Non-technical summary
The enteric nervous system of the gastrointestinal tract regulates all the functions of the gut. Enteric neurons are surrounded and outnumbered by enteric glial cells, whose functions are not well understood. Digestive and defensive functions of the gut require the secretion of fluid into the lumen in a regulated manner. Fluid is controlled by the secretion or absorption of ions across the epithelium. Here we have identified a physiological role for enteric glia of the colon in the regulation of fluid balance, through the production of the gaseous mediator nitric oxide. Enteric glia work in concert with enteric nerves by using nitric oxide to regulate the movement of ions across the wall of the colon, thereby affecting water movement and hence digestion and host defence.
Abstract
Abstract
Enteric glia are increasingly recognized as important in the regulation of a variety of gastrointestinal functions. Here we tested the hypothesis that nicotinic signalling in the myenteric plexus results in the release of nitric oxide (NO) from neurons and enteric glia to modulate epithelial ion transport. Ion transport was assessed using full-thickness or muscle-stripped segments of mouse colon mounted in Ussing chambers. The cell-permeant NO-sensitive dye DAR-4M AM and amperometry were utilized to identify the cellular sites of NO production within the myenteric plexus and the contributions from specific NOS isoforms. Nicotinic receptors were localized using immunohistochemistry. Nicotinic cholinergic stimulation of colonic segments resulted in NO-dependent changes in epithelial active electrogenic ion transport that were TTX sensitive and significantly altered in the absence of the myenteric plexus. Nicotinic stimulation of the myenteric plexus resulted in NO production and release from neurons and enteric glia, which was completely blocked in the presence of nitric oxide synthase (NOS) I and NOS II inhibitors. Using the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), neuronal and enteric glial components of NO production were demonstrated. Nicotinic receptors were identified on enteric neurons, which express NOS I, and enteric glia, which express NOS II. These data identify a unique pathway in the mouse colon whereby nicotinic cholinergic signalling in myenteric ganglia mobilizes NO from NOS II in enteric glia, which in coordinated activity with neurons in the myenteric plexus modulates epithelial ion transport, a key component of homeostasis and innate immunity.
Introduction
The control of water movement across the epithelium of the gastrointestinal (GI) tract is driven by vectorial electrogenic ion transport and is central to health and well-being (Barrett & Keely, 2000). Water movement is required to hydrate the surface of the epithelium for contact digestion and nutrient absorption, and as an essential component of the epithelial barrier and hence innate immunity (Barrett & Keely, 2000). Neurons of the submucosal plexus of the enteric nervous system (ENS) represent the main physiological control mechanism regulating epithelial ion transport (Cooke, 1989). In contrast, neurons of the myenteric plexus that are well known to control GI motility have been largely overlooked when considering the regulation of epithelial ion transport. Our understanding of the control of epithelial barrier function has taken on a new dimension recently because it was shown that not only were neurons of the ENS involved, but also the enteric glial cells (Bush et al. 1998; Savidge et al. 2007; Flamant et al. 2010). Enteric glia are analogous to astrocytes of the central nervous system, protecting and supporting enteric neurons (Gabella, 1981). In addition to regulating barrier function, enteric glia actively participate in neurotransmission within the ENS (Gulbransen & Sharkey, 2009; Gulbransen et al. 2010). Whether enteric glia play a role in the regulation of ion transport has yet to be determined.
Nitric oxide (NO) is tonically produced under physiological conditions by the constitutively expressed nitric oxide synthase (NOS) I (neuronal NOS), and in higher amounts during inflammation when inducible NOS II (inducible NOS) is mobilized (Moncada & Bolanos, 2006). Nitric oxide liberated from a variety of cell types including neurons can affect enteric epithelial ion transport by acting directly upon the epithelium and through the submucosal plexus of the ENS (Tamai & Gaginella, 1993; Wilson et al. 1993; Rao et al. 1994; Stack et al. 1996; Mourad et al. 1999; Rolfe & Milla, 1999; Reddix et al. 2000). NO synthases have been identified within the myenteric plexus, in populations of enteric neurons which express NOS I and in enteric glia which express NOS II under basal conditions (Sang & Young, 1996; Neunlist et al. 2001; Green et al. 2004; Qu et al. 2008). The release of NO from guinea pig myenteric plexus in vitro has been demonstrated following nicotinic receptor stimulation (Patel et al. 2008); however, the cell types and isoforms of NOS contributing to this response have not been identified. In a model of colitis in which ex vivo analysis of colonic tissue from mice treated with dextran sodium sulphate (DSS) was performed, a role for myenteric plexus-derived NO in nicotinic regulation of epithelial ion transport was revealed (Green et al. 2004). The localization of NOS II in enteric glia in the myenteric plexus led to the speculation that these cells were the source of NO. The role of enteric glial-derived NO under physiological conditions remains to be elucidated.
Here we focus on the novel and largely unappreciated role of the myenteric plexus in the control of epithelial ion transport. Employing the complementary techniques of amperometry, immunohistochemistry, NO imaging and Ussing chamber electrophysiology, we tested the hypothesis that nicotinic signalling in the myenteric plexus results in the release of NO from neurons and enteric glia to modulate epithelial ion transport. We have discovered that epithelial active electrogenic ion transport in the mouse colon can be modulated by NO-mediated cross-talk between neurons and enteric glia of the myenteric plexus.
Methods
Ethical approval
Animal use protocols were approved by the University of Calgary Animal Care Committee, and were carried out in accordance with the guidelines of the Canadian Council on Animal Care. All animals were killed by cervical dislocation under deep isofluorane anaesthesia.
Animals
Animals were housed in plastic cages with sawdust on the floor, with free access to Purina Laboratory Chow and tap water. Male CD1 mice were utilized for the Ussing chamber studies. Male and female S100β-GFP mice bred at the University of Calgary (Vives et al. 2003) were utilized for the NO imaging studies. Male CD1 mice, male knockout mice for the NOS I isoform (NOS I−/−; B6;129S4-Nos1tm1Plh/J) and NOS II isoform (NOS II−/−; B6.129P2-Nos2tm1Lau/J) were from The Jackson Laboratory (Bar Harbor, ME, USA) and were utilized for the electrochemical detection studies. NOS I and II−/− mice were also used for the NO imaging studies.
Measurement of electrogenic ion transport
The distal colon (distal 2.5 cm of colon from the midpoint between the caecum and rectum) was removed and full thickness segments or those stripped of the external muscle layers and attendant myenteric plexuses (confirmed by H&E staining) mounted in Ussing chambers (0.6 cm2 opening). The tissue was equilibrated for 30 min in oxygenated (95% O2–5% CO2; pH 7.4) Krebs buffer that contained (in mM): NaCl (117), KCl (4.8), CaCl2 (2.5), MgCl2 (1.2), NaHCO3 (25), NaH2PO4 (1.2) and D-glucose (11), warmed to 37°C (Green et al. 2004). The tissue was held under voltage-clamp conditions and the net movement of ions across the epithelium recorded as short-circuit current (ISC, μA cm−2). Drugs were added serosally unless otherwise specified and remained in the bath for the duration of the experiment. The following drugs were used: (Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino] diazen-1-ium-1,2-diolate (PAPA NONOate; 10, 50, 100, 500 μm; Cayman Chemical Co., Ann Arbor, MI, USA), dimethylphenylpiperazinium (DMPP; 10 μm, Sigma-Aldrich, St Louis, MO, USA); hexamethonium (10 μm, Sigma-Aldrich); S-methyl-l-thiocitrulline (SMTC; 10 μm, Calbiochem, La Jolla, CA, USA); tetrodotoxin (TTX; 500 nm, Tocris Bioscience, Ellisville, MO, USA). The NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO; 100 μm, Calbiochem) was also utilized. ISC responses were recorded at the maximum increase (i.e. peak of Phase I) and decrease (i.e. trough of Phase II) to occur within 15 min of drug application. At the conclusion of each experiment, the secretagogue forskolin (10 μm, Sigma-Aldrich) was added to assess tissue viability. Current (μA) and voltage (mV) measurements were taken at the beginning, mid-point and end of each experiment, and the resulting conductance calculated (mS cm−2). Tissues with a conductance greater than 100 mS cm−2 were excluded (n = 1/80). The involvement of specific ions was assessed using the sodium channel blocker amiloride, which was added mucosally (10 μm, Sigma-Aldrich) and chloride-free Krebs buffer that contained (in mm): D-gluconic acid, sodium salt (117), D-gluconic acid, potassium salt (4.8), NaHCO3 (25), NaH2PO4 (1.2), D-gluconic acid, hemimagnesium salt (1.2), D-gluconic acid, calcium salt (2.5), and D-glucose (11), pH 7.4.
NO imaging
The procedure for live tissue preparation and enzyme dissociation has been described elsewhere (Gulbransen & Sharkey, 2009; Gulbransen et al. 2010). Tissue was subsequently rinsed with Krebs buffer and loaded with 5 μm diaminorhodamine-4M acetoxymethyl ester (DAR-4M AM; Calbiochem) in the presence of 0.02% Pluronic F-127 (Invitrogen, Eugene, OR, USA) and 100 μm probenecid (Invitrogen Canada Inc., Burlington, ON, Canada) at 37°C in a moist, oxygenated environment. Loaded tissue was rinsed and equilibrated (20 min) in oxygenated Krebs buffer containing 0.02% Pluronic F-127, then mounted on an upright Olympus BX61WI motorized fixed stage microscope (Olympus, Markham, ON, Canada) and continually perfused with oxygenated Krebs buffer (30–33°C). Initial experiments were conducted with carbachol (CCh; 100 μm, Sigma-Aldrich) in the presence of atropine (1 μm, Sigma-Aldrich). However, this caused significant muscle contractions in the longitudinal muscle myenteric plexus (LMMP) preparations, which made accurate analysis of these data difficult. Subsequent experiments were conducted with the specific nicotinic agonist DMPP (10 μm), which had no effect on muscle contraction. CCh or DMPP were bath applied for 5 min after a 5 min baseline period. Responses were also evoked by electrical stimulation of interganglionic fibre tracts (Electrical field stimulation, EFS; 20 Hz 5V, 3 s). Inhibitors were bath applied for the duration of the experiment and included Hex (100 μm), NG-nitro-l-arginine methyl ester (l-NAME; 100 μm; Tocris), SMTC (10 μm), N-(3-aminomethyl) benzylacetamidine (1400W; 10 μm), and PTIO (100 μm). PAPA NONOate (100 μm) was utilized as a positive control, and was applied alone and in the presence of NOS inhibitors SMTC and 1400 W to ensure that inhibitor combinations were not causing an efflux of DAR-4M AM from neurons and enteric glia. Images were captured every 10 s through a 20× water-immersion objective (UMPlanFl, 0.5 n.a.) using Imaging Workbench software (INDEC Biosystems, Santa Clara, CA, USA) and a CCD Hamamatsu ORCA-ER digital camera (Hamamatsu Photonics K.K., Hamamatsu-City, Japan) at an excitation/emission wavelength of 560/607 nm for DAR-4M AM.
DAR-4M AM provides useful information regarding the spatial release of NO in live tissue. However, a limitation of DAR-4M AM that should be noted is that it binds irreversibly with NO in the presence of oxygen, preventing multiple experiments on the same sample (Wang et al. 2006). Experiments involving the electrochemical detection of NO were utilized to observe the in vitro time-course of NO release from these preparations.
Cell identification
S100β is a calcium-binding protein that has been localized solely within enteric glial cells in the mouse myenteric plexus (Ferri et al. 1982). S100β-GFP mice have green fluorescent protein (GFP) tagged to the β subunit of the S100 protein. Immunohistochemistry has demonstrated that only two cell types exist within mouse myenteric ganglia – neurons and enteric glial cells (Gabella & Trigg, 1984). Therefore all non-GFP positive cells are taken to be neurons, which were also defined by their size and the presence of a nucleus (Gulbransen & Sharkey, 2009; Gulbransen et al. 2010).
Image analysis
For NO imaging, raw image files were analysed using Imaging Workbench software. Regions of interest (ROIs) were drawn around enteric glia and neurons as identified by the presence or absence of GFP fluorescence in S100β-GFP mice, and via cellular characteristics, including size and shape, in all other mice (Gabella & Trigg, 1984). The percentage relative change in fluorescence intensity was calculated for each neuron and glia ROI using the equation:
where Fx is the fluorescence at a single data point at time x between −60 s and 600 s; Frest is the average fluorescence of 90 s prior to −60 s; Fb is the background fluorescence at time x between −60 s and 600 s; Fb,rest is the average background fluorescence 90 s prior to −60 s
Responders were defined as individual cells whose increase in %ΔF/F was greater than 2 standard deviations from the average %ΔF/F value for Frest. The average %ΔF/F was then calculated for all responding neurons and enteric glia within an individual ganglion for each experimental condition. Therefore n = 1 represents the average of all responding neurons or enteric glia from a preparation. These data were graphed using GraphPad Prism (v. 4, GraphPad Software Inc., San Diego, CA, USA) where the %ΔF/F was represented for each condition at 600 s (300 s post-stimulus). Because the interaction between NO and DAR-4M AM is irreversible, the %ΔF/F is cumulative over time and therefore this measure is representative of the magnitude of the response.
Electrochemical detection of NO
Continuous amperometric monitoring of NO release was conducted in myenteric ganglia using a three-electrode configuration. The NO oxidation current was recorded using a 40 μm diameter boron-doped diamond microelectrode. The procedure for fabricating the electrode has been described elsewhere (Patel et al. 2007, 2008; Patel, 2008). A stainless steel wire served as the counter electrode and a ‘no leak’ Ag|AgCl electrode was used as the reference (EE009, ESA Biosciences, Inc., Chelmsford, MA, USA) electrode. These latter two electrodes were placed in the flow bath but away from the ganglion where a measurement was being conducted. All amperometric measurements were carried out using a BioStat multi-mode potentiostat (ESA Biosciences, Inc.). The boron-doped diamond microelectrode was reproducibly positioned near a ganglion using a micromanipulator (model 25033, Fine Scientific Tools (USA) Inc., Foster City, CA, USA).
The electrode was held at a detection potential of 1.0 V vs. Ag|AgCl, which was sufficient to oxidize NO at a mass-transfer limited rate. The methodology applied to stimulate the tissue was based upon that described previously (Patel et al. 2008). Tissues were continually perfused with Krebs buffer at a flow rate of 2 ml min−1. The cholinergic agonist carbachol (CCh; 10 μm, Sigma-Aldrich) was applied to elicit release of NO by means of a local superfusion pipette placed within 100 μm of the tissue for a duration of 20 s. The muscarinic antagonist atropine (1 μm) was used to inhibit muscular contractions, while hexamethonium (10 μm) was used to prevent nicotinic stimulation. l-NAME (10 μm) was used to block all NOS enzyme activity, while the specific NOS inhibitors SMTC (10 μm) and 1400W (10 μm) were used to block NOS I and NOS II enzyme activity, respectively. Veratridine (10 μm; Sigma-Aldrich) was used to depolarize neurons to examine the release of NO under physiological conditions.
Immunohistochemistry
Whole-mount immunohistochemistry was performed as described elsewhere (Nasser et al. 2007). Briefly, colon was fixed for 12 h in Zamboni's fixative or 4% w/v paraformaldehyde and the myenteric plexus was dissected atop the longitudinal muscle myenteric plexus (LMMP). To observe the percentage of NOS I-positive neurons, LMMPs were incubated with neuronal marker, mouse anti-HuC/HuD (Hu, Invitrogen, Carlsbad, CA, USA, 1:100) followed by goat anti-mouse FITC (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, 1:100), and subsequently incubated with rabbit anti-NOS I antiserum (BD Biosciences, San Jose, CA, USA, 1:500) followed by donkey anti-rabbit CY3 (Jackson, 1:100). For nicotinic receptor immunohistochemistry, paraformaldehyde-fixed LMMP was incubated with goat anti-α3R (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100), followed by donkey anti-goat CY3 (Jackson, 1:100) (to test antibody specificity, preabsorption of the antibody with the blocking peptide was performed). The tissue was subsequently incubated with either rabbit anti-glial fibrillary acidic protein (GFAP) (Dako, Mississauga, ON, Canada, 1:500), followed by donkey anti-rabbit CY5 (BioCan Scientific Inc., Mississauga, ON, Canada, 1:50) to observe enteric glia, or rabbit anti-PGP (Neuromics, Edina, MN, USA, 1:500), followed by donkey anti-rabbit CY5 (Biocan, 1:50) to observe neurons. Labelled LMMP samples were mounted on glass slides with bicarbonate buffered glycerol (pH 8.6) and were visualized with an Olympus BX50 Fluoview laser scanning confocal microscope (Olympus America Inc., Melville, NY, USA) using a 60× (UPlanApo, 1.42 n.a.) oil immersion lens. Optical sections (1 μm) were acquired sequentially for each of the three fluorescence channels.
Statistics
Data are presented as means ± standard error of the mean (SEM) and were compared using one-way ANOVA followed by post hoc pair-wise comparisons with Tukey's test, where P < 0.05 was accepted as a level of statistically significant difference.
Results
Nicotinic cholinergic stimulation of the myenteric plexus regulates ion transport
The serosal application of the nicotinic agonist DMPP (n = 10) to full-thickness segments of mouse colon resulted in a biphasic short-circuit current (ISC) response (Fig. 1A): an initial increase in ISC (phase I) that peaked 79 ± 24s after administration of the drug followed by a decrease in ISC below baseline (phase II) that took 394 ± 58 s from the peak response to recover. Forskolin elicited a robust increase in ISC following DMPP application in all preparations (data not shown). When DMPP was added mucosally, no change in ISC was noted (n = 4). Amiloride had no effect on the ISC response to DMPP (n = 6; Phase I and Phase II), whereas in Cl−-free buffer, no DMPP-evoked increase in ISC was noted (n = 6; P < 0.05 vs. Phase I, P < 0.01 vs. Phase II). The effects of DMPP on the ISC response were reduced by NOS I or NOS II inhibitors but the response was not statistically significant (n = 5, P > 0.05). However, the simultaneous inhibition of NOS I and II abolished both phases of the DMPP response (n = 5; P < 0.01; Fig. 1B). Similarly, when preparations were pretreated with the NO scavenger PTIO, both phases of the DMPP response were abolished (n = 3; P < 0.01 vs. Phase I and Phase II). The NO donor PAPA NONOate resulted in an increase in ISC at 10 and 50 μm (n = 4), and a decrease in ISC at 100 and 500 μm (n = 4; Fig. 1C). PAPA NONOate (10 and 500 μm) induced a change in ISC that was blocked by the neurotoxin TTX treatment (n = 3; 10 μmP < 0.001; 500 μmP < 0.05). The tissue response to forskolin was unaltered following the application of NOS I and II inhibitors (n = 5, P > 0.5) or PAPA NONOate (n = 3, P > 0.5).
Figure 1. Cholinergic regulation of ion transport in the mouse colon.

A, original recording demonstrating the biphasic short-circuit current (ISC) response measured in a full-wall thickness preparation of the colon after addition of DMPP. Dotted line = baseline ISC. B, the biphasic change in short circuit current in response to DMPP, which is unchanged by SMTC or 1400W alone. Together, these inhibitors significantly reduced Phase I and eliminate Phase II of the DMPP response (n = 5; **P < 0.01 vs. Phase I, #P < 0.05 vs. Phase II). C, the NO donor PAPA NONOate resulted in an increase in ISC at 10 μm (n = 4; **P < 0.01 vs. 100 and 500 μm) and 50 μm (n = 4; **P < 0.01 vs. 100 and 500 μm) and a decrease in ISC at 100 and 500 μm (n = 4). D, the biphasic DMPP response converted to a smaller monophasic response in the presence of TTX (n = 5; P > 0.05 vs. DMPP Phase I; #P < 0.05 vs. DMPP Phase II). This response is unchanged by SMTC but is completely blocked by 1400W (n = 5; ***P < 0.001 vs. DMPP Phase I).
Following pretreatment with TTX, the DMPP response was altered from a biphasic to a smaller monophasic increase in ISC (n = 5; P < 0.05 Phase I, P < 0.05 Phase II). This effect was not altered by the NOS I inhibitor SMTC (n = 5, P > 0.05), but was completely blocked by the NOS II inhibitor SMTC (n = 5; P < 0.001; Fig. 1D).
To assess the role of the myenteric plexus in the epithelial ISC, analyses were conducted on tissues from which the external muscle layers and myenteric plexus had been removed (Fig. 2A and B). DMPP application resulted in an increase in ISC that was sustained throughout the experiment and was 2-fold greater compared to phase I of the normal DMPP response (n = 4; P < 0.5 vs. DMPP Phase I, P < 0.001 vs. DMPP Phase II) and completely sensitive to hexamethonium (n = 3; P < 0.001). Phase II was completely abolished. Forskolin-evoked changes in ISC (peak = 99 ± 17 μA cm−2; n = 4, P > 0.05) were unchanged compared to myenteric plexus-intact tissues (peak = 113 ± 16 μA cm−2; n = 10). When MP-removed tissue was pretreated with PTIO (100 μm; n = 4), the response to DMPP (16 ± 8 μA cm−2) was reduced compared to DMPP alone in MP-removed tissue (75 ± 11 μA cm−2; P < 0.01). The application of PAPA NONOate at 10 μm (n = 6) or 100 μm (n = 6) to MP-removed tissue resulted in no change in ISC.
Figure 2. The nicotinic regulation of epithelial ion transport relies on the myenteric plexus.

A, the biphasic DMPP response converted to a monophasic response following removal of the myenteric plexus and muscle layers from the mouse colon. n = 4; *P < 0.5 vs. DMPP Phase I, ###P < 0.001 vs. DMPP Phase II, †††P < 0.001 MP removed DMPP vs. DMPP + Hex. B, full-thickness section of the mouse colon stained with H&E. Right panel shows successful removal of myenteric plexus with both the circular and longitudinal muscle layers stained with H&E. Scale bar: 50 μm.
Cellular NO production following cholinergic stimulation: neurons
To determine the cellular source of NO production, LMMP preparations loaded with the NO sensitive dye DAR 4M AM were examined (Kojima et al. 2001). Preparations exhibited low baseline fluorescence. In the absence of stimulation, there was no increase in fluorescence in the preparation over a 15 min observation period, suggesting undetectably low levels of endogenous NO production. PAPA NONOate was used as a positive control and resulted in an increase in fluorescence in 98 ± 2% of neurons and 97 ± 3% of enteric glia.
DMPP stimulated a robust increase in NO fluorescence in 67 ± 6% of myenteric neurons (n = 5; P < 0.001 vs. control) which was completely blocked by hexamethonium (n = 5; P < 0.05; Fig. 3A–C). SMTC reduced the fluorescence activation compared to DMPP alone; however, 45 ± 6% of neurons continued to demonstrate a response (n = 5; P < 0.01; Fig. 3B and C). Pretreatment with 1400W resulted in a reduction of the DMPP-induced neuronal response, where 49 ± 3% of neurons demonstrated an increase in fluorescence (n = 5; P < 0.01; Fig. 3B and C). However, when DMPP was applied in the presence of SMTC and 1400W, only 6 ± 2% of neurons demonstrated a response (n = 5; P < 0.001 vs. DMPP) with a %ΔF/F comparable to control, suggesting that NOS I and NOS II were the sole sources of NO production within the myenteric plexus. In order to ensure that SMTC and 1400W were not causing an efflux of DAR-4M AM from cells, PAPA NONOate was applied in the presence of both inhibitors. The percentage of responding neurons and enteric glia was unchanged from PAPA NONOate alone (n = 4; P > 0.05).
Figure 3. Nitric oxide imaging of the myenteric plexus following nicotinic stimulation.

A, change in fluorescence over time of enteric neurons under control conditions and following DMPP application alone or in the presence of Hex (20–30 neurons per condition). B, the %ΔF/F for neurons 300 s following DMPP application. C, the percentage of neurons demonstrating an increase in fluorescence. n = 5; ***P < 0.001 vs. control; #P < 0.05, ###P < 0.001 vs. DMPP.
Cellular NO production following nicotinic cholinergic stimulation: enteric glia
Enteric glia from non-stimulated tissue showed no increase in NO fluorescence over time. DMPP evoked a robust increase in fluorescence with 76 ± 3% of enteric glia responding (n = 5; P < 0.001 vs. control; Fig. 4A and B). This response was completely blocked by hexamethonium (n = 5; P < 0.001; Fig. 4B and C). Both SMTC and 1400W reduced the fluorescence for enteric glia compared to DMPP alone (n = 5; Fig. 4B), although only 1400W significantly influenced the percentage of responding cells (n = 5; P < 0.001; Fig. 4C). In combination, SMTC and 1400W completely prevented the ability of DMPP to elicit an NO response from enteric glia (n = 5; P < 0.001; Fig. 4B and C).
Figure 4. NO imaging of enteric glia loaded with DAR-4M AM following cholinergic stimulation.

A, micrographs of S100β-GFP colonic myenteric plexus comparing GFP labelling with DAR-4M AM-loaded preparations before and after stimulation with DMPP. Arrows indicate enteric glia demonstrating an increase in fluorescence following DMPP stimulation. Scale bar: 20 μm. B, the %ΔF/F for enteric glia 300 s following DMPP application. C, the percentage of enteric glia demonstrating an increase in fluorescence. n = 5; *P < 0.05, ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. DMPP.
These data were supported by NO imaging with carbachol (CCh) in the presence of atropine, which evoked a 91% increase in fluorescence in neurons (n = 5) and a 90% increase in fluorescence in enteric glia within the myenteric plexus (n = 5), an effect completely blocked by hexamethonium and L-NAME in neurons and enteric glia (n = 5; P < 0.001; Fig. 5A–C). When CCh was applied in the presence of SMTC, stimulated fluorescence in neurons was reduced by ∼38% (n = 5; P < 0.01), a finding corroborated in NOS I−/− mice (n = 3; P < 0.001; Fig. 5D). Cholinergic stimulation in the presence of the NOS II specific antagonist 1400W reduced the glial response by ∼63% (n = 5; P < 0.001), which was confirmed by utilizing NOS II−/− mice (n = 3; P < 0.001; Fig. 5E).
Figure 5. Nitric oxide imaging of the myenteric plexus following cholinergic stimulation.

A, micrographs of colonic myenteric plexus following stimulation with carbachol (CCh) in the presence of atropine (Atr), and hexamethonium (Hex). Scale bar: 50 μm. B, the change in fluorescence of enteric neurons under control conditions and following CCh application in the presence of atropine, and hexamethonium or l-NAME. C, the change in fluorescence of enteric glia under control conditions and following stimulation with CCh in the presence of atropine and in the presence of Hex or l-NAME. D, the change in fluorescence in myenteric neurons following the application of CCh alone or in the presence of the NOS I inhibitor SMTC and in NOS I−/− animals. E, the change in fluorescence in enteric glia following the application of CCh alone or in the presence of the NOS II inhibitor 1400W and in NOS II−/− animals. ***P < 0.001 vs. control; ##P < 0.01, ###P < 0.001 vs. CCh + Atr.
Additionally, these data were supported by results using amperometry to detect the production of NO from NOS I and NOS II following nicotinic cholinergic stimulation (Fig. 6). Amperometry was used to measure NO release from the mouse colonic myenteric plexus following CCh stimulation of muscarinic and nicotinic receptors. When the working electrode was positioned over the myenteric ganglia and held at a potential of 1.0 V vs. Ag|AgCl, CCh induced a significant increase in NO release from the myenteric plexus within 5.3 ± 0.4 s that returned to baseline levels by 11.9 ± 1.5 s following the end of the CCh application (n = 5). When the electrode was held at voltages lower than 1.0 V, no signal was detected. If the stimulation was applied when the electrode was removed from the tissue surface or placed over a non-ganglionic region, virtually no signal was detected (Fig. 6A).
Figure 6. Electrochemical detection of nitric oxide.
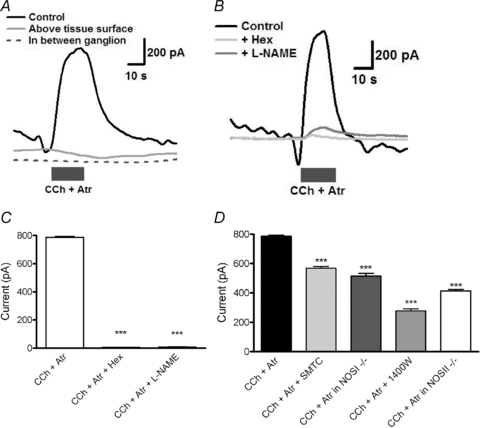
A, original recordings demonstrating the current recorded during carbachol (CCh) applications (grey bar) in the presence of atropine over an aganglionic portion of the plexus, when the electrode was removed from the tissue, and when the electrode is touching the tissue surface over a myenteric ganglion. B, original recordings from a single myenteric ganglion demonstrating the current recorded while contacting the myenteric plexus. C, the average peak current recorded during the application of CCh in the presence of atropine, hexamethonium or l-NAME. D, CCh application in the presence of atropine (Atr) compared with specific NOS isoform inhibitors in control animals and NOS isoform knockout (−/−) mice. ***P < 0.001 vs. CCh + Atr.
The cholinergic response was not significantly affected by the muscarinic antagonist atropine, suggesting that the effect was primarily nicotinic in nature (data not shown). All subsequent experiments were performed in the presence of atropine. When CCh was applied in the presence of hexamethonium or the pan-NOS inhibitor l-NAME, NO production was almost completely inhibited (n = 4; P < 0.001; Fig. 6B and C). SMTC reduced the cholinergic response by ∼30%, while 1400W reduced the response by ∼66% (n = 4; P < 0.001). Similar ratios were observed in tissues from NOS I and II knockout mice (n = 3; P < 0.001; Fig. 6D).
Evidence for the interganglionic movement of NO between neurons and glia in the myenteric plexus
Due to the ready diffusion of NO across cell membranes, determining the cellular source of NO (producers vs. responders) is a significant challenge. In order to limit cellular fluorescence only to cells producing NO, the extracellular NO scavenger PTIO was employed. The number of neurons demonstrating an increase in fluorescence following DMPP application in the presence of PTIO was decreased when compared to DMPP alone, strongly suggesting movement of NO between neurons and glia (Fig. 7A and B). The percentage of neurons responding to DMPP in the presence of PTIO was supported by immunohistochemistry in the S100β-GFP mouse colon, in which 37 ± 5% of neurons were NOS I immunoreactive (n = 12), with no enteric glial cells positive for NOS I. When PTIO was applied in the presence of SMTC, the neuronal response was abolished, while 1400W had no effect compared to DMPP and PTIO (Fig. 7A). The reciprocal pattern was observed in enteric glia where PTIO and 1400W almost abolished the effect of DMPP and PTIO combined (Fig. 7B).
Figure 7. Interganglionic movement of nitric oxide (NO) within the myenteric plexus following cholinergic stimulation.

A, percentage of neurons demonstrating an increase in fluorescence where the movement of NO is restricted within the ganglia by NO scavenger PTIO. B, percentage of enteric glia demonstrating an increase in fluorescence in the presence of PTIO. n = 5; ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DMPP.
Finally, when DMPP was applied in the presence of TTX (n = 3), 54 ± 7% of neurons and 68 ± 5% of enteric glia demonstrated an increase in fluorescence, illustrating that nicotinic stimulation can act directly on enteric glia to elicit NO production.
Immunohistochemical Identification of nicotinic receptors
Immunohistochemistry revealed co-localization of the nicotinic receptor α3 subunit with the neuronal marker PGP 9.5 in the myenteric plexus of the S100β-GFP mouse colon (Fig. 8A). Additionally, these mice showed α3 receptor localization with GFAP immunoreactivity and the glial marker S100β-GFP (Fig. 8B). These findings demonstrate nicotinic receptors on neurons and enteric glia in the myenteric plexus. Greater than 90% of neurons and enteric glia were positive for the nicotinic receptor α3 subunit, which correlates with other work that has described the α3 receptor subunit as the most abundant nicotinic receptor subunit within the guinea pig myenteric plexus, and confirmed to be nearly ubiquitous in its expression on neurons (Kirchgessner & Liu, 1998; Obaid et al. 2005). Labelling with this antibody was completely abolished by pre-absorption with the blocking peptide.
Figure 8. Localization of the nicotinic receptor subunit α3 within the myenteric plexus.

A, confocal images demonstrating S100β-positive enteric glia (green), neuron-specific marker PGP 9.5 (blue), and α3 receptor (red). The overlay illustrates colocalization between neuron cell bodies and the α3 receptor subunit, but reveals additional α3 labelling not in neurons. B, confocal images demonstrating S100β-positive enteric glia (green), glial fibrillary acidic protein (GFAP, blue), and α3 receptor (red). The overlay demonstrates colocalization of the α3 receptor subunit with both the glial-specific S100 protein and GFAP. Scale bar: 20 μm.
Physiological NO production and release
When EFS was used to stimulate myenteric ganglia loaded with DAR-4M AM, neurons and enteric glia demonstrated an increase in fluoresence following activation (Fig. 9A and B). When EFS was applied in the presence of hexamethonium or SMTC and 1400W, the percentage of responding neurons and glia was significantly reduced, but was not completely abolished (Fig. 9A and B). These results were confirmed with amperometry, using veratridine to depolarize enteric neurons (Mawe & Gershon, 1986) (Fig. 9C and D).
Figure 9. Physiological stimulation of nitric oxide release and production.

A, nitric oxide imaging demonstrating the percentage of neurons increasing in fluorescence following electrical field stimulation (EFS; 20 Hz 5V stimulus, 3 s duration) to an interganglionic fibre tract of the myenteric plexus being imaged. B, the percentage of enteric glia increasing in fluorescence in enteric glia following EFS. n = 5 for EFS, n = 3 for all other conditions; *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; ###P < 0.001 vs. EFS. C, electrochemical detection experiment showing original recording from a single myenteric ganglion demonstrating the current recorded over plexus during veratridine (Ver) application (grey bar) in the presence of hexamethonium (Hex) or l-NAME. D, the average peak current recorded during the application of veratridine in the presence of Hex or l-NAME (n = 4).
Discussion
Enteric glia have traditionally been thought of as supportive cells in the ENS. However, recent work has suggested that the diversity and importance of their functions have been underestimated (Bush et al. 1998; Savidge, 2007; Savidge et al. 2007; Gulbransen & Sharkey, 2009; Gulbransen et al. 2010; Van Landeghem et al. 2009). The goal of this work was to investigate the role of enteric glia in the regulation of electrogenic ion transport in the mouse colon, and in doing so we have uncovered a novel role for them in regulating epithelial ion transport in response to nicotinic stimulation via the synthesis of NO and communication with enteric neurons in the myenteric plexus.
The regulation of enteric epithelial ion transport contributes to body fluid homeostasis and the coordination of this with the motor and vascular systems in the gut is the primary role of the ENS (Cooke, 1989; Furness, 2006). The submucosal plexus is considered the primary neural regulator of active ion transport and hence directed water movements (Furness, 2006). In the current study we identified a myenteric plexus-dependent control of epithelial ion transport in mouse colon that involves NO release from both neurons and enteric glia. It has been demonstrated that enteric neurons contain NOS I and release NO (Sang & Young, 1996; Patel et al. 2008; Qu et al. 2008) and that enteric glia express NOS II, with indirect evidence suggesting that they may also release NO under pathophysiological conditions (Green et al. 2004). Extending these data, we found that both isoforms of NOS in the myenteric plexus contribute to a biphasic ISC response following nicotinic stimulation, with NOS I activated in enteric neurons and NOS II activated in enteric glia.
The myenteric plexus is seldom considered in the control of epithelial ion transport. Indeed, the majority of Ussing chamber analyses of enteric epithelial ion transport do so following the removal of both external muscle layers and the myenteric plexus. However, several studies have demonstrated clear differences between intact and mucosal–submucosal tissue in vivo and in vitro (Jodal et al. 1993; See & Bass, 1993; Rolfe & Levin, 1998). In the rat jejunum in vivo, the rate of Na+ absorption in response to glucose was significantly decreased in tissues lacking the myenteric plexus compared to controls (See & Bass, 1993). Similarly, removal of the myenteric plexus resulted in a decreased net fluid secretion in response to cholera toxin in the rat jejunum in vivo. This result suggested that the myenteric plexus was responsible for mediating the large net fluid secretion in response to cholera toxin under control conditions (Jodal et al. 1993). In the rat ileum in vitro, the addition of serotonin resulted in comparable secretory responses from intact and myenteric plexus-removed tissue. However, the response in intact tissue was highly sensitive to TTX and the NO inhibitor l-NAME, whereas tissue in which the myenteric plexus had been removed was not (Rolfe & Levin, 1998). Thus, it is important to highlight that analyses of epithelial ion transport in tissues from which the external muscle layers have been removed can overlook a potential modulating effect of the myenteric plexus.
The effects of NO on ion transport have been studied both in vitro and in vivo and the data have often been conflicting: NO has been shown to be prosecretory, proabsorptive, and to have no effect in other studies (Izzo et al. 1998). This may be due in part to data acquired with or without the myenteric plexus intact. In two studies, the myenteric plexus has been implicated as a source of NO that contributes to the modulation of enteric epithelial ion transport during intestinal inflammation (Green et al. 2004; Mourad et al. 2006).
A biphasic change in ISC following nictotinic stimulation with DMPP has previously been observed (Tapper & Lewand, 1981; Diener et al. 1989), although the role of NO was not explored. The biphasic nature of the response could be a concentration-dependent effect of NO, as NO concentrations above a specific threshold could reverse the secretory response below baseline levels. Concentration-dependent differences of NO on ion transport have been noted by others (Wapnir et al. 1997; Mourad et al. 2003), and here we found that at low concentrations NO increased Isc, while at higher concentrations NO reduced Isc. When the myenteric plexus was removed, no response to the application of the NO donor was observed. Additionally, NO has been demonstrated to inhibit acetylcholine release (Hebeiss & Kilbinger, 1998; Mang et al. 2002), potentially providing a feedback loop to control the production of NO following nicotinic stimulation. Recently, biphasic cholinergic responses have also been described from the epithelium in the rat distal colon (Yajima et al. 2011).
The current findings suggest that both neurons and enteric glia are directly stimulated by nicotinic cholinergic stimulation. We report the presence of the nicotinic receptor α3 subunit on enteric glia and suggest that these cells have the ability to respond directly to nicotinic stimulation. However, our findings do not exclude the possibility that the surrounding neurons, once stimulated, release a neurotransmitter which then induces glial NO production. Using a physiologically relevant stimulation to depolarize neurons, a small portion of the response remained after cholinergic inhibition, suggesting that acetylcholine is not the sole neurotransmitter capable of eliciting the production and release of NO within the mouse colonic myenteric plexus; the goal of future studies will be to identify these additional neurotransmitters.
Another important and unexpected finding was the release of NO from NOS II within enteric glia. NOS I is constitutively active under physiological conditions (Knowles & Moncada, 1994) while NOS II is generally considered to be active only following a calcium-independent induction under pathophysiological conditions (Galea et al. 1992; Simmons & Murphy, 1992; Murphy et al. 1993; Knowles & Moncada, 1994). However, our results suggest that NOS II may also be constitutively active within the ENS and regulate enteric function under normal conditions. This finding is not unprecedented, as evidence exists to suggest that NOS II expression may be constitutive in other tissues, such as the hypothalamus and the spinal cord (Tang et al. 2007; Amitai, 2010). While it is known that NOS II is capable of producing larger quantities of NO compared to NOS I, the degree of activation of NOS II is not fully understood under physiological conditions. Our data suggest that NOS I and NOS II produce comparable levels of NO and are activated on a similar time scale, if not simultaneously, which suggests that NOS I is proportionally more active than NOS II. This observation could be important in diseases where NOS II expression is up-regulated, such as inflammatory bowel disease (Miampamba & Sharkey, 1999; Cross & Wilson, 2003). While it is known that NO production is increased during inflammation, the role of NO in inflammation is still controversial, as evidence exists to suggest that NO can be either cytoprotective or cytotoxic (Cross & Wilson, 2003).
Our findings are consistent with the model of cholinergic control of secretomotor function previously proposed (Green et al. 2004), extending it to include direct nicotinic stimulation of both neurons and enteric glia within the myenteric plexus, resulting in NO release. NOS I is activated in neurons and NOS II is activated in enteric glia. Once released, NO moves bidirectionally between neurons and enteric glia within the ganglia. NO also modulates a signalling cascade that influences epithelial ion transport directly or through neurons and enteric glia of the submucosal plexus, as demonstrated by the application of an NO scavenger, which eliminated the biphasic nicotinic response under control conditions. The mechanism by which myenteric NO modulates epithelial ion transport has yet to be fully described. Direct stimulation of the epithelium is unlikely, as NO would have to penetrate multiple tissue layers to reach the enterocytes. Indirect stimulation would likely involve connections between neurons and enteric glia from the myenteric plexus to those in the submucosal plexus, and may involve NO. This is supported both by immunohistochemistry, which has identified NOS I positive neurons within the guinea pig submucosal plexus (Furness et al. 1994) and NOS II positive enteric glia within the mouse submucosal plexus (Green et al. 2004), and by our data that describes a modulation of the DMPP response in myenteric plexus-removed tissue by an NO scavenger.
This study illustrates the concept of neuronal–glial communication within the myenteric plexus, through the demonstration of nicotinic receptors on enteric glia and their role in the production and release of NO. Specifically, we propose that activation of nicotinic receptors on myenteric neurons and enteric glia results in the liberation of NO from NOS I and NOS II, respectively, where NO acts as a mediator of bi-directional communication between the two cell types. In conclusion, these findings significantly advance knowledge of the interaction between neurons and enteric glia in the myenteric plexus, describe an important new function for enteric glia, and underscore the importance of the myenteric plexus in the regulation of epithelial active ion transport. A deeper understanding of the neural and enteric glial regulation of physiological water balance may permit further advances in understanding the perturbations during pathophysiological reactions such as intestinal inflammation.
Acknowledgments
We thank Dr Wallace MacNaughton and Dr Brian Gulbransen for advice and support, Matthew Klompus and Winnie Ho for technical assistance, and Drs Catherine Legraverend (Institut de Génomique Fonctionnelle, Montpellier) and Richard Dyck (University of Calgary) for supplying the S100β-GFP mice. This work was supported by the Canadian Institutes of Health Research, the Canadian Foundation for Innovation and the Alberta Science and Research Authority. S.J.M. is supported by the Dr T. Chen Fong Doctoral Scholarship in Neuroscience through the Hotchkiss Brain Institute. B.A.P. was supported by the EPSRC LSI Overseas Fellowship (EP/C532058/1). D.M.M. is an Alberta Heritage Foundation for Medical Research (AHFMR) Medical Scientist and Canada Research Chair (Tier 1). K.A.S. is an AHFMR Medical Scientist and the Crohn's and Colitis Foundation of Canada Chair in IBD Research.
Glossary
Abbreviations
- EFS
electrical field stimulation
- ENS
enteric nervous system
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- Hb
haemoglobin
- Hex
hexamethonium
- ISC
short-circuit current
- LMMP
longitudinal muscle myenteric plexus
- NOS
nitric oxide synthase
Author contributions
S.M. – study concept and design; acquisition, analysis and interpretation of data; writing the manuscript; final approval of the manuscript to be published. B.P. – acquisition, analysis and interpretation of data; critical revision of the manuscript. D.M. – study design; interpretation of data; writing the manuscript. K.S. – study concept and design; writing the manuscript; grant funding. All authors approved the final version for publication. The authors have no competing interests.
References
- Amitai Y. Physiologic role for “inducible” nitric oxide synthase: A new form of astrocytic-neuronal interface. Glia. 2010;58:1775–1781. doi: 10.1002/glia.21057. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Cooke HJ. Role of the “little brain” in the gut in water and electrolyte homeostasis. FASEB J. 1989;3:127–138. doi: 10.1096/fasebj.3.2.2464517. [DOI] [PubMed] [Google Scholar]
- Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179–189. doi: 10.1097/00054725-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W. Cholinergic-mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol. 1989;168:219–229. doi: 10.1016/0014-2999(89)90568-2. [DOI] [PubMed] [Google Scholar]
- Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahe MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2010;60:473–484. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Malden, MA: Blackwell Publishing; 2006. [Google Scholar]
- Furness JB, Li ZS, Young HM, Forstermann U. Nitric oxide synthase in the enteric nervous system of the guinea-pig: a quantitative description. Cell Tissue Res. 1994;277:139–149. doi: 10.1007/BF00303090. [DOI] [PubMed] [Google Scholar]
- Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. J Neurocytol. 1984;13:49–71. doi: 10.1007/BF01148318. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CL, Ho W, Sharkey KA, McKay DM. Dextran sodium sulfate-induced colitis reveals nicotinic modulation of ion transport via iNOS-derived NO. Am J Physiol Gastrointest Liver Physiol. 2004;287:G706–G714. doi: 10.1152/ajpgi.00076.2004. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci. 2010;30:6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Hebeiss K, Kilbinger H. Nitric oxide-sensitive guanylyl cyclase inhibits acetylcholine release and excitatory motor transmission in the guinea-pig ileum. Neuroscience. 1998;82:623–629. doi: 10.1016/s0306-4522(97)00308-4. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Capasso F. Nitric oxide as a modulator of intestinal water and electrolyte transport. Dig Dis Sci. 1998;43:1605–1620. doi: 10.1023/a:1018887525293. [DOI] [PubMed] [Google Scholar]
- Jodal M, Holmgren S, Lundgren O, Sjoqvist A. Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology. 1993;105:1286–1293. doi: 10.1016/0016-5085(93)90130-5. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390:497–514. [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Hirotani M, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Hirata Y, Nagano T. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal Chem. 2001;73:1967–1973. doi: 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- Mang CF, Truempler S, Erbelding D, Kilbinger H. Modulation by NO of acetylcholine release in the ileum of wild-type and NOS gene knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1132–G1138. doi: 10.1152/ajpgi.00192.2002. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Gershon MD. Functional heterogeneity in the myenteric plexus: demonstration using cytochrome oxidase as a verified cytochemical probe of the activity of individual enteric neurons. J Comp Neurol. 1986;249:381–391. doi: 10.1002/cne.902490305. [DOI] [PubMed] [Google Scholar]
- Miampamba M, Sharkey KA. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol Motil. 1999;11:193–206. doi: 10.1046/j.1365-2982.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Mourad FH, Barada KA, bdel-Malak N, Bou Rached NA, Khoury CI, Saade NE, Nassar CF. Interplay between nitric oxide and vasoactive intestinal polypeptide in inducing fluid secretion in rat jejunum. J Physiol. 2003;550:863–871. doi: 10.1113/jphysiol.2003.043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad FH, Barada KA, Bou Rached NA, Khoury CI, Saade NE, Nassar CF. Inhibitory effect of experimental colitis on fluid absorption in rat jejunum: role of the enteric nervous system, VIP, and nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2006;290:G262–G268. doi: 10.1152/ajpgi.00271.2005. [DOI] [PubMed] [Google Scholar]
- Mourad FH, Turvill JL, Farthing MJ. Role of nitric oxide in intestinal water and electrolyte transport. Gut. 1999;44:143–147. doi: 10.1136/gut.44.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Simmons ML, Agullo L, Garcia A, Feinstein DL, Galea E, Reis DJ, Minc-Golomb D, Schwartz JP. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- Nasser Y, Keenan CM, Ma AC, McCafferty DM, Sharkey KA. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia. 2007;55:859–872. doi: 10.1002/glia.20507. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Michel K, Aube AC, Galmiche JP, Schemann M. Projections of excitatory and inhibitory motor neurones to the circular and longitudinal muscle of the guinea pig colon. Cell Tissue Res. 2001;305:325–330. doi: 10.1007/s004410100387. [DOI] [PubMed] [Google Scholar]
- Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol. 2005;208:2981–3001. doi: 10.1242/jeb.01732. [DOI] [PubMed] [Google Scholar]
- Patel BA. Continuous amperometric detection of co-released serotonin and melatonin from the mucosa in the ileum. Analyst. 2008;133:516–524. doi: 10.1039/b717034c. [DOI] [PubMed] [Google Scholar]
- Patel BA, Bian X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- Patel BA, Galligan JJ, Swain GM, Bian X. Electrochemical monitoring of nitric oxide released by myenteric neurons of the guinea pig ileum. Neurogastroenterol Motil. 2008;20:1243–1250. doi: 10.1111/j.1365-2982.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- Rao RK, Riviere PJ, Pascaud X, Junien JL, Porreca F. Tonic regulation of mouse ileal ion transport by nitric oxide. J Pharmacol Exp Ther. 1994;269:626–631. [PubMed] [Google Scholar]
- Reddix RA, Liu X, Miller MJ, Niu X, Powell A. Constitutive nitric oxide release modulates neurally-evoked chloride secretion in guinea pig colon. Auton Neurosci. 2000;86:47–57. doi: 10.1016/S1566-0702(00)00206-X. [DOI] [PubMed] [Google Scholar]
- Rolfe VE, Levin RJ. Neural and non-neural activation of electrogenic secretion by 5-hydroxytryptamine in the rat ileum in vitro. Acta Physiol Scand. 1998;162:469–474. doi: 10.1046/j.1365-201X.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- Rolfe VE, Milla PJ. Nitric oxide stimulates cyclic guanosine monophosphate production and electrogenic secretion in Caco-2 colonocytes. Clin Sci (Lond) 1999;96:165–170. [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Savidge TC. MIND the gap: an astroglial perspective on barrier regulation. Neuron Glia Biol. 2007;3:191–197. doi: 10.1017/S1740925X08000124. [DOI] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- See NA, Bass P. Glucose-induced ion secretion in rat jejunum: a mucosal reflex that requires integration by the myenteric plexus. J Auton Nerv Syst. 1993;42:33–40. doi: 10.1016/0165-1838(93)90339-v. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992;59:897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Stack WA, Filipowicz B, Hawkey CJ. Nitric oxide donating compounds stimulate human colonic ion transport in vitro. Gut. 1996;39:93–99. doi: 10.1136/gut.39.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai H, Gaginella TS. Direct evidence for nitric oxide stimulation of electrolyte secretion in the rat colon. Free Radic Res Commun. 1993;19:229–239. doi: 10.3109/10715769309056511. [DOI] [PubMed] [Google Scholar]
- Tang Q, Svensson CI, Fitzsimmons B, Webb M, Yaksh TL, Hua XY. Inhibition of spinal constitutive NOS-2 by 1400W attenuates tissue injury and inflammation-induced hyperalgesia and spinal p38 activation. Eur J Neurosci. 2007;25:2964–2972. doi: 10.1111/j.1460-9568.2007.05576.x. [DOI] [PubMed] [Google Scholar]
- Tapper EJ, Lewand DL. Actions of a nicotinic agonist, DMPP, on intestinal ion transport in vitro. Life Sci. 1981;28:155–162. doi: 10.1016/0024-3205(81)90547-6. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Mahe MM, Teusan R, Leger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics. 2009;10:507. doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol. 2003;457:404–419. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- Wang S, Paton JF, Kasparov S. The challenge of real-time measurements of nitric oxide release in the brain. Auton Neurosci. 2006;126–127:59–67. doi: 10.1016/j.autneu.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Wapnir RA, Wingertzahn MA, Teichberg S. L-Arginine in low concentration improves rat intestinal water and sodium absorption from oral rehydration solutions. Gut. 1997;40:602–607. doi: 10.1136/gut.40.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KT, Xie Y, Musch MW, Chang EB. Sodium nitroprusside stimulates anion secretion and inhibits sodium chloride absorption in rat colon. J Pharmacol Exp Ther. 1993;266:224–230. [PubMed] [Google Scholar]
- Yajima T, Inoue R, Matsumoto M, Yajima M. Non-neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol. 2011;589:953–962. doi: 10.1113/jphysiol.2010.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]


