Non-technical summary
The maintenance of healthy blood pressure is influenced by the activity of the sympathetic perivascular nerves which surround small arteries and arterioles. Increases in sympathetic neurovascular control may play a role in the genesis and progression of cardiovascular disease during obesity. Our data show that sympathetic nerve-mediated vasoconstriction is augmented during diet-induced obesity and that ATP and purinergic mechanisms play a significant role. Changes occur due to an increase in sympathetic nerve density and release of ATP, with a potential contribution from a decreased sensitivity to sensory vasodilatory neurotransmitters. Improving control of sympathetic function through the identification of potential therapeutic targets is likely to lead to increased cardiovascular health benefits for overweight and obese individuals.
Abstract
Abstract
While a close correlation exists in obese humans between sympathetic, adrenergic hyperactivity and structural and functional organ damage, a role for the co-transmitter, ATP, in vascular function remains unexplored. We therefore studied sympathetic nerve-mediated responses of pressurised small mesenteric arteries from control and obese rats. Diet-induced obesity significantly increased the amplitude of vasoconstriction to transmural nerve stimulation (1–10 Hz; P < 0.05). At 1 and 5 Hz, both adrenergic and purinergic responses were significantly augmented, while only the purinergic component was increased at 10 Hz (P < 0.05). Nerve stimulation at 1 Hz evoked contractions and underlying excitatory junction potentials (EJPs), which were both significantly increased in amplitude during obesity (P < 0.05) and abolished by αβ-methylene ATP (1 μm; desensitises purinergic receptors). The rise time and rate of decay of these EJPs were significant decreased (P < 0.05), without change in resting membrane potential. Amplitude and frequency of spontaneous EJPs and the density of perivascular sympathetic nerves were also significantly increased (P < 0.05). Inhibition of sensory neurotransmitter release (capsaicin; 10 μm) significantly increased the amplitude of nerve-mediated contraction (P < 0.05), with a greater effect in control than obese animals, although the density of sensory nerves was unaffected by obesity. We demonstrate that sympathetic nerve-mediated vasoconstriction is enhanced by diet-induced obesity due to upregulation of purinergic, in addition to adrenergic, neurotransmission. Changes result from increased perivascular sympathetic innervation and release of ATP. We conclude that augmented sympathetic control of vasoconstriction induced by obesity could contribute directly to hypertension and global organ damage. A decrease in sensitivity to sensory vasodilatory neurotransmitters may also affect these processes.
Introduction
A clear relationship exists between the current obesity epidemic, hypertension and increased cardiovascular risk, with hyperactivity of the sympathetic nervous system emerging as an important contributing factor (Snitker et al. 2000; Morris, 2008; Grassi et al. 2009; Lambert et al. 2010). In obese humans, sympathetic outflows to the kidney and skeletal muscle are augmented and a close correlation exists between sympathetic hyperactivity and structural changes resulting in arterial stiffness, endothelial dysfunction, cardiac hypertrophy and renal damage (Grassi et al. 2010; Lambert et al. 2010). These correlations have led to the suggestion that pharmacological targeting of sympathetic nervous activity would be of benefit in obese individuals, even in the absence of overt hypertension (Grassi et al. 2010; Lambert et al. 2010). However, it is not clear to what extent the mechanisms underlying this augmentation of sympathetic activity operate at a peripheral or organ level. Furthermore, no study has demonstrated whether diet-induced obesity directly affects the sympathetic control of vasoconstriction under physiological conditions. Such a peripherally located effect could exacerbate the cardiac and renal organ damage accompanying obesity, as well as contribute to the development of hypertension.
Increased release of noradrenaline has long been considered to be a marker of sympathetic hyperactivity. Indeed, techniques measuring plasma noradrenaline concentration and increased urinary noradrenaline excretion have been used in humans, along with studies of muscle sympathetic nerve firing rates, to assess sympathetic nerve activity during obesity and hypertension (see Lambert et al. 2010). However, sympathetic nerves release other cotransmitters, among which ATP is especially important in the control of vascular tone (Sjoblom-Widfeldt, 1990; Burnstock, 2009). Furthermore, in isolated and pressurised small mesenteric arteries of rats, ATP and the associated purinergic receptor signalling pathways predominate over adrenergic mechanisms at physiological pressures (Rummery et al. 2007). Thus, potential pharmaceutical targeting of sympathetic hyperactivity through adrenergic pathways may not be entirely beneficial since noradrenaline and ATP control vasoconstriction through separate mechanisms. While bath-applied noradrenaline activates α1-adrenoceptors, leading to release of calcium from intracellular stores, a slow depolarisation and prolonged constriction, a mechanical response can still be evoked by focal application of noradrenaline in the absence of any membrane potential change (Hashimoto et al. 1986; Hirst & Edwards, 1989; Tran et al. 2009). In contrast, ATP elicits an electromechanical response, by activating ligand-gated P2X1 purinoceptors on vascular smooth muscle and generating a fast depolarisation (Benham & Tsien, 1987; Brock & Cunnane, 1993; Lamont et al. 2006). The unitary depolarisation evoked by ATP release from sympathetic nerves following a single stimulus is called an excitatory junction potential or EJP (Brock & Cunnane, 1993). Individual EJPs are brief with a rise time of <100 ms and duration of <1 s and consequently do not normally elicit vasoconstriction. However, repetitive sympathetic nerve stimulation evokes phasic EJPs, which produce a focal increase in smooth muscle calcium, adjacent to the neurotransmitter release sites at varicosities, and fast transient constriction (Brain et al. 2002; Lamont & Wier, 2002). In mesenteric arteries, the depolarisation produced by EJPs activates voltage-dependent calcium channels and the resulting calcium influx causes constriction (Rummery et al. 2007).
Alterations in purinergic sympathetic neurotransmission have been described in spontaneously hypertensive and deoxycorticosterone acetate (DOCA)-salt hypertensive rats (Vidal et al. 1986; Brock & Van Helden, 1995; Demel & Galligan, 2008) yet despite established links between obesity and hypertension, no studies have examined the effects of diet-induced obesity on sympathetic nerve-mediated control of vascular tone at physiologically relevant pressures, nor the contribution of adrenergic versus purinergic mechanisms. While the effects of exogenous application of noradrenaline were reported to be enhanced in the skeletal and renal circulations of a genetic animal model of obesity (Hayashi et al. 2002; Stepp & Frisbee, 2002), these data may not tell us about the actions of neurally released sympathetic neurotransmitters, since studies in the heart and intestine have shown that global application of neurotransmitter activates intracellular pathways which are different to those initiated by neurally released neurotransmitter (Hirst et al. 1996).
Thus, the present study has utilised a more physiologically relevant approach, involving neural stimulation of pressurised small mesenteric resistance arteries taken from control and long-term diet-induced obese rats, to probe the effects of obesity on sympathetic nerve-mediated vasomotor function. We hypothesised that local sympathetic nerve-mediated vasoconstriction of mesenteric resistance arteries, held at physiologically relevant intravascular pressures, would be augmented in obesity and that release of ATP would play a prominent role in this process.
Methods
Animals and dietary intervention
All experiments were performed in accordance with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, under a protocol approved by the Australian National University Animal Experimentation and Ethics Committee.
Male Sprague–Dawley rats (aged 6 weeks) were randomly assigned into two diet groups. The control diet groups were exposed to standard laboratory chow, consisting of 12% calories as fat, while the obese diet groups were fed a high-fat, simple carbohydrate, semi-pure diet consisting of 43% calories as fat (Specialty Feeds, Australia). Animals were given ad libitum access to food during the 20–24 weeks of diet intervention.
Isolated artery preparation
Rats were anaesthetized with isofluorane and decapitated. Small mesenteric arteries (third-order) were isolated and placed into cold (5–7°C) dissection buffer containing (mmol l−1): 3 Mops, 1.2 NaH2PO4, 4.6 glucose, 2 pyruvate, 0.02 EDTA (Na), 0.15 albumin, 145 NaCl, 4.7 KCl, 2 CaCl2 and 1.2 MgSO4. Artery segments were cannulated between two glass micropipettes, secured using 10–0 nylon surgical sutures (Alcon Laboratories, Australia) and mounted in a recording chamber (Living Systems Instrumentation Inc., USA), in which platinum stimulating electrodes were positioned on either side of the vessel, perpendicular to the vessel axis. The recording chamber was perfused at a constant flow rate (3 ml min−1) with physiological salt solution (PSS) containing (mmol l−1): 120 NaCl, 5 KCl, 25 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 2 MgCl2 and 11 glucose, gassed with 5% CO2 in air and maintained at 36–37°C.
Pressure myography
Segments of arteries were pressurized to 40 mmHg by connecting the inflow pipette to a pressure servo and peristaltic pump (Living Systems Instrumentation Inc.) and allowed to equilibrate. Following the equilibration period, pressure was increased in 10 mmHg increments from 40 to 80 mmHg, every 10 min. Spontaneous tone was typically seen to develop at the completion of this protocol. In order to obtain optimal myogenic and drug responsiveness, the stability of the cannulation was tested at the onset of experiments by transiently increasing luminal pressure to 110 mmHg. Only vessels without pressure leaks and displaying spontaneous myogenic tone were studied. Videomicroscopy and the DIAMTRAK computer tracking program (Neild, 1989) were used to continuously measure vessel diameter (10×, 0.4 NA objective; resolution ∼1 μm). All drug solutions were superfused and, at the completion of each experiment, the maximum vessel diameter (Dmax) was recorded following perfusion with 0 mmol l−1 Ca2+ PSS containing 2 mmol l−1 EGTA.
Electrophysiology
Intracellular recordings were made using sharp microelectrodes (120–200 MΩ; Flaming Brown Micropipette Puller, Sutter Instrument Co.) filled with carboxyfluorescein (5% in 0.5 mol l−1 KCl) to confirm cellular identity. In this study, membrane potential recordings were obtained from smooth muscle cells. Records were amplified with an Axoclamp 200A (Molecular Devices, USA) while simultaneous changes in membrane potential and vessel diameter in the region where the cell was impaled were stored for analysis using pCLAMP software (Molecular Devices). Successful recordings were characterized by an abrupt signal deflection upon cell impalement and a stable membrane potential more negative than the initial potential. Resting membrane potential was determined upon withdrawal of the electrode.
In all experiments, perivascular nerves were excited by electrical field stimulation (0.1 ms pulse width). In some experiments, supramaximal stimulation parameters (40–90 mA) were established so that increasing the strength of a single stimulus did not cause further increase in the size of the evoked excitatory junction potential. Under these conditions, nerves were stimulated with a single stimulus every 30 s. EJP peak amplitude and time course were determined from measurements of a minimum of three EJPs in each preparation.
Trains of electrical stimuli were also delivered at 1, 3, 5 and 10 Hz for 30 s every 3 min or until vessel diameter returned to baseline. However, due to the large contractions, it was not possible to maintain impalements during repetitive nerve stimulation at frequencies greater than 1 Hz. αβ-methylene ATP, prazosin and capsaicin were used to determine the relative contribution of ATP and noradrenaline, and the role of sensory nerves to the nerve-mediated responses observed in the pressurized small mesenteric arteries, respectively. A single concentration of prazosin (0.1 μm) was chosen since it has previously been shown to selectively abolish responses mediated by α1–adrenoceptors, the main subtype of smooth muscle adrenoceptor causing nerve-mediated vasoconstriction in small mesenteric arteries (Angus et al. 1988; Lamont et al. 2003). Single concentrations of αβ-methylene ATP (1 μm) and capsaicin (10 μm) were also used for their known ability to cause P2X receptor desensitisation and inhibition of sensory neurotransmitter release, respectively (Ralevic et al. 2000; Pakdeechote et al. 2007). Drugs were superfused and allowed to equilibrate for approximately 30 min before nerve stimulation protocols were initiated.
Confocal immunohistochemisty
Rats were anaesthetized as above and segments of mesentery containing first, second and third order arteries and recurrent loops were removed and fixed for 10 min with 2% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. Tissues were washed in phosphate-buffered saline (PBS) and permeabilised with DMSO (3 × 10 min) followed by PBS washes. Arteries were dissected free of all fat and pre-incubated in 2% BSA and 0.2% Triton-X in PBS for 30 min, followed by incubation overnight at room temperature in anti-synaptophysin (1:200, Dako Denmark A/S, Glostrup, Denmark) or anti-nNOS (1:100, Zymed Laboratories, USA) or for 2 nights at room temperature in anti-CGRP (1:1000, Auspep, Australia). Immunofluorescence was visualised with Cy3 conjugated, goat anti-rabbit IgG (1:100, 2 h; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Preparations were mounted in buffered glycerol and viewed with a Zeiss LSM PASCAL 5 confocal microscope (Zeiss Instruments, Heidelberg, Germany).
Sympathetic adrenergic nerves were identified using the Faglu method (Furness et al. 1978), whereby isolated mesenteric arteries were immersion fixed in 4% formaldehyde and 0.5% glutaraldehyde in 0.1 m sodium phosphate buffer (pH 7.0) at 4°C for 3 h. Preparations were transferred to slides, dehydrated over phosphorus pentoxide for 1.5 h at room temperature and mounted in paraffin oil. Catecholamine fluorescence was viewed using a UV laser on a Leica TCS SP5 microscope (Leica Instruments, North Ryde, Australia).
Third order mesenteric arteries, corresponding to those used for physiological studies, were identified and confocal image series were collected at 1 μm intervals, from the upper surface of each vessel to a point midway through the vessel (40×, 1.3 NA objective). Each image series was recombined to a single projection for quantification of nerve networks using ImageJ (NIH). Line profiles were determined using PASCAL software (Zeiss Instruments). Background levels were set so that all positively labelled varicosities along isolated fibres or small fibre bundles on the vessel surface were visualised. The area in which fibres were quantified in each vessel was determined in μm2 and normalised to the area occupied in 100 μm of vessel length. This normalisation provides a measure of the functional nerve density for comparison with data from physiological experiments in which vessel segments of constant length were used. The total labelling per area of artery occupied within 100 μm of axial length for each animal was the average of at least three image series. Means ± SEM were calculated using these averaged values such that n represents the number of animals.
Analysis of results
Physiological data were collected using pCLAMP software (Molecular Devices) and analysed using Clampfit (Molecular Devices) and GraphPad Prism (GraphPad Software). EJPs recorded in each preparation and during each experimental protocol were averaged so that statistical comparisons could be made. Spontaneous EJP (sEJP) amplitudes were determined from a background noise level of 0.5 mV.
The amplitudes of the individual nerve-mediated responses were normalised to maximum vessel diameter (Dmax) recorded in 0 mm Ca2+ PSS, as (Do−Dpeak)/Dmax× 100, where Do represents the resting vessel diameter before nerve stimulation and Dpeak represents the diameter at the peak of the response. In order to determine the relative contribution of adrenergic versus purinergic mechanisms, for experiments in which prazosin was added first, followed by prazosin in combination with α,β-methylene ATP, the adrenergic component was determined by (Dpraz−Dpeak)/Dmax× 100, where Dpraz represents the diameter at the peak response in the presence of prazosin. The purinergic component was subsequently determined by (Dpraz+α,βmATP−Dpraz)/Dmax× 100, where Dpraz+α,βmATP represents the diameter at the peak response in the combined presence of prazosin and α,β-methylene ATP. For experiments in which α,β-methylene ATP was added first, followed by α,β-methylene ATP in combination with prazosin, the purinergic component of the response was determined by (Dα,βmATP−Dpeak)/Dmax× 100, while the adrenergic component was determined by (Dα,βmATP+praz−Dα,β-mATP)/Dmax× 100.
Results are means ± SEM of n preparations, where each preparation was from a different rat, unless otherwise stated. Data were analysed as indicated for specific protocols, with Student's t test for paired or unpaired data, or ANOVA, followed by t tests with Bonferroni correction for multiple comparisons. Statistical significance was defined as P < 0.05.
Drugs
Prazosin HCl and 8-methyl-N-vanillyl-6-non-enamide (capsaicin) were obtained from Sigma. αβ-methylene ATP was obtained from RBI. αβ-methylene ATP and prazosin were prepared as stock solutions in distilled water. Stock solutions of capsaicin were prepared using 100% ethanol. Appropriate dilution showed no effect of ethanol on vascular responses.
Results
Effect of obesity: general observations
Following the 20–24 week period of high-fat diet intervention, body weight was significantly greater compared with controls, with body length, kidney and liver weight and retroperitoneal and gonadal fat mass being increased; retroperitoneal and gonadal fat mass were also significantly increased relative to body weight (Table 1). The fat content of this diet and of a 34% fat, cafeteria-style diet have been shown to cause a significant increase in blood pressure and blood glucose (Fan et al. 2008; Ng et al. 2010; Rajia et al. 2010). Arteries from both control and obese rats developed a graded myogenic constriction, although there was no significant difference in the level of vascular tone at 80 mmHg (control: 72.3 ± 5.7%Dmax, n = 30; obese: 72.6 ± 3.3%Dmax, n = 30; unpaired t test) or in the maximum vessel diameter (Dmax) recorded in 0 mm Ca2+ PSS (control: 337 ± 10 μm, n = 30; obese: 349.3 ± 8.3 μm, n = 30). The smooth muscle cell resting membrane potential recorded from arteries exhibiting the myogenic response was also not significantly different between the control and obese animals (control: −40.5 ± 0.9 mV, n = 20; obese: −41.0 ± 0.6 mV, n = 25).
Table 1.
Control and diet-induced obese rat general characteristics
| Control (n = 30) | Obese (n = 30) | |
|---|---|---|
| Body weight (g) | 533 ± 9 | 698 ± 17* |
| Body length (cm) | 25.5 ± 0.2 | 26.6 ± 0.2* |
| Kidney (g) | 1.6 ± 0.1 | 1.8 ± 0.1* |
| Liver (g) | 17.6 ± 0.5 | 23.6 ± 1.0* |
| Retroperitoneal fat (g) | 6.6 ± 0.4 | 21.1 ± 1.4* |
| Gonadal fat (g) | 9.3 ± 0.5 | 23.3 ± 1.2* |
P < 0.05, unpaired t test.
Nerve-mediated vasoconstriction
In arteries from control (Fig. 1A) and obese animals (Fig. 1B), electrical stimulation evoked a nerve-mediated contraction that increased in amplitude with increase in stimulation frequency. At all frequencies examined, the amplitude of the evoked contractions was significantly greater in arteries taken from obese animals (Fig. 1C, n = 12, P < 0.0001, two-way ANOVA; unpaired t tests). Tetrodotoxin (TTX: 1 μm) blocked electrically evoked contractions (10 Hz control: 23 ± 4%Dmax, TTX: 0 ± 0, n = 3; obese: 40.3 ± 8.2%Dmax, TTX: 0 ± 0, n = 3; P < 0.05, paired t test) demonstrating that contractions were not due to direct stimulation of the vascular smooth muscle. Controls to demonstrate the stability of nerve-mediated responses over time showed no significant difference in the amplitude of contractions during three consecutive periods of nerve stimulation comprising 1, 3, 5 and 10 Hz frequencies in the absence of drugs, using arteries from either control animals or high-fat diet animals, (n = 4, P < 0.05, data not shown).
Figure 1. Obesity increases nerve-mediated vasoconstriction in pressurized rat mesenteric arteries.
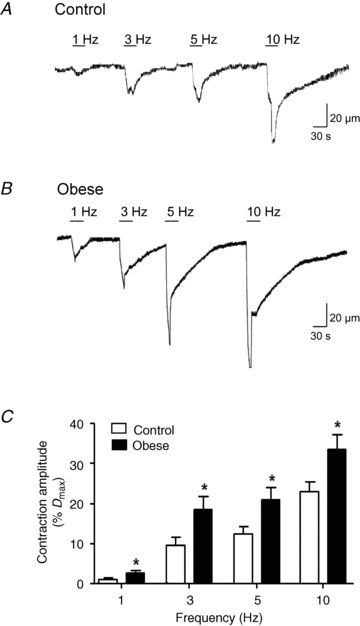
Representative traces showing neurally evoked vasoconstriction to 1, 3, 5 and 10 Hz stimuli (30 s duration) in arteries from control (A) and diet-induced obese rats (B). C, summary data show that the amplitude of nerve-mediated responses is increased during obesity. The period of stimulation at each frequency is represented by the stimulus bar. Columns represent mean ± SEM. *P < 0.05 control versus obese, unpaired t test.
In order to examine the noradrenergic component of the nerve-mediated vasoconstriction evoked by 10 Hz stimulation, arteries were pre-incubated in prazosin (0.1 μm). In control arteries, prazosin abolished the evoked constriction (Fig. 2A, n = 4, P < 0.05, two-way ANOVA; Bonferroni post hoc test), while in arteries from obese animals, the augmented nerve-mediated constriction was significantly decreased in amplitude, but not abolished (Fig. 2B, n = 9, P < 0.05, two-way ANOVA; Bonferroni post hoc test). The subsequent addition of αβ-methylene ATP (1 μm) to arteries already exposed to prazosin was then used to examine the purinergic component of the sympathetic vasoconstriction. Thus, in the presence of prazosin and αβ-methylene ATP, stimulation of perivascular nerves (10 Hz) unmasked a small nerve-mediated dilatation in arteries from both control and obese animals, although this was only significant in the obese animals (Fig. 2A and B, P < 0.05, two-way ANOVA; Bonferroni post hoc test). Analysis of the size of the vasoconstriction due to noradrenaline and ATP showed that the purinergic, but not the adrenergic, component of the sympathetic nerve-mediated vasoconstriction was significantly increased in arteries taken from diet-induced obese animals, while the adrenergic component was unchanged (Fig. 2C, P < 0.05, two-way ANOVA; Bonferroni post hoc test).
Figure 2. Obesity enhances only the purinergic component of nerve-mediated vasoconstriction evoked by 10 Hz stimulation.
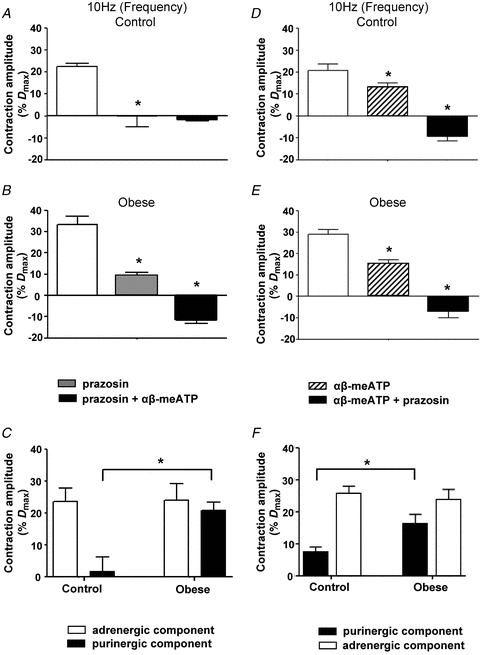
Effects of prazosin (0.1 μm) followed by αβ-meATP (1 μm) (A and B) or of αβ-meATP followed by prazosin (D and E) on nerve-mediated vasoconstrictions evoked by 10 Hz stimulation in arteries from control (A and D) and obese animals (B and E). Columns represent mean ± SEM. *P < 0.05 compared to previous treatment; two-way ANOVA; Bonferroni post hoc test. C, summary data showing the amplitude of neurally evoked responses of control and obese arteries blocked by prazosin (0.1 μm), to reveal the adrenergic component, and by αβ-meATP (1 μm), to reveal the purinergic component of the sympathetically mediated vasoconstriction in A and B. *P < 0.05, compared to control; two-way ANOVA; Bonferroni post hoc test. F, summary data showing the amplitude of neurally evoked responses of control and obese arteries blocked by αβ-meATP (1 μm), to reveal the purinergic component, and by prazosin (0.1 μm), to reveal the adrenergic component, of the sympathetically mediated vasoconstriction in D and E. *P < 0.05, compared to control; two-way ANOVA; Bonferroni post hoc test. Columns represent mean ± SEM.
When inhibitors were applied in the reverse order, αβ-methylene ATP (1 μm) significantly reduced the amplitude of vasoconstriction evoked in arteries from control rats and there was a larger vasoconstriction in arteries from obese animals, leaving a similar residual constriction in both groups (Fig. 2D, n = 6 and Fig. 2E, n = 4; P < 0.05, two-way ANOVA; Bonferroni post hoc test). The combined application of αβ-methylene ATP and prazosin (0.1 μm) subsequently uncovered a comparable nerve-mediated dilatation in both groups (Fig. 2D, n = 6 and Fig. 2E, n = 4; P < 0.05, two-way ANOVA; Bonferroni post hoc test). Analysis of the adrenergic and purinergic components again confirmed a significant increase in the purinergic component of the nerve-mediated vasoconstriction in arteries taken from diet-induced obese animals, without change in the adrenergic component (Fig. 2F, n = 4, P < 0.05, two-way ANOVA; Bonferroni post hoc test). While bath application of αβ–methylene ATP (1 μm) was used to desensitise purinergic receptors, a transient constriction was initially evoked in all vessels which returned to baseline diameters. This constriction was significantly larger (P < 0.05, unpaired t test) in arteries taken from obese animals (60.6 ± 4.9%Dmax, n = 4) compared to control (47.4 ± 1.7%Dmax, n = 6).
The contribution of purinergic and adrenergic components to nerve-mediated vasoconstriction evoked by mid-range (3 and 5 Hz) stimulation frequencies was also examined (Fig. 3). Application of αβ-methylene ATP (1 μm) alone significantly reduced the amplitude of the nerve-mediated vasoconstriction evoked by 3 and 5 Hz stimulation frequencies in arteries from control rats (Fig. 3A and D; P < 0.05, two-way ANOVA; Bonferroni post hoc test) and augmented vasoconstrictions evoked in arteries from obese rats (Fig. 3B and E; P < 0.05, two-way ANOVA; Bonferroni post hoc test). In the combined presence of αβ-methylene ATP and prazosin (0.1 μm), stimulation of the perivascular nerves at both 3 and 5 Hz uncovered a nerve-mediated vasodilatation (3 Hz: Fig. 3A, control; Fig. 3B, obese; 5 Hz: Fig. 3D, control; Fig. 3E, obese), similar to that recorded during 10 Hz stimulation. Analysis of the purinergic and adrenergic components contributing to the nerve-mediated vasoconstriction evoked by 3 Hz stimulation, showed that the adrenergic component was significantly increased in arteries from obese rats, compared to those from control rats (Fig. 3C; P < 0.05, two-way ANOVA; Bonferroni post hoc test), while at 5 Hz stimulation, both adrenergic and purinergic components were significantly increased (Fig. 3F; P < 0.05, two-way ANOVA; Bonferroni post hoc test).
Figure 3. Obesity enhances the adrenergic and purinergic components of nerve-mediated vasoconstriction evoked by 3 Hz and 5 Hz stimulation.
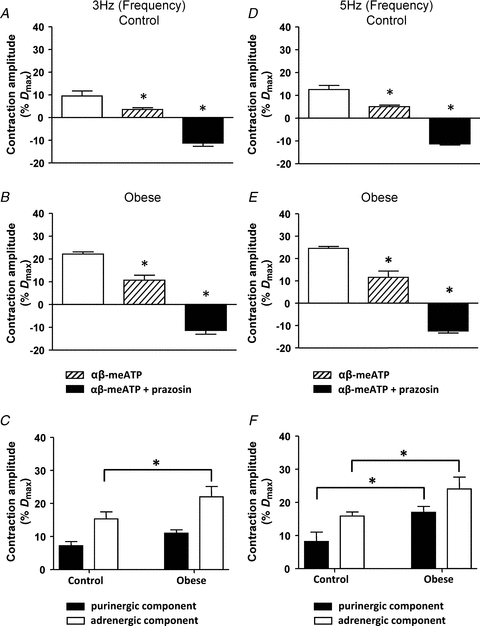
Effects of αβ-meATP (1 μm) followed by prazosin (0.1 μm) on nerve-mediated vasoconstriction evoked by 3 Hz (A and B) and 5 Hz (D and E) stimulation in arteries from control (A and D) and obese (B and E) animals. Columns represent mean ± SEM. *P < 0.05 compared to previous treatment, two-way ANOVA; Bonferroni post hoc test. C and F, summary data showing the amplitude of neurally evoked responses of control and obese arteries blocked by αβ-meATP (1 μm), to reveal the purinergic component, and by prazosin (0.1 μm), to reveal the adrenergic component, of the sympathetically mediated vasoconstriction to 3 Hz and 5 Hz stimulation, respectively. *P < 0.05, two-way ANOVA; Bonferroni post hoc test. Columns represent mean ± SEM.
Neurally released ATP and EJPs
We recorded the electrophysiological responses underlying nerve-mediated vasoconstriction at 1 Hz. Consistent with data in Fig. 1, the amplitude of nerve-mediated vasoconstriction was significantly increased (Fig. 4A and B, control: 1.1 ± 0.2%Dmax, n = 8; obese: 4.6 ± 0.9%Dmax, n = 6; P < 0.05, unpaired t test). In arteries from both control and obese animals, constriction was associated with trains of EJPs which were also increased in amplitude during obesity (Fig. 4A and B, control: 7.2 ± 0.8 mV, n = 7; obese: 9.5 ± 1.0 mV, n = 6; P < 0.05, unpaired t test). Both the nerve-evoked constrictions and underlying electrical responses were abolished by αβ-methylene ATP (Fig. 4A and B, P < 0.05, paired t test) in arteries from control and obese rats, confirming that both resulted entirely from the release of ATP.
Figure 4. αβ-meATP (1 μm) abolishes nerve-mediated vasoconstriction and underlying EJPs evoked by 1 Hz stimulation.
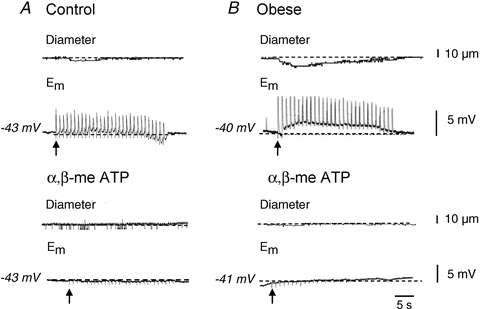
Representative traces showing the effects of αβ-meATP (1 μm) on neurally evoked vasoconstriction and underlying EJPs (1 Hz stimulation frequency, 30 s duration) in arteries from control (A) and diet-induced obese rats (B). The arrows represent onset of stimulus.
A role for noradrenaline release during 1 Hz nerve stimulation was tested by the subsequent addition of prazosin to αβ-methylene ATP. Nerve stimulation in the presence of both drugs elicited a very small vasodilatation, which showed a small but significant increase in arteries from obese rats (control: −0.6 ± 0.2%Dmax, n = 4; obese: −1.3 ± 0.1%Dmax, n = 4; P < 0.05, unpaired t test). These data showed that a small adrenergic component was evoked by 1 Hz stimulation in control arteries and that this was also increased in arteries from obese rats. Nevertheless, the augmented vasoconstriction to 1 Hz stimulation in the arteries from obese rats was mediated predominantly by the purinergic component.
Effect of obesity on EJPs
In order to determine whether obesity affected pre- or postsynaptic mechanisms, we analysed individual EJPs arising from a single supramaximal stimulation of the perivascular nerves. Single EJPs were shown to have significantly larger amplitudes in arteries taken from obese animals (Fig. 5A and B; control: 10.1 ± 0.6 mV, n = 64; obese: 13.2 ± 0.5 mV, n = 53) and this was accompanied by a significant decrease in both rise time (Fig. 5C) and rate of decay (Fig. 5D). Single EJPs were followed by a transient hyperpolarisation in arteries from both diet groups (Fig. 5A) and obesity also caused a significant increase in the amplitude of this hyperpolarisation (Fig. 5E).
Figure 5. Obesity increases EJP amplitude in rat small mesenteric arteries.
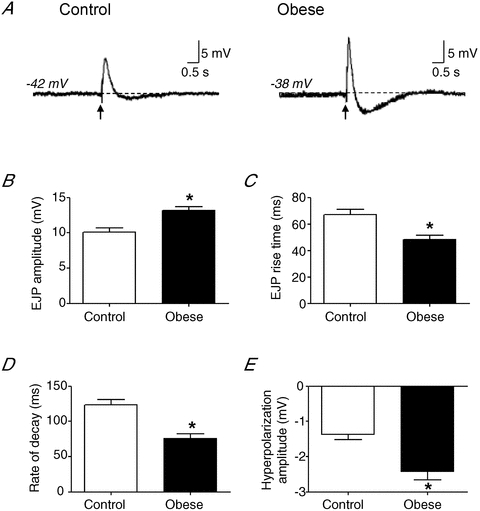
A, representative traces showing EJPs evoked by a single supramaximal stimulus from control and diet-induced obese rats. The arrows represent onset of stimulus. Summary data show that the EJP amplitude is increased during obesity (B), with a significant decrease in rise time (C) and rate of decay (D). Obesity increases the amplitude of the transient hyperpolarisation following EJPs (E). Columns represent mean ± SEM. *P < 0.05 control versus obese, unpaired t test.
Effect of obesity on spontaneous EJPs
Small, spontaneous EJPs (sEJPs) can be recorded in the absence of nerve stimulation and their amplitude distribution is considered to represent the quantal nature of neurotransmitter release (Brock & Cunnane, 1993). sEJPs were recorded in ∼14% of arteries from control animals and ∼23% of arteries from obese animals. Membrane potential recordings showed that the frequency (Fig. 6A–C, n = 6, P < 0.05, unpaired t test) and amplitude (Fig. 6A and B) of spontaneous events was greater in arteries from obese animals, compared to control. To compare amplitudes of sEJPs between arteries of both diet groups, the peak amplitude of the first 100 sEJPs was analysed from control arteries and obese arteries and the probability of these events determined (Fig. 6D). Probability–amplitude distributions showed that sEJPs were significantly larger in arteries from obese animals than from controls (P < 0.05, two-way ANOVA). These data indicate that the probability of transmitter release and/or the quantal content of individual packets of transmitter is increased in obesity.
Figure 6. Obesity increases amplitude and frequency of spontaneous EJPs.
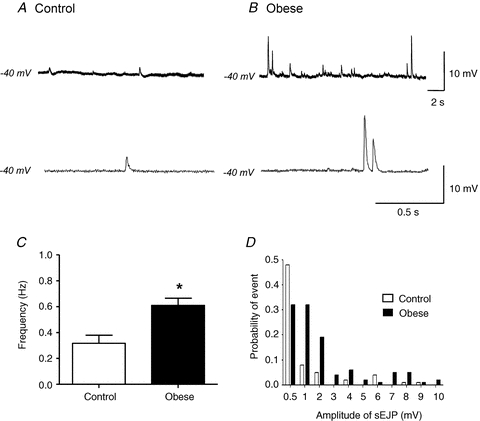
Representative traces showing spontaneous EJPs recorded in arteries from control (A) and obese animals (B) demonstrate an increase in frequency (B, top trace) and amplitude (B, bottom trace) in obesity. C, summary data showing the increase in spontaneous EJP frequency in obese animals. *P < 0.05, unpaired t test. Columns represent mean ± SEM. D, probability–amplitude distribution of spontaneous EJPs recorded from arteries of both diet groups. Analysis included the first 100 spontaneous EJPs recorded from control (n = 5) and obese (n = 3) arteries. P < 0.05, two-way ANOVA.
Contribution of noradrenaline and ATP to the transient hyperpolarisation
The contribution of noradrenaline and ATP to the transient hyperpolarisation associated with single EJPs was assessed. In the first instance, prazosin (0.1 μm) had no effect on smooth muscle cell membrane potential in arteries from control (−41.6 ± 1.4 mV, prazosin: –42.9 ± 1.0 mV; n = 8) or obese animals (−38.9 ± 1.0 mV, prazosin: −39 ± 1.3 mV, n = 9). Prazosin also had no significant effect on the amplitude of EJPs evoked by a single supramaximal stimulation in arteries from control animals (Fig. 7A, 8.7 ± 0.8 mV, prazosin: 7.7 ± 1.0 mV; n = 6). However, EJP amplitude was decreased in arteries from obese animals (Fig. 7B, 11.8 ± 0.3 mV, prazosin: 10.4 ± 0.5 mV; n = 7, P < 0.05, paired t test), although the EJP amplitude was still significantly larger in arteries from obese rats compared to those from control rats (P < 0.05, unpaired t test).
Figure 7. αβ-meATP (1 μm), but not prazosin (0.1 μm), abolishes EJPs and transient hyperpolarisations.
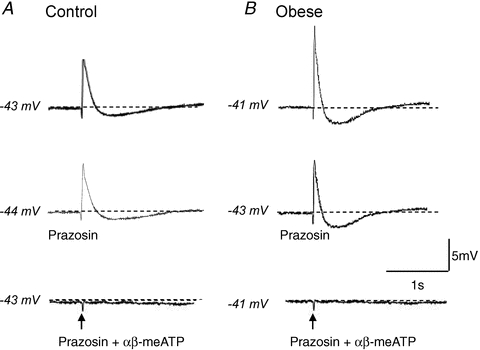
EJPs and transient hyperpolarisations recorded from control (A) and obese (B) arteries evoked by a single supramaximal stimulus are blocked by addition of prazosin (0.1 μm) and αβ–meATP (1 μm), but not by prazosin alone. The arrows represent onset of stimulus.
Prazosin caused no significant change to the amplitude of the transient hyperpolarisation in either diet group (control: 1.5 ± 0.4 mV, prazosin: 1.9 ± 0.5 mV, n = 6; obese: 2.7 ± 0.5 mV, prazosin: 2.5 ± 0.2 mV, n = 5; paired t test). In contrast, addition of αβ–methylene ATP abolished EJPs and the transient hyperpolarisations recorded in arteries from both diet groups (Fig. 7, P < 0.05, paired t test), without significant effects on resting membrane potential recorded from control arteries (+αβ-methylene ATP: −38.9 ± 1.0 mV, n = 7) or obese arteries (+αβ-methylene ATP: −42.0 ± 3.6 mV, n = 6; paired t test).
Role of sensory nerves
Application of capsaicin (10 μm), which causes an initial excitation of sensory nerves via TRPV1 channels and subsequent desensitisation and inhibition of neurotransmitter release (Baamonde et al. 2005; Peng & Li, 2010), produced a transient hyperpolarisation, although no lasting effect on resting membrane potential was observed after this initial period (control: −41.6 ± 1.4 mV, n = 8; obese: −42.1 ± 3.5 mV, n = 10; unpaired t test).
Incubation in capsaicin caused a significant increase in the amplitude of the nerve-mediated vasoconstriction evoked by 10 Hz stimulation in arteries from both control (Fig. 8A; n = 8, P < 0.05, paired t test) and obese animals (n = 10, paired t test, P < 0.05); however, this increase was significantly greater in arteries taken from control animals (control: 22.3 ± 9.9%Dmax, n = 8; obese: 7.3 ± 1.4%Dmax, n = 10; P < 0.05, unpaired t test). The smaller increase in constriction seen in the obese rats after capsaicin was not due to the vessel already having reached its minimum diameter, since the transient constriction following application of αβ-methylene ATP exceeded these values in both diet groups (as described above).
Figure 8. Obesity decreases sensory nerve-mediated vasodilatation.
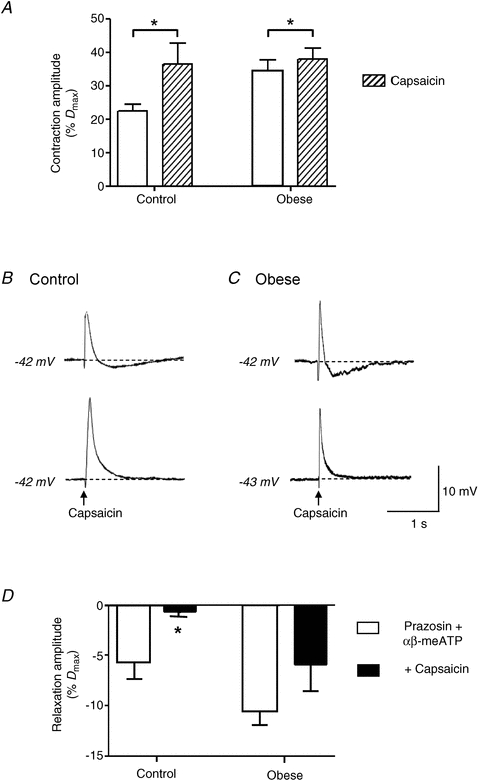
A, capsaicin (10 μm), which inhibits sensory neurotransmitter release, caused a significant increase in nerve-mediated vasoconstriction evoked by 10 Hz stimulation in arteries from both diet groups, although the effect was greater in control rats. Columns represent mean ± SEM. *P < 0.05, paired t test. B and C, capsaicin also abolished transient hyperpolarisations following EJPs in both diet groups. The arrows represent onset of stimulus. D, nerve-mediated vasodilatations, uncovered by blockade of adrenergic and purinergic vasoconstriction (0.1 μm prazosin and 1 μmαβ-meATP), were abolished by capsaicin in arteries from control rats, but not in arteries from obese rats.
Capsaicin abolished the transient hyperpolarisation following EJPs evoked by a single supramaximal stimulus in arteries from both control and obese animals (Fig. 8B and C, P < 0.05, paired t test). Capsaicin also abolished the residual vasodilatation evoked by 10 Hz stimulation in the presence of prazosin (0.1 μm) and αβ-methylene ATP (1 μm) in control arteries, but this was not seen in arteries from obese rats (Fig. 8D).
Changes in perivascular nerve plexuses
Immunohistochemistry using antibodies against synaptophysin revealed a substantial plexus of nerve fibres over the surface of primary, secondary, tertiary and recurrent mesenteric arteries taken from both control and obese rats. However, the plexus was denser over arteries from the obese rats (Fig. 9A and B) and line profiles demonstrated a higher incidence of fibre bundles in these arteries compared with those from control rats (Fig. 9C and D). At higher magnification, within the fibre bundles, the density of varicosities was also greater over mesenteric arteries from obese rats (Fig. 9E and F).
Figure 9. Obesity increases synaptophysin-containing nerves surrounding small mesenteric arteries.
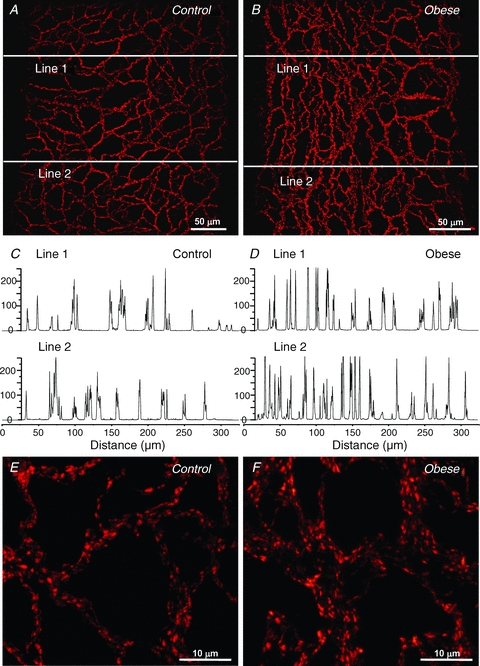
Nerve plexuses were denser over arteries taken from obese (B) compared to control rats (A). Line profiles determined at the sites shown in A and B demonstrated that nerve bundles were more common on arteries from obese (D) than control rats (C). Ordinate denotes arbitrary units of fluorescence intensity. Varicosities were more numerous on arteries from obese (F) compared to control rats (E).
Catecholamine histochemistry confirmed that the increased nerve plexus over the mesenteric vessels from the obese rats resulted from an increase in density of adrenergic nerves (Fig. 10A and B). In contrast, immunohistochemistry with antibodies against CGRP to visualise sensory nerves (Fig. 10C and D) and against nNOS to visualise nitrergic nerves (Fig. 10E and F) revealed only sparse nerve plexuses which did not differ between arteries from control and obese rats.
Figure 10. Catecholaminergic nerves surrounding small mesenteric arteries are increased in obesity, without change in CGRP- and nNOS-containing nerves.
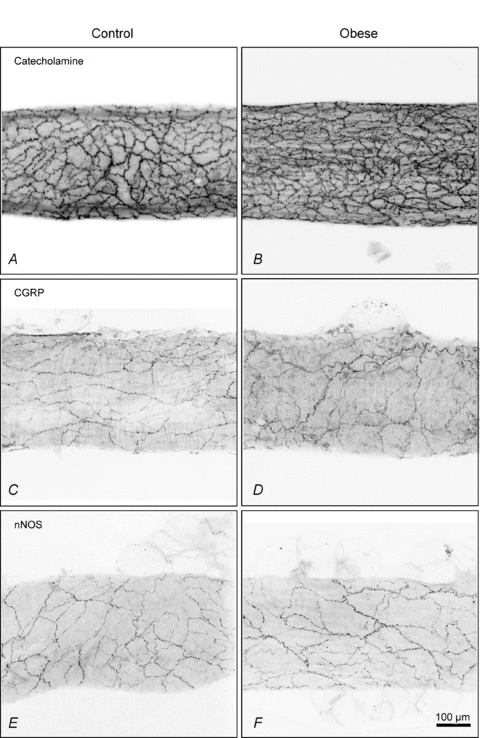
Perivascular sympathetic nerves containing catcholamines were denser around arteries taken from obese (B) compared to control (A) rats. Plexuses of sensory nerves showing immunoreactivity for CGRP (C and D) and nitrergic nerves showing immunoreactivity for nNOS (E and F) were similar in density between arteries from control and obese rats.
Quantification of the plexuses stained for synaptophysin and catecholamines showed a significant increase in density over the arteries from the obese rats compared to controls (Fig. 11; P < 0.05, n = 4 for each group), but no significant change in the densities of the plexuses of sensory or nitrergic nerves (Fig. 11; n = 4 for each group).
Figure 11. Obesity increases catecholaminergic but not sensory or nitrergic perivascular nerve plexuses.
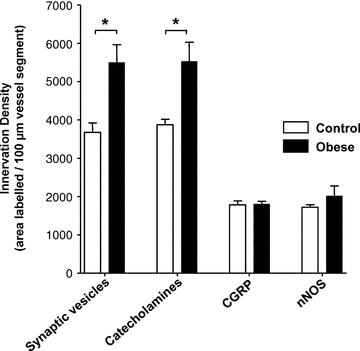
Quantification of the density of perivascular nerves labelled with synaptophysin (Synaptic vesicles) or containing catecholamines confirmed an increase in arteries taken from obese compared to control rats. In contrast, no significant difference was found in the density of CGRP- or nNOS-containing nerve fibres between diet groups. Columns represent mean ± SEM. *P < 0.05 control versus obese, unpaired t test.
Discussion
The present study has shown that diet-induced obesity directly enhances sympathetic nerve-mediated vasoconstriction of small, pressurised, resistance sized, mesenteric arteries of the rat over a range of stimulation frequencies. We show that this sympathetic hyperactivity in the mesenteric resistance vasculature in obesity involves enhanced purinergic neurotransmission, in addition to increased adrenergic function. Changes in amplitude and time course of the underlying purinergic EJPs suggest that effects are largely presynaptic, with increased amount of neurotransmitter released from sympathetic varicosities. Consistent with these data, we found a significant increase in the density of sympathetic, perivascular nerve plexuses, although additional alterations in postsynaptic reactivity may also contribute to the responses. Inhibition of transmitter release from sensory nerves, following exposure to capsaicin, augmented sympathetic nerve-mediated vasoconstriction in both control and obese animals; however, this effect was less prominent with obesity. Since we found no change in the density of the sensory nerve plexus during obesity, the data may suggest a reduced sensitivity of the vasculature to sensory neurotransmitters, which could contribute to augmentation of sympathetic vasoconstriction and reduction in the ability to regulate blood flow to the gastrointestinal tract.
Electrical stimulation of perivascular nerves surrounding pressurized mesenteric arteries of control rats evoked a vasoconstriction which increased in amplitude with increased stimulation frequency, as previously described (Rummery et al. 2007). In arteries taken from diet-induced obese animals, the evoked contractile response was significantly enhanced at all frequencies (Fig. 1), consistent with the sympathetic over-activity associated with the obese state. Diet-induced obesity significantly increased the purinergic component at 1, 5 and 10 Hz, while the noradrenergic component of this constriction was increased at all but 10 Hz, where activity was maximal (Figs 2 and 3). Microneurography studies from resting conscious humans have shown that sympathetic vasoconstrictor neurones in vivo fire at low frequencies (<1 Hz) but that this rate increases significantly in hypertension, obesity and chronic asphyxia (Mancia et al. 2007; Ashley et al. 2010). Interestingly, our data confirmed that the purinergic component of the nerve-mediated vasoconstriction in mesenteric arteries dominates at these low frequencies (Sjoblom-Widfeldt & Nilsson, 1990) and we extend this observation to nerve stimulation of arteries from obese animals. In contrast to our results, recent studies in primary mesenteric arteries from obese rats following short-term diet intervention reported that an enhanced adrenergic component of nerve-mediated vasoconstriction accounted for augmented vasoconstriction following 1–16 Hz stimulation (Blanco-Rivero et al. 2011). However, in the latter study the absence of intraluminal pressure may have resulted in underestimation of the purinergic contribution to neurally evoked responses, as described by Rummery et al. (2007). Since upregulation of purinergic neurotransmission has previously been described to underpin enhanced sympathetic nerve-mediated vasoconstriction in mesenteric arteries from a rat hypertensive model (Nilsson & Folkow, 1982) and sympathetic activity is upregulated via purinergic mechanisms in the present study, it is possible that changes in purinergic neurotransmission may contribute significantly to the hypertensive condition underlying cardiovascular disease in overweight and obese individuals (Kotsis et al. 2010).
The mechanism by which ATP and purinergic mechanisms are upregulated during diet-induced obesity was investigated using trains of low-frequency stimulation. Our data demonstrate that EJPs have significantly larger amplitudes but are still abolished by αβ-methylene ATP (Figs 4 and 5), confirming an expanded role for ATP in this artery during diet-induced obesity. In addition, exogenous αβ-methylene ATP evoked a transient contractile response which was increased in arteries taken from diet-induced obese animals. While these data may suggest postsynaptic changes in P2X receptor density and distribution in the obese state, it should be noted that neurally released ATP may only reach these extrasynaptic sites following changes to the mechanisms controlling ATP breakdown.
In vascular tissues, the time course of the exponential decay phase of the EJP reflects the passive membrane properties of the smooth muscle cells, and is governed by the membrane resistance and capacitance. In the present study the decay phase of the EJPs evoked by a single supramaximal stimulus was significantly reduced in arteries taken from obese animals and the EJPs were followed by a transient after-hyperpolarisation which was larger in the arteries from obese than control rats (Fig. 5). These data are consistent with the activation, during the falling phase of the EJP, of an outward conductance which was larger in the obese animals, resulting in a significant reduction in membrane resistance and, consequently, the time constant of decay of the EJP (Hirst & Edwards, 1989). However, we cannot exclude the possibility that a reduction in smooth muscle cell size or a decrease in cell coupling in obesity could produce the same effect, although our recent studies failed to find any such change in myoendothelial coupling in these vessels (Haddock et al. 2011). In control and obese rats, the transient after-hyperpolarisation was abolished by αβ-methylene ATP, but unaffected by prazosin (Fig. 7), confirming that it also arose through enhanced purinergic but not adrenergic activation. After-hyperpolarisations have previously been recorded in rat tail and mesenteric arteries where they were also abolished by purinergic receptor antagonists (Cheung, 1982; Rummery et al. 2007). Activation of sympathetic nerves thus elicits a depolarisation and vasoconstriction followed by a hyperpolarisation which may normally act as a feedback brake to further constriction. The augmentation of this latter mechanism in obesity may be a fortuitous consequence of the upregulation of sympathetic function.
In order to determine if obesity-related changes were occurring presynaptically or postsynaptically, we examined the characteristics of single EJPs evoked by a supramaximal stimulus. While EJPs recorded in arteries from obese animals were increased in amplitude, the time course to peak response was decreased (Fig. 5). As smooth muscle cells are electrically coupled and receive innervation from multiple synapses in the perivascular nerve plexus, the EJP represents the composite electrical activity resulting from ATP release at sites both near and far from the recording electrode (Brock & Cunnane, 1993). An increase in amplitude associated with a decrease in rise time in arteries from obese rats would therefore result from the source of current lying closer to the recording electrode (Hill et al. 1983). These changes are consistent with either an increase in nerve density or an increase in the probability of transmitter release from sympathetic varicosities (Bennett & Gibson, 1995). In line with this prediction, we found a significant increase in innervation density of the perivascular sympathetic nerves around arteries from obese compared to control rats (Figs 9–11). Alterations in innervation density of the rat tail and mesenteric arteries have also been reported in a number of disease states, particularly under hypertensive conditions (Kawamura et al. 1989; Smeda, 1990; Kunes et al. 1991; Hobara et al. 2004; Parkington et al. 2004). These data suggest that one component contributing to sympathetic hyperactivity during obesity is an expansion of peripheral neural plexuses and increased release of ATP.
Analysis of the incidence and amplitude of spontaneous EJPs in the sympathetic nervous system has been used to define the quantal nature of neurotransmitter release (Brock & Cunnane, 1992). However, increase in innervation density would also be expected to increase the incidence and amplitude of spontaneous EJPs, as the distance between transmitter release sites and the recording electrode would be decreased leading to reduction in current loss through the vascular syncytium (Bennett, 1972; Cheung, 1982). Obesity-induced increases in spontaneous EJP amplitude and frequency found here (Fig. 6), can therefore be assigned to presynaptic effects on neurotransmitter release, although the observed increase in innervation density makes it difficult to determine whether quantal content is actually increased. While it is possible that changes in postsynaptic receptor expression could also contribute to increases in spontaneous EJP amplitude (Brock & Cunnane, 1993), the reduction in rise time of EJPs in obese rats provides strong evidence for the involvement of presynaptic mechanisms (Bennett, 1972; Cheung, 1982). Interestingly, increases in quantal content have previously been described in the vas deferens of spontaneous hypertensive rats although no assessment of innervation density was made (Guitart et al. 2002).
Activation of perivascular capsaicin-sensitive sensory nerves resulting in vasodilatation and increased flow in the mesenteric circulation is likely to occur in response to chemical or mechanical stimulation of adjacent segments of intestine. Indeed, distention of the distal colon has been shown to induce hyperpolarisation of the inferior mesenteric artery via capsaicin-sensitive sensory nerves which would thus operate to match blood flow to gut motility (Meehan & Kreulen, 1992). For such a role to be important in counteracting the tonic vasoconstrictor influence of the extensive perivascular sympathetic nerve plexus, activation of sensory nerves would be expected to antagonise the effects of ongoing sympathetic nerve activity. In support of this proposal, functional interaction between sensory vasodilatory action and sympathetic vasoconstriction has been previously demonstrated in mesenteric resistance vessels (Meehan et al. 1991; Ahluwalia & Vallance, 1996; Coffa & Kotecha, 1999; Lomax & Vanner, 2010). In the present study, we found that inhibition of sensory neurotransmitter release increased the amplitude of nerve-mediated vasoconstriction significantly more in arteries from control than obese animals, although we did not find any significant difference in the density of the perivascular sensory nerve plexus between the diet groups (Figs 8, 10 and 11). These data suggest that the sensitivity of the vasculature to sensory neurotransmitters may be reduced during diet-induced obesity. Such impairment may lead to additional complications in obesity, resulting from a reduction in the ability to regulate blood flow to the gastrointestinal tract in response to distention. Impaired sensory nerve function has also been shown to contribute to increased sympathetic neurotransmission in the renal vasculature during hypertension (Wyss et al. 1986; DiBona et al. 1999; Kopp et al. 2008).
Two different neurally mediated vasodilatory components were found in the present study. One was independent of either P2X-purinergic or α1-adrenergic mechanisms (Figs 2 and 3), while the other was activated directly or indirectly through P2X-purinergic pathways (Fig. 7). Neither of these components was attenuated in obesity; however, the contribution of sensory nerves to these two responses differed between the diet groups. The P2X-purinergically activated vasodilatation was mediated entirely by capsaicin-sensitive sensory nerves in arteries from both control and obese rats (Fig. 8B and C), while these nerves were only involved in the non-α1-adrenergic, non-P2X-purinergic vasodilatation in arteries from control rats (Fig. 8D). The persistence of a capsaicin-insensitive vasodilatory component in obesity suggests that compensatory mechanisms involving non-sensory vasodilator nerves are activated. Several different possibilities exist for these effects, including the release of noradrenaline from sympathetic nerves acting on β-adrenoceptors, the release of ATP from sympathetic nerves acting on P2Y purinoceptors or the release of nitric oxide from the perivascular nitrergic nerve plexus demonstrated in the present study. Future studies directed to identification of the nerves, neurotransmitters and downstream mechanisms responsible for these vasodilatory responses may provide additional therapeutic targets to counteract the detrimental effects of obesity-induced sympathetic over-constriction.
In conclusion, sympathetic nerve-mediated vasoconstriction is augmented in rat mesenteric resistance arteries during diet-induced obesity. This hyperactivity results from upregulation of purinergic in addition to adrenergic signalling mechanisms, through an increase in the density of the sympathetic perivascular nerve plexus and release of ATP. A decrease in the sensitivity to sensory vasodilatory neurotransmitters may also compound these effects. A summary of the changes occurring in obesity can be found in Fig. 12. We suggest that similar changes may underpin the hypertensive condition characteristic of cardiovascular disease in overweight and obese humans and may thus provide novel data to assist in identification of new therapeutic targets to reduce and control cardiovascular disease.
Figure 12. Mechanisms underlying augmented neurally evoked vasoconstriction during obesity.
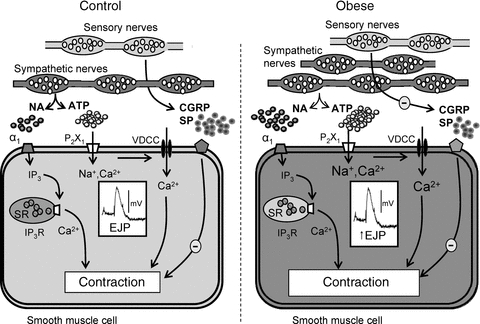
In the mesenteric artery from control animals, sympathetically mediated vasoconstriction is due to (1) activation of P2X1 purinoceptors by ATP, depolarisation due to an inward current recorded as an EJP, opening of voltage-dependent calcium channels (VDCCs) leading to Ca2+ influx and vasoconstriction, and (2) activation of α1-adrenoceptors by noradrenaline (NA) leading to production of IP3 and release of Ca2+ from intracellular stores and sustained contraction. Sensory nerve activity negatively modulates neurally evoked vasoconstriction. Mesenteric arteries from obese animals have a denser sympathetic perivascular nerve plexus with increased number of varicosities. EJPs are larger due to increased ATP release and the amplitude of purinergically mediated and adrenergically mediated vasoconstriction is amplified. Although sensory nerve density is not changed, negative modulation by sensory neurotransmitters is less effective leading to augmentation of the amplitude of nerve-mediated vasoconstriction.
Acknowledgments
We thank Mr Joshua Inglis for technical assistance. This study was supported by a grant from the National Health and Medical Research Council of Australia to R.E.H. (ID 466009).
Glossary
Abbreviations
- αβ-meATP
αβ-methylene ATP
- Dmax
maximum diameter
- EJP
excitatory junction potential
- Em
membrane potential
- NPY
neuropeptide Y
- sEJP
spontaneous excitatory junction potential
- SMC
smooth muscle cell
- TTX
tetrodotoxin
Author contributions
R.E.H. and C.E.H. contributed to experimental conception and design; data acquisition and interpretation; manuscript drafting and revision. Both authors approved the final manuscript for publication.
References
- Ahluwalia A, Vallance P. Interaction between sympathetic and sensory nerves in rat small arteries: involvement of nitric oxide. Am J Physiol Heart Circ Physiol. 1996;271:H969–H976. doi: 10.1152/ajpheart.1996.271.3.H969. [DOI] [PubMed] [Google Scholar]
- Angus JA, Broughton A, Mulvany MJ. Role of α-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J Physiol. 1988;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C, Burton D, Sverrisdottir YB, Sander M, McKenzie DK, Macefield VG. Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J Physiol. 2010;588:701–712. doi: 10.1113/jphysiol.2009.185348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baamonde A, Lastra A, Juarez L, Hidalgo A, Menendez L. TRPV1 desensitisation and endogenous vanilloid involvement in the enhanced analgesia induced by capsaicin in inflamed tissues. Brain Res Bull. 2005;67:476–481. doi: 10.1016/j.brainresbull.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Autonomic neuromuscular transmission. Monogr Physiol Soc. 1972:1–271. [PubMed] [Google Scholar]
- Bennett MR, Gibson WG. On the contribution of quantal secretion from close-contact and loose-contact varicosities to the synaptic potentials in the vas deferens. Philos Trans R Soc Lond B Biol Sci. 1995;347:187–204. doi: 10.1098/rstb.1995.0021. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, De Las Heras N, Martin-Fernandez B, Cachofeiro V, Lahera V, Balfagon G. Rosuvastatin restored adrenergic and nitrergic function in mesenteric arteries from obese rats. Br J Pharmacol. 2011;162:271–285. doi: 10.1111/j.1476-5381.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol. 2002;541:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Impulse conduction in sympathetic nerve terminals in the guinea-pig vas deferens and the role of the pelvic ganglia. Neuroscience. 1992;47:185–196. doi: 10.1016/0306-4522(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Neurotransmitter release mechanisms at the sympathetic neuroeffector junction. Exp Physiol. 1993;78:591–614. doi: 10.1113/expphysiol.1993.sp003709. [DOI] [PubMed] [Google Scholar]
- Brock JA, Van Helden DF. Enhanced excitatory junction potentials in mesenteric arteries from spontaneously hypertensive rats. Pflugers Arch. 1995;430:901–908. doi: 10.1007/BF01837403. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Autonomic neurotransmission: 60 years since Sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- Cheung DW. Spontaneous and evoked excitatory junction potentials in rat tail arteries. J Physiol. 1982;328:449–459. doi: 10.1113/jphysiol.1982.sp014276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffa PF, Kotecha N. Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea-pig small intestine. J Auton Nerv Syst. 1999;77:125–132. [PubMed] [Google Scholar]
- Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2008;52:322–329. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF, Jones SY, Kopp UC. Renal mechanoreceptor dysfunction: an intermediate phenotype in spontaneously hypertensive rats. Hypertension. 1999;33:472–475. doi: 10.1161/01.hyp.33.1.472. [DOI] [PubMed] [Google Scholar]
- Fan LH, Tian HY, Ma AQ, Hu Z, Huo JH, Cao YX. Altered ATP-sensitive potassium channels may underscore obesity-triggered increase in blood pressure. Acta Pharmacol Sin. 2008;29:1167–1174. doi: 10.1111/j.1745-7254.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Heath JW, Costa M. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochemistry. 1978;57:285–295. doi: 10.1007/BF00492664. [DOI] [PubMed] [Google Scholar]
- Grassi G, Arenare F, Quarti-Trevano F, Seravalle G, Mancia G. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Prog Cardiovasc Dis. 2009;52:31–37. doi: 10.1016/j.pcad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'oro R. Sympathetic activation in obesity: a noninnocent bystander. Hypertension. 2010;56:338–340. doi: 10.1161/HYPERTENSIONAHA.110.156596. [DOI] [PubMed] [Google Scholar]
- Guitart M, Jimenez M, Giraldo J, Gonalons E, Badia A. Changes in electrophysiological properties in the prostatic portion of vas deferens from spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:425–430. doi: 10.1007/s00210-002-0614-2. [DOI] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hirata M, Itoh T, Kanmura Y, Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, Tatematsu S, Kumagai H, Saruta T. Altered renal microvascular response in Zucker obese rats. Metabolism. 2002;51:1553–1561. doi: 10.1053/meta.2002.36311. [DOI] [PubMed] [Google Scholar]
- Hill CE, Hirst GD, van Helden DF. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Choate JK, Cousins HM, Edwards FR, Klemm MF. Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience. 1996;73:7–23. doi: 10.1016/0306-4522(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hobara N, Nakamura A, Goda M, Kawasaki H. Malfunction of vascular control in lifestyle-related diseases: distribution of adrenomedullin-containing perivascular nerves and its alteration in hypertension. J Pharmacol Sci. 2004;96:391–394. doi: 10.1254/jphs.fmj04006x2. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Ando K, Takebayashi S. Perivascular innervation of the mesenteric artery in spontaneously hypertensive rats. Hypertension. 1989;14:660–665. doi: 10.1161/01.hyp.14.6.660. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Yorek MA. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: role of angiotensin. Am J Physiol Regul Integr Comp Physiol. 2008;294:R858–R866. doi: 10.1152/ajpregu.00830.2007. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- Kunes J, Leontjeva GR, Byskova E, Pohlova I, Govyrin VA, Zicha J. Adrenergic innervation of blood vessels in Dahl rats with salt hypertension. Clin Exp Hypertens A. 1991;13:1343–1355. doi: 10.3109/10641969109048797. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome – causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–H3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Vanner SJ. Presynaptic inhibition of neural vasodilator pathways to submucosal arterioles by release of purines from sympathetic nerves. Am J Physiol Gastrointest Liver Physiol. 2010;298:G700–G705. doi: 10.1152/ajpgi.00291.2009. [DOI] [PubMed] [Google Scholar]
- Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- Meehan AG, Hottenstein OD, Kreulen DL. Capsaicin-sensitive nerves mediate inhibitory junction potentials and dilatation in guinea-pig mesenteric artery. J Physiol. 1991;443:161–174. doi: 10.1113/jphysiol.1991.sp018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan AG, Kreulen DL. A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J Physiol. 1992;448:153–159. doi: 10.1113/jphysiol.1992.sp019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ. Cardiovascular and metabolic effects of obesity. Clin Exp Pharmacol Physiol. 2008;35:416–419. doi: 10.1111/j.1440-1681.2008.04912.x. [DOI] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Folkow B. Vasoconstrictor nerve influence on isolated mesenteric resistance vessels from normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1982;116:205–208. doi: 10.1111/j.1748-1716.1982.tb07131.x. [DOI] [PubMed] [Google Scholar]
- Pakdeechote P, Rummery NM, Ralevic V, Dunn WR. Raised tone reveals purinergic-mediated responses to sympathetic nerve stimulation in the rat perfused mesenteric vascular bed. Eur J Pharmacol. 2007;563:180–186. doi: 10.1016/j.ejphar.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Dodd J, Luff SE, Worthy K, Coleman HA, Tare M, Anderson WP, Edgley AJ. Selective increase in renal arcuate innervation density and neurogenic constriction in chronic angiotensin II-infused rats. Hypertension. 2004;43:643–648. doi: 10.1161/01.HYP.0000117140.52220.85. [DOI] [PubMed] [Google Scholar]
- Peng J, Li YJ. The vanilloid receptor TRPV1: role in cardiovascular and gastrointestinal protection. Eur J Pharmacol. 2010;627:1–7. doi: 10.1016/j.ejphar.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Rajia S, Chen H, Morris MJ. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J Neuroendocrinol. 2010;22:905–914. doi: 10.1111/j.1365-2826.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol. 2007;582:745–754. doi: 10.1113/jphysiol.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom-Widfeldt N. Neuro-muscular transmission in blood vessels: phasic and tonic components. An in-vitro study of mesenteric arteries of the rat. Acta Physiol Scand Suppl. 1990;587:1–52. [PubMed] [Google Scholar]
- Sjoblom-Widfeldt N, Nilsson H. Sympathetic transmission in small mesenteric arteries from the rat: influence of impulse pattern. Acta Physiol Scand. 1990;138:523–528. doi: 10.1111/j.1748-1716.1990.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Smeda JS. Analysis of cerebrovascular sympathetic nerve density in relation to stroke development in spontaneously hypertensive rats. Stroke. 1990;21:785–789. doi: 10.1161/01.str.21.5.785. [DOI] [PubMed] [Google Scholar]
- Snitker S, Macdonald I, Ravussin E, Astrup A. The sympathetic nervous system and obesity: role in aetiology and treatment. Obes Rev. 2000;1:5–15. doi: 10.1046/j.1467-789x.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282:H816–H820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- Tran CH, Vigmond EJ, Plane F, Welsh DG. Mechanistic basis of differential conduction in skeletal muscle arteries. J Physiol. 2009;587:1301–1318. doi: 10.1113/jphysiol.2008.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Hicks PE, Langer SZ. Differential effects of ab-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:384–390. doi: 10.1007/BF00500092. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol Heart Circ Physiol. 1986;250:H82–H86. doi: 10.1152/ajpheart.1986.250.1.H82. [DOI] [PubMed] [Google Scholar]


