontaneous SR calcium release (Hove-Madsen et al. 2006b), and recent data from our laboratory show that inhibition of this receptor selectively reduces spontaneous SR calcium release without compromising ICa or SR calcium loading in myocytes from patients with atrial fibrillation (Llach et al. 2011). Finally, angiotensin and endothelin receptors also have the potential to intervene in the regulation of beat-to-beat stability through modulation of SR calcium release (Zima & Blatter, 2004; Gassanov et al. 2006).
Limitations
A relevant limitation when using isolated cardiomyocytes is that the arrhythmogenic response of a single myocyte may be diluted in a multicellular preparation due to cardiotonic effects of neighbouring myocytes, making the extrapolation from isolated myocytes to cardiac tissue or the intact heart difficult. By examining the beat-to-beat response in a population of 133 human atrial myocytes, the present study provides a means to address this issue. Thus, as previously mentioned, the majority of the myocytes had uniform beat-to-beat responses at physiologically relevant stimulation frequencies, suggesting that cardiotonic effects would be likely to eliminate the effects of a few irregular responses. In contrast, alternating and particularly irregular responses predominated at stimulation frequencies relevant to atrial arrhythmias, making cardiotonic protection from neighbouring myocytes impossible and hampering synchronized activation of contraction.
The physiological background for such heterogeneity could potentially arise from adverse conditions associated with myocyte isolation from human atrial tissue samples. However, tissue samples were transferred to cold oxygenated Tyrode solution immediately after excision, and myocyte isolation was begun within less than 10 min in order to minimize potential myocyte damage. In support of a minimal influence of the isolation protocol, different beat-to-beat patterns were observed among myocytes isolated from the same atrial tissue sample, and there were no significant differences in the beat-to-beat responses recorded on days with only one successful myocyte (62% had alternating responses and 20% had irregular responses) and those recorded on days with higher yield/success rate (59% had alternating responses and 16% had irregular responses). In fact, loading of myocytes with fluo-4, which is a commonly used and accepted approach to study intracellular calcium handling, had a much more dramatic impact on the beat-to-beat response, reducing the fraction of alternating responses from 58 to 19% and increasing the fraction of irregular responses from 19 to 45%. The observed heterogeneity in the beat-to-beat response among isolated myocytes is therefore more likely to be related to local structural and electrical remodelling of the atria, which can occur prior to atrial arrhythmia (Deroubaix et al. 2004; Dinanian et al. 2008). Deficient atrial perfusion (Bitigen et al. 2007) and local atrial ischaemia (Sinno et al. 2003) could also contribute to the development of local heterogeneities in ICa, SR calcium loading and spontaneous SR calcium release.
Conclusion
Previous studies on mammalian cardiomyocytes have shown that beat-to-beat alternation in the intracellular calcium transient is caused by alternations in the amount of calcium released from the SR on each beat. The present findings extend this to include human atrial myocytes, but the important novelty of this study is that an elevation of the stimulation frequency to values encountered during arrhythmic episodes produces a heterogeneous beat-to-beat response in a population of isolated human atrial myocytes, demonstrating that heterogeneity in itself is a relevant arrhythmogenic cellular substrate in human atria undergoing a prolonged elevation of the beating rate. Moreover, our findings show that the ability of a human atrial myocyte to maintain a uniform beat-to-beat response upon elevation of the stimulation frequency is determined by its ICa density, by the lability of calcium release from the SR and by its SR calcium load. Specifically, beat-to-beat stability was favoured by moderate ICa, SR calcium load and calcium release, while myocytes with frequent spontaneous calcium release and/or high SR calcium load were more prone to present alternating or irregular beat-to-beat responses, suggesting that pharmacological correction of abnormally high ICa, SERCA and/or RyR2 activity may be therapeutic strategies of particular relevance to stabilizing the beat-to-beat response in patients prone to present pacing-induced atrial arrhythmias.
Acknowledgments
We greatly appreciate the collaboration of the surgeons at the Cardiac Surgery Department at Hospital de Sant Pau, and Miss Berta Ballester for technical assistance with myocyte isolation. This work was funded by the Spanish Ministry of Science and Innovation (SAF2007-60174) and (CNIC2007-12) Instituto de Salud Carlos III (network REDINSCOR). C.E.M. received a predoctoral grant from Generalitat de Catalunya.
Glossary
Abbreviations
- ICa
L-type calcium current
- Itail
tail current
- ITI
transient inward current
- RyR2
ryanodine receptor
- SERCA
sarco-endoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- τfast
fast time constant for ICa inactivation
Author contributions
A.L., C.E.M., J.F. and L.H.-M. contributed to the conception and design of the experiments. Collection, analysis and interpretation of data were done by A.L., C.E.M., J.F., J.P. and L.H.-M. The article was written and revised by J.C. and L.H.-M. All authors contributed to this work and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The authors declare no conflict of interest.
References
- Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:e65–e73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bitigen A, Bulut M, Tanalp AC, Kirma C, Barutcu I, Pala S, Erkol A, Boztosun B. Left atrial appendage functions in patients with severe rheumatic mitral regurgitation. Int J Cardiovasc Imaging. 2007;23:693–700. doi: 10.1007/s10554-007-9207-y. [DOI] [PubMed] [Google Scholar]
- Boknik P, Unkel C, Kirchhefer U, Kleideiter U, Klein-Wiele O, Knapp J, Linck B, Luss H, Muller FU, Schmitz W, Vahlensieck U, Zimmermann N, Jones LR, Neumann J. Regional expression of phospholamban in the human heart. Cardiovasc Res. 1999;43:67–76. doi: 10.1016/s0008-6363(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Bolter CP, Atkinson KJ. Influence of temperature and adrenergic stimulation on rat sinoatrial frequency. Am J Physiol Regul Integr Comp Physiol. 1988;254:R840–R844. doi: 10.1152/ajpregu.1988.254.5.R840. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- Cinca J, Sassine A, Deceuninck P, Roca J, Gagne P, Morena H, Puech P. The dependence of T wave alternans on diastolic resting period duration. Eur J Cardiol. 1978;7:299–309. [PubMed] [Google Scholar]
- Deroubaix E, Folliguet T, Rucker-Martin C, Dinanian S, Boixel C, Validire P, Daniel P, Capderou A, Hatem SN. Moderate and chronic hemodynamic overload of sheep atria induces reversible cellular electrophysiologic abnormalities and atrial vulnerability. J Am Coll Cardiol. 2004;44:1918–1926. doi: 10.1016/j.jacc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Diaz ME, Eisner DA, O'Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- Diaz ME, O'Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- Dinanian S, Boixel C, Juin C, Hulot JS, Coulombe A, Rucker-Martin C, Bonnet N, Le Grand B, Slama M, Mercadier JJ, Hatem SN. Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation. Eur Heart J. 2008;29:1190–1197. doi: 10.1093/eurheartj/ehn140. [DOI] [PubMed] [Google Scholar]
- Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. 12p following 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassanov N, Brandt MC, Michels G, Lindner M, Er F, Hoppe UC. Angiotensin II-induced changes of calcium sparks and ionic currents in human atrial myocytes: potential role for early remodeling in atrial fibrillation. Cell Calcium. 2006;39:175–186. doi: 10.1016/j.ceca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Bers DM. Modulation of excitation–contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreyer BN, Kastor JA, Kershbaum KL. The hemodynamic effects of induced supraventricular tachycardia in man. Circulation. 1976;54:783–789. doi: 10.1161/01.cir.54.5.783. [DOI] [PubMed] [Google Scholar]
- Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, Christini DJ. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation. 2007;115:2094–2102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M. Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circ J. 2005;69:1368–1373. doi: 10.1253/circj.69.1368. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Bers DM. Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J. 1992;63:89–97. doi: 10.1016/S0006-3495(92)81597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Llach A, Molina CE, Prat-Vidal C, Farré J, Roura S, Cinca J. The proarrhythmic antihistaminic drug terfenadine increases spontaneous calcium release in human atrial myocytes. Eur J Pharmacol. 2006a;553:215–221. doi: 10.1016/j.ejphar.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Prat-Vidal C, Llach A, Ciruela F, Casadó V, Lluis C, Bayes-Genis A, Cinca J, Franco R. Adenosine A2A receptors are expressed in human atrial myocytes and modulate spontaneous sarcoplasmic reticulum calcium release. Cardiovasc Res. 2006b;72:292–302. doi: 10.1016/j.cardiores.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation–contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524:795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Zhang L, Chen SR. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ Res. 2002;91:218–225. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockskamper J, Zima AV, Blatter LA. Modulation of sarcoplasmic reticulum Ca2+ release by glycolysis in cat atrial myocytes. J Physiol. 2005;564:697–714. doi: 10.1113/jphysiol.2004.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Kato K, Hirono S, Okura Y, Hanawa H, Ito M, Fuse K, Shiono T, Watanabe K, Aizawa Y. Mechanical alternans in patients with chronic heart failure. J Card Fail. 2001;7:138–145. doi: 10.1054/jcaf.2001.24122. [DOI] [PubMed] [Google Scholar]
- Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, Huang SK, Tseng YZ, Lien WP. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca2+-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33:1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Lee YS. Pathophysiological mechanisms of altered transmembrane potentials in diseased human atria. J Electrocardiol. 1986;19:41–49. doi: 10.1016/s0022-0736(86)80006-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Diaz ME, Eisner DA, O'Neill S. The effects of membrane potential, SR Ca2+ content and RyR responsiveness on systolic Ca2+ alternans in rat ventricular myocytes. J Physiol. 2009;587:1283–1292. doi: 10.1113/jphysiol.2008.164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casadó V, Ciruela F, Lluís C, Franco R, Cinca J, Hove-Madsen L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J. 2011;32:721–729. doi: 10.1093/eurheartj/ehq464. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- Navarro-Lopez F, Cinca J, Sanz G, Magrina J, Betriu A. Isolated T wave alternans elicited by hypocalcemia in dogs. J Electrocardiol. 1978a;11:103–108. doi: 10.1016/s0022-0736(78)80098-3. [DOI] [PubMed] [Google Scholar]
- Navarro-Lopez F, Cinca J, Sanz G, Periz A, Magrina J, Betriu A. Isolated T wave alternans. Am Heart J. 1978b;95:369–374. doi: 10.1016/0002-8703(78)90369-1. [DOI] [PubMed] [Google Scholar]
- Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem. 2003a;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, Burkhoff D, Marks AR. β-Blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003b;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- Shiferaw Y, Karma A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc Natl Acad Sci USA. 2006;103:5670–5675. doi: 10.1073/pnas.0511061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman V, Goldberg A, London B. Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113:2880–2887. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]
- Sinno H, Derakhchan K, Libersan D, Merhi Y, Leung TK, Nattel S. Atrial ischemia promotes atrial fibrillation in dogs. Circulation. 2003;107:1930–1936. doi: 10.1161/01.CIR.0000058743.15215.03. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphatedependent Ca2+ signalling in cat atrial excitation–contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]


