Non-technical summary
Sympathetic activity is altered by the baroreflex in response to changes in blood pressure. Thus, the sympathetic baroreflex is essential in preventing dangerous changes in blood pressure. The sympathetic baroreflex is more responsive to falling blood pressure than rising blood pressure in young men and women. This changes with age where older men and women become more sensitive to rising blood pressure. Our findings indicate that in young and older men and older women, the sensitivity of the sympathetic baroreflex depends on the level of sympathetic activity at rest. Therefore, individuals with high sympathetic activity respond better to rising than falling blood pressure and vice versa for people with low resting sympathetic activity. This might explain why older men and women cannot tolerate large decreases in blood pressure, as they have high sympathetic activity. In young women, factors other than sympathetic activity may be more important in determining baroreflex sensitivity.
Abstract
Abstract
Sympathetic baroreflex sensitivity (BRS) is greater during decreasing compared to increasing diastolic blood pressure (DBP) in young men and women. In older men and women there is no difference in sympathetic BRS to increasing and decreasing DBP. We investigated whether the sensitivity of the central nervous system to increasing and decreasing DBP is dependent upon baseline muscle sympathetic nerve activity (MSNA). We hypothesised that the difference in sympathetic BRS between falling and rising segments of DBP would be positively related to baseline MSNA in 30 young men, 21 young women, 14 older men and 14 postmenopausal women. MSNA was measured using peroneal microneurography and BRS was measured using the spontaneous baroreflex threshold technique. On average, sympathetic BRS was greater during decreasing compared to increasing DBP in young men (P < 0.05) and women (P < 0.05). In older men and women, mean sympathetic BRS was similar in response to increasing and decreasing DBP. The difference (delta) between the falling and rising BRS correlated with baseline MSNA in young (r = 0.58, P < 0.05) and older men (r = 0.66, P < 0.05) and postmenopausal women (r = 0.74, P < 0.05). Thus, all men, and older women, with higher BRS to falling DBP had lower baseline MSNA. This relationship was not observed in young women (r = 0.14, P > 0.05). In summary, baseline MSNA plays a role in determining sympathetic BRS to falling and rising DBP in young and older men and postmenopausal women, but not in young women. This relationship is consistent with a decreased potential for sympathoexcitation in people with higher resting MSNA. Furthermore, the lack of relationship in young women suggests important contributions of sex hormones to differential responses of MSNA to falling and rising pressures.
Introduction
Sympathetic nerve activity is tightly linked to arterial pressure via the baroreflex: acute decreases in arterial pressure cause reflex sympatho-excitation and increases in arterial pressure cause sympatho-inhibition (Sundlof & Wallin, 1978). The activity of sympathetic nerves can be measured directly in humans as muscle sympathetic nerve activity (MSNA) via peroneal microneurography. The responsiveness of MSNA to a given change in arterial pressure is defined as the sensitivity of the sympathetic component of the baroreflex. Importantly, this sensitivity has direct relevance to human daily activities: individuals with low sympathetic baroreflex sensitivity have less ability to buffer transient changes in arterial pressure and are more likely to experience symptoms of orthostatic intolerance (Mosqueda-Garcia et al. 1997).
Interestingly, the sensitivity of the sympathetic baroreflex appears to depend upon the direction of the change in arterial pressure. Sundlof & Wallin (1978) first demonstrated that, for a given level of arterial pressure, MSNA was more ‘responsive’ to falling pressures compared to rising pressures. The authors indicated that changes in burst amplitude were greater in response to spontaneous decreases in diastolic blood pressure (DBP) compared to spontaneous increases in DBP. More recent evidence suggests that when changes in arterial pressure are induced pharmacologically (via the modified Oxford technique), MSNA (burst area) is also more responsive to falling pressures compared to rising pressures in young men and women (Studinger et al. 2009). Interestingly, Studinger et al. reported that in older humans the sympathetic baroreflex responsiveness to falling pressures was actually decreased when compared to younger men and women. Other studies have also demonstrated that older men may not respond well to hypotensive stimuli because they operate near their maximal ability to increase arterial pressure via the baroreflex (Fisher et al. 2010). That is, there is a limit to the extent to which the system can increase sympathetic neural activity when resting activity is already relatively high in older people.
There are several mechanisms which might explain the difference in baroreflex responsiveness to falling and rising arterial pressures in young humans and why this changes with age. Studinger et al. (2009) reported that differences in the sensitivity of the mechanical (stretch at the carotid sinus) and neural components of the sympathetic baroreflex partially explained the difference in baroreflex sensitivity to falling and rising pressures in young and older humans. In addition, it is possible that baseline sympathetic nerve activity influences the responsiveness of the sympathetic baroreflex to falling and rising pressures. In this context, because resting MSNA exhibits such wide inter-individual variability even in young health humans, individuals with high MSNA may not be able to activate a large increase in MSNA to buffer decreases in arterial pressure.
The aim of the present study, therefore, was to examine whether baseline MSNA is related to the differences in sympathetic baroreflex responsiveness to increasing and decreasing arterial pressures in young and older men and women. We hypothesised that there would be a positive correlation between baseline MSNA and the difference (delta) between baroreflex sensitivity to falling and rising arterial pressures. That is, individuals with low MSNA will show relatively more responsiveness to decreasing arterial pressures compared to increasing arterial pressures.
Methods
Participants
Some of the data used in this study were retrospectively analysed from baseline trials obtained from previous studies (Charkoudian et al. 2005b, 2006; Hart et al. 2009a,b;). Other data were taken from ongoing studies in the laboratory. The investigations from which the data were obtained had ethical approval from the Institutional Review Board of the Mayo Clinic. In total, 72 subjects (30 young men, 14 older men, 21 young women and 14 postmenopausal women) gave their informed consent to participate in the specific studies. Table 1 outlines demographic data for each group. The subjects were healthy, recreationally physically active non-smokers with no history of cardiovascular or other chronic diseases. Participants were not taking any medications with the exception of the oral contraceptive pill. However, one postmenopausal woman was taking a statin (Simvastatin).
Table 1.
Demographic information for separate study groups
| Men | Women | |||
|---|---|---|---|---|
| Demographics | Young | Older | Young | Older |
| Age (years) | 25 ± 1 | 61 ± 2*† | 26 ± 1 | 58 ± 2*† |
| Height (cm) | 175 ± 3 | 179 ± 1 | 167 ± 1‡ | 166 ± 2‡ |
| Body mass (kg) | 79 ± 2 | 82 ± 2 | 64 ± 2‡ | 67 ± 2‡ |
| BMI (kg m−2) | 24.2 ± 0.3 | 25.6 ± 0.8 | 23.1 ± 0.6 | 24.4 ± 0.6 |
P < 0.05 vs. young sex matched
P < 0.05 vs. young opposite sex
P < 0.05 vs. same age opposite sex. BMI, body mass index. mean ± SEM
Participants were asked to not consume anything except small volumes of water within 2 h of the experiment and were asked to abstain from caffeine or alcohol consumption 24 h before the study. To minimize the effects of the reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use (Minson et al. 2000). Postmenopause was defined as amenorrhoea for >12 months (Gracia et al. 2005).
Measurements and protocol
All studies were performed in a Clinical Research Unit laboratory at the Mayo Clinic. On arrival to the laboratory, subjects rested in the supine position during instrumentation. Following local anaesthesia with 2% lidocaine, a 5 cm, 20-gauge catheter was inserted into the brachial artery of the non-dominant arm, using aseptic technique. The catheter was connected to a pressure transducer and interfaced with a personal computer to monitor arterial pressure. A three-lead ECG was used for continuous monitoring of heart rate.
Multi-unit MSNA was measured from the right peroneal nerve at the fibular head using tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses (Sundlof & Wallin, 1977). The recorded signal was amplified 80,000-fold, band passed filtered (700 to 2000 Hz), rectified and integrated (time constant 0.1 s) by a nerve traffic analyser.
Following instrumentation, 5 min of resting baseline data were collected in all subjects (all protocols record baseline data at the same time during the study). Heart rate, arterial pressure and MSNA were measured and recorded continuously using a data acquisition program (Windaq).
Data analysis
Data were analysed only from control periods, i.e. prior to any manipulations associated with a specific study. Data were sampled at 240 Hz and stored on a personal computer for off-line analysis. Mean arterial pressure was calculated as the time integral over the pressure pulse.
Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program (Kienbaum et al. 2001); burst identification was then corrected by inspection by a single observer (E.C.H.). The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
Spontaneous baroreflex sensitivity
Baroreflex sensitivity was calculated by associating spontaneous fluctuations in DBP to the occurrence of bursts of MSNA. The analysis that we used has been termed ‘threshold analysis’ and has been described in detail (Kienbaum et al. 2001; Hart et al. 2010). Briefly, diastolic blood pressures for each cardiac cycle (recorded during the baseline period) were grouped into blood pressure bins of 1 mmHg. The percentage of heart beats associated with a burst in each of these blood pressure bins was calculated and associated with the mean DBP in the corresponding bin. The slope of the relationship between DBP and MSNA was calculated using linear regression. The slope of this relationship has been previously shown to agree with the baroreflex sensitivity calculated during a modified Oxford baroreflex test (Hart et al. 2010).
We completed this analysis on all DBP values over the full 5 min baseline to obtain a baroreflex sensitivity which was not dependent on whether DBP was increasing or decreasing (this reflected overall baroreflex sensitivity). We then completed the same analysis during spontaneous increases or decreases in DBP. More specifically, to calculate spontaneous baroreflex sensitivity to rising arterial pressure, we used cardiac cycles that were preceded by a cardiac cycle with a lower DBP. For spontaneous sympathetic baroreflex sensitivity to falling arterial pressures we used cardiac cycles which were preceded by cardiac cycles with a higher DBP. This meant that two total cardiac cycles were included for each segment.
As a follow-up analysis, we used cardiac cycles that were preceded by two or more cardiac cycles with either a lower DBP or a higher DBP (for a total of 3 cardiac cycles per segment). We used this to check whether this analysis supported the baroreflex sensitivity calculated when using only one preceding cardiac cycle. We did not use this as our main analysis since the inclusion of more cardiac cycles meant we had to remove subjects from the analysis due to lack of data points. In total we removed 48% of the younger men and 38% of the younger women with less than five data points. All figures illustrate findings using the main analysis which includes one preceding cardiac cycles. We tried a similar analysis using three preceding cardiac cycles. We could not construct baroreflex curves for most of the young women during rising DBP since the average number of data points was ∼2. We also had to remove ∼75% of the young men as they also did not produce enough data points. Thus we were not able to include these results. These criteria for cardiac baroreflex sensitivity can be used since there is always a heart beat (R-R interval), whereas there is not always a sympathetic burst.
Linear regression analyses were weighted for the number of cardiac cycles within each DBP bin, and a minimum r value of 0.5 was used as the criterion for accepting slopes. From the linear regression analysis we also calculated the DBP at which 50% of the cardiac cycles were associated with a burst, which is defined as the T50 value (Kienbaum et al. 2001). We then calculated the difference between the actual mean DBP for each individual and the T50 value (T50-DBP); this was defined as the ‘error signal’ (Wehrwein et al. 2010).
We decided to use spontaneous baroreflex analysis instead of the gold standard modified Oxford analysis for two reasons: (a) modified Oxford trials were not performed on the majority of the subjects and (b) when we tried splitting the modified Oxford trials into increasing and decreasing arterial pressures, we did not obtain enough cardiac cycles with sympathetic bursts in one or both of the split portions to construct statistically appropriate baroreflex slopes (i.e. there were not enough points for linear regression analysis).
Our main aim was to examine whether baseline MSNA contributed to the difference between an individual's baroreflex responsiveness during increasing and decreasing arterial pressures. Therefore, the difference between the baroreflex slopes measured during falling and rising arterial pressures (e.g. falling baroreflex slope minus rising baroreflex slope) was calculated for each participant and then associated to their baseline MSNA. Baroreflex slopes are negative, and thus negative numbers were being subtracted from each other. This meant that when an individual's baroreflex sensitivity to falling arterial pressures was greater than that to rising arterial pressures, the difference was negative. On the other hand, when an individual's baroreflex sensitivity to rising arterial pressure was greater than that to falling arterial pressures, the difference was positive.
Statistics
Data were analysed statistically using commercially available software (SigmaStat 2.03, SPSS Inc, Chicago, IL, USA). Group data are expressed as means ± SEM. A one-way analysis of variance (ANOVA) was used to measure differences in baseline haemodynamic variables. Two-way repeated measures ANOVA was used to examine whether mean baroreflex sensitivity (slope of linear regression) was different in response to increasing and decreasing arterial pressures within and between each group. To measure whether there was a relationship between the differences in baroreflex sensitivity to increasing and decreasing arterial pressure and baseline MSNA, linear regression analysis was performed and Pearson's correlation coefficients calculated. The α-level was set at 0.05.
Results
Haemodynamic, neural and respiratory variables
Table 2 outlines average haemodynamic and neural variables in the young and older men and women. MSNA, expressed as burst incidence and burst frequency, was higher in the older vs. the young men and women (P < 0.05). Arterial blood pressures were similar between the young men and women and the older men. In contrast, SBP and MAP were greater in the older women compared to the young men and women and the older men. There was no difference in the overall slope of the spontaneous sympathetic baroreflex or the T50 blood pressure between the groups. Interestingly, the error signal (T50-DBP) was different between the young and older men and women, where the error signal was positive in the older men and women but negative in the younger men and women (P < 0.05). The error signal was not different between the young men and women. Respiratory rate (breaths min−1) was not different among the groups.
Table 2.
Resting neural-haemodynamic variables in young and older men and women
| Men | Women | |||
|---|---|---|---|---|
| Neural-haemodynamic variables | Young (n = 30) | Older (n = 14) | Young (n = 21) | Older (n = 14) |
| MSNA (bursts (100 heart beats)−1) | 38 ± 2 | 60 ± 4*† | 37 ± 3 | 62 ± 4*† |
| MSNA (bursts min−1) | 20 ± 1 | 35 ± 3*† | 22 ± 2 | 38 ± 3*† |
| HR (beats min−1) | 57 ± 1 | 58 ± 2 | 60 ± 1 | 62 ± 3 |
| MAP (mmHg) | 91 ± 2 | 93 ± 2 | 88 ± 2 | 102 ± 5*† |
| SBP (mmHg) | 132 ± 2 | 137 ± 4 | 124 ± 3 | 146 ± 9*† |
| DBP (mmHg) | 72 ± 1 | 70 ± 2 | 69 ± 2 | 75 ± 4 |
| sBRS (% bursts mmHg−1) | −7.3 ± 0.5 | −6.0 ± 0.5 | −6.3 ± 0.4 | −5.2 ± 0.8 |
| T50 (mmHg) | 68 ± 1 | 73 ± 2 | 67 ± 2 | 77 ± 4*† |
| T50-DBP (mmHg) | −3.4 ± 0.8 | 1.9 ± 0.7*† | −2.5 ± 0.9 | 2.1 ± 0.2 |
| Respiration rate (breaths min−1) | 14 ± 1 | 12 ± 1 | 15 ± 1 | 13 ± 1 |
P < 0.05 vs. young sex matched
P < 0.05 vs. young opposite sex
For example, older men had a higher MSNA compared to young men (*) and young women (†). MSNA; muscle sympathetic nerve activity, HR; heart rate, MAP; mean arterial pressure, SBP; systolic blood pressure, DBP; diastolic blood pressure, sBRS; spontaneous baroreflex sensitivity. mean & SEM
Average group sympathetic baroreflex sensitivity to falling and rising blood pressure
Baroreflex sensitivity based on one preceding cardiac cycle
Figure 1 shows an example of the difference between the sympathetic baroreflex slopes in response to falling and rising DBP within a young man. In the young men and women, average sympathetic baroreflex sensitivity was greater when arterial pressure was falling compared to when arterial pressure was rising (Fig. 2). In the older men and women, average sympathetic baroreflex sensitivity to increasing and decreasing arterial pressures was similar. Interestingly, sympathetic baroreflex sensitivity to falling arterial pressures was greater in the younger men compared to the older men (P < 0.05). Sympathetic baroreflex sensitivity to falling arterial pressures was also greater in young vs. older women. In young women, there was a greater sympathetic baroreflex sensitivity to falling arterial pressures compared to that in older men (P = 0.05). Average baroreflex sensitivity to rising arterial pressures was similar among young and older men and women. The average number of DBP bins used to calculate baroreflex sensitivity to increasing and decreasing DBP was not different between groups and did not depend on whether DBP was falling or rising (ANOVA; P > 0.05, Table 3).
Figure 1. Sympathetic baroreflex slopes in response to spontaneous falling and rising diastolic blood pressures (DBP) in a young male participant.
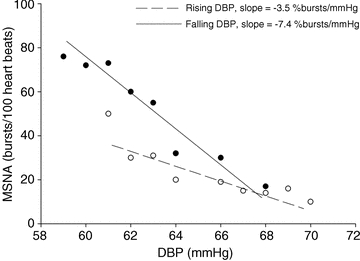
The slope of the linear regression during falling and rising DBP shows that sympathetic baroreflex sensitivity was greater during falling DBP compared to when it was rising.
Figure 2. Average group sympathetic baroreflex responses to falling (filled bars) and rising diastolic blood pressures (DBP, open bars) in young and older men (YM; n = 30, OM; n = 14) and young and older women (YW; n = 21, OW; n = 14).
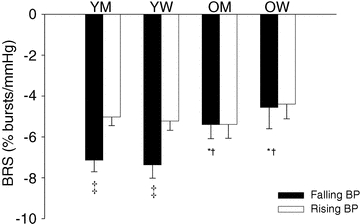
Sympathetic baroreflex responsiveness (BRS) was greater in response to falling DBP compared to that during rising DBP in young men and women but, on average, BRS was not different between falling and rising pressures in older men and women. Overall, sympathetic baroreflex responses to falling arterial pressure were reduced in the older men and women vs., young men and women. *P≤ 0.05 vs. young sex matched, †P≤ 0.05 vs. young opposite sex, ‡P≤ 0.05 vs. rising DBP within group.
Table 3.
Average number of DBP used to calculate baroreflex sensitivity during falling and rising DBP in young and older men and women
| BRS based on one preceding cardiac cycle | BRS based on two preceding cardiac cycles | |||
|---|---|---|---|---|
| Rising DBP (number of DBP bins) | Falling DBP (number of DBP bins) | Rising DBP (number of DBP bins) | Falling DBP (number of DBP bins) | |
| YW | 17 ± 1 | 16 ± 1 | 15 ± 1 | 12 ± 1 |
| OW | 17 ± 3 | 12 ± 1 | 15 ± 2 | 10 ± 1 |
| YM | 20 ± 2 | 17 ± 1 | 17 ± 1 | 13 ± 1 |
| OM | 15 ± 2 | 14 ± 1 | 13 ± 1 | 12 ± 1 |
Data are means ± SEM.
Baroreflex sensitivity calculated using two preceding cardiac cycles
As a follow-up analysis we measured differences in baroreflex sensitivity to falling and rising DBP based on cardiac cycles which were followed by two cardiac cycles with a lower or higher DBP compared to the preceding cardiac cycle. Since using this approach results in fewer data points used to calculate baroreflex sensitivity we had to remove subjects with too few data points from subsequent analysis. We were able to use 16 of the 30 young men, 13 of the 21 young women, 13 of the 14 older women and 13 of the 14 older men. The results of this follow up analysis were similar to those using one preceding cardiac cycle. In the young men and women, average sympathetic baroreflex sensitivity was greater when arterial pressure was falling compared to when arterial pressure was rising (young men: fall, −8.2 ± 0.9 vs. rise, 5.1 ± 0.6%bursts mmHg−1 and young women: fall, −7.0 ± 0.9 vs. rise, −4.7 ± 1.2%bursts mmHg−1, P < 0.05). In the older men and women, average sympathetic baroreflex sensitivity to increasing and decreasing arterial pressures were similar (older men: fall, −4.0 ± 2.2 vs. rise, −6.4 ± 1.1%bursts mmHg−1 and older women: fall, −5.5 ±vs. rise, −5.7 ±%bursts mmHg−1, P > 0.05).
Relationship of baseline MSNA to the difference in BRS to falling and rising pressures
We found that there was a wide inter-individual variability in the difference between baroreflex sensitivity to falling and rising arterial pressures in the young men and women (Fig. 3). This persisted in the older men and women, despite the fact that on average there was no difference between the baroreflex responsiveness to falling and rising arterial pressure in these two groups. Individual delta values between baroreflex responsiveness to falling and rising arterial pressures were positively related to baseline MSNA in the young (r = 0.58, P < 0.05) and older men (r = 0.68, P < 0.05, Fig. 3). This meant that a man with higher baseline MSNA had a more positive delta value (i.e. his baroreflex slope during rising pressures was greater than that during falling pressures). In the young women there was no relationship between baseline MSNA and the difference in baroreflex responsiveness to falling and rising pressure (r = 0.14, P > 0.05). Conversely, in the postmenopausal women, there was a strong positive correlation (r = 0.74, P > 0.05, Fig. 3).
Figure 3. Relationship between baseline MSNA (BI) and the difference between sympathetic baroreflex reflex sensitivities (BRS) to falling and rising blood pressures in young and older men and women.
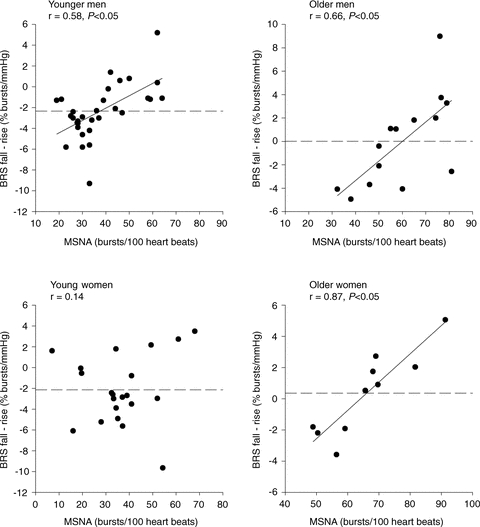
Dashed line represents mean difference between BRS to falling and rising blood pressure. There was a positive relationship between baseline MSNA and the difference in BRS to falling and rising blood pressure in all groups except for the younger women.
In our follow-up analysis (2 preceding cardiac cycles), the relationships between baseline MSNA and the difference in baroreflex sensitivity to increasing and decreasing DBP persisted. In this context, individual delta values between baroreflex responsiveness to falling and rising arterial pressures were positively related to baseline MSNA in the young men (r = 0.73, n = 16), older men (r = 0.79, n = 13) and older women (r = 0.65, n = 13, P < 0.05). In the young women, there was no relationship between baseline MSNA and the difference in baroreflex responsiveness to falling and rising arterial pressure (r = 0.23, n = 13, P > 0.05).
To further explore potential mechanisms underlying the hysteresis in the sympathetic baroreflex, we completed a secondary analysis which focused on whether breathing frequency (breaths min−1) was related to the difference in baroreflex sensitivity to falling and rising DBP. Respiratory rates were not related to the difference in sympathetic baroreflex sensitivity to falling and rising arterial pressures in young men (r = 0.03), young women (r = −0.27) and older women (r = 0.10, P > 0.05). Interestingly, there was a relationship between respiratory rate and the difference in baroreflex sensitivity to falling and rising arterial pressures in older men (r = 0.74, P < 0.05).
Discussion
The main finding of this study was that the difference in baroreflex responsiveness to falling and rising arterial pressures was related to baseline MSNA in young and older men, and in postmenopausal women. In contrast, there was no such relationship in the young women. This suggests that baseline MSNA plays a role in determining how sensitive the sympathetic baroreflex is to increasing and decreasing DBP in young and older men and in postmenopausal women.
Group average responses to falling and rising arterial pressures
As expected, we found that in the young men and women, on average, sympathetic baroreflex sensitivity was greater when arterial pressure was falling compared to when it was rising. However, in the older men and women, baroreflex sensitivity to falling arterial pressure was reduced and, on average, became similar to the baroreflex sensitivity to rising arterial pressure. These data were also supported by our follow-up analysis where we used two preceding cardiac cycles to calculate baroreflex sensitivity to falling or rising DBP.
The group mean differences in baroreflex sensitivity (or lack thereof) to falling and rising arterial pressure in younger and older men and women are similar to those reported by Studinger et al. (2009), where baroreflex sensitivity (total burst area) to falling and rising arterial pressures was calculated during a modified Oxford technique. Studinger et al. (2009) also reported that in older men, there was a higher sympathetic baroreflex sensitivity to rising arterial pressure compared to the younger men. We did not find any difference between the baroreflex sensitivity to rising arterial pressure between the young and older men in this study. This may be explained by difference in the methodology used, for example our data are based on burst incidence, whereas Studinger et al. (2009) used total integrated sympathetic nerve activity.
Baseline MSNA and individual baroreflex responses to falling and rising arterial pressure
We found a positive correlation between individual values for resting MSNA and the difference in baroreflex sensitivity to falling and rising arterial pressures in young and older men and in postmenopausal women. Thus, individuals with lower baseline MSNA were more responsive to falling arterial pressure than to rising arterial pressure (i.e. a more negative delta value). This is consistent with the idea that these individuals can ‘turn on’ sympathetic nerve activity more effectively than they can inhibit sympathetic nerve activity because they already have a low MSNA. Conversely, individuals who had higher baseline MSNA were more responsive to increasing arterial pressure than to decreasing arterial pressures. These individuals can exhibit sympatho-inhibition more effectively than sympatho-excitation because they already have a higher MSNA. They have less capacity to increase MSNA because it is already high at baseline. This is consistent with the fact that baroreflex curves are sigmoidal (Rea & Eckberg, 1987) and suggests that humans who are less responsive to falling arterial pressures operate at a point of the baroreflex curve at which its responsiveness is already at or near maximal (Fisher et al. 2010).
In the older men and women, there was no difference in group mean baroreflex sensitivity to falling and rising arterial pressure. However, when the older subjects were considered as individuals, we found a large range in the differences in sympathetic baroreflex sensitivity to falling and rising arterial pressure (Fig. 3). This indicated that some older men and women were more responsive to falling than rising arterial pressure and vice versa in others. Therefore, there is hysteresis in the sympathetic baroreflex in older individuals, which may be masked when only group mean data are considered. This further emphasizes the importance of considering inter-individual differences when evaluating sympathetic control mechanisms in cardiovascular physiology (Charkoudian et al. 2005a, 2006; Hart et al. 2009a, b).
In young women, there was no relationship between baseline MSNA and the difference in baroreflex sensitivity to falling and rising arterial pressures. This suggests that dynamic neural factors have lesser impact on mechanisms for burst generation in young women. The relationship between baseline MSNA and the difference in baroreflex sensitivity to falling and rising arterial pressures became positive in the postmenopausal women. The striking difference between premenopausal and postmenopausal women implies that the female sex hormones modify the sensitivity of the baroreflex to falling and rising arterial pressures.
Indeed, the female sex hormones have the potential to modify central autonomic processes due to oestrogen and androgen receptors present in the autonomic nuclei (Murphy et al. 1999; Spary et al. 2009). The female sex hormones alter the ‘excitability’ of the autonomic neurones in response to baroreceptor input into the brainstem (Fatehi et al. 2006). Therefore, it is possible that the female sex hormones primarily influence alterations in sympathetic nerve activity in response to baroreceptor input. Such effects may also explain why an inverse relationship between cardiac output and MSNA, seen in young men (Charkoudian et al. 2005b), is not present in young women (Hart et al. 2009a).
Respiration and sympathetic BRS to falling and rising arterial pressure
Respiration has a strong influence the control of autonomic outflow (Macefield & Wallin, 1995; Macefield & Elam, 2002; Narkiewicz et al. 2006), and cardiac baroreflex responses appear to be strongly influenced by respiration (Rothlisberger et al. 2003). Therefore, in an exploratory analysis, we examined whether there was a relationship between breathing frequency and the difference in baroreflex sensitivity to falling and rising DBP (hysteresis) in our groups. We found no relationship between these variables in any group except in older men, where a positive relationship existed. Although this analysis was not the main focus of the present study, the observed relationship in the older men is consistent with the general idea that breathing can modulate sympathetic control mechanisms (Seals et al. 1990, 1993; Wallin et al. 2010). The basis for this relationship in older men is unclear at this time, particularly since we were only able to evaluate breathing frequency and not other aspects of respiration. It would be interesting in future work to more comprehensively characterize the interactions between respiratory control and sympathetic baroreflex hysteresis.
Older men and women and orthostatic hypotension
Average group baroreflex responses to falling arterial pressures in the older men and women were blunted compared to their younger counterparts. Studinger et al. (2009) reported that this was partially due to a decrease in the mechanical component of the sympathetic baroreflex in older men and women. Vascular stiffening in the older participants meant that there was a smaller change in vessel diameter for a given change in arterial pressure. Our data suggest that neural factors also influence the responsiveness of the sympathetic baroreflex to falling and rising arterial pressure. Therefore, older men and women may have lower baroreflex responsiveness to decreasing arterial pressures partly because they have higher average resting MSNA. Consequently, older individuals have less capacity for sympathoexcitation in response to falling arterial pressures. This is consistent with previous reports that older men operate at an arterial pressure which is closer to the maximal responsiveness of their carotid baroreflex curve (Fisher et al. 2010). This might also be the case in older women, but has not been investigated.
Elderly men and women have a higher prevalence of orthostatic intolerance and orthostatic hypotension compared to younger individuals (Rutan et al. 1992). Orthostatic hypotension in older people contributes directly to the increased number of falls and thus hospitalisations in the US older adult population (Ooi et al. 2000; Shibao et al. 2007). Our data suggest that higher baseline sympathetic nerve activity among elderly individuals might predispose this population to more orthostatic events due to the inability to further activate sympathetic neurogenic vasoconstriction from an already high baseline value.
Limitations
We used a spontaneous baroreflex technique to measure sympathetic baroreflex sensitivity which might have several limitations when assessing baroreflex function. At rest, other factors besides changes in arterial pressure may affect the alterations in MSNA (for example, respiration). Non-baroreflex factors have less relative influence during a modified Oxford technique, where the changes in MSNA are more strongly related to the changes in arterial pressure because the arterial pressure error signal becomes much greater than all other influences. Interestingly, a recent investigation in anaesthetised rabbits indicates that closed loop baroreflex analysis (e.g. spontaneous baroreflex analysis) does not necessarily reflect baroreflex function measured using open loop analysis (e.g. modified Oxford technique) and that the two methods may in fact be measuring different components of the baroreflex (Kamiya et al. 2011). However, a previous study demonstrates that the technique we used to analyse baroreflex sensitivity to spontaneous changes in arterial pressure does agree with the baroreflex sensitivity obtained during a modified Oxford technique (Hart et al. 2010).
Conclusions
In summary, baseline sympathetic nerve activity appears to play an important role in determining baroreflex responses to increasing and decreasing arterial pressures in young and older men and postmenopausal women. We report that individuals with higher MSNA had a lower baroreflex response to falling arterial pressures than they did to rising arterial pressures. However, in young women this relationship did not exist, suggesting that tonic neural factors are less important in this group. This may relate to influences of the female sex hormones on central autonomic nuclei. Furthermore, these interactions between MSNA and baroreflex responsiveness may help explain why older men and women, with higher levels of sympathetic nerve activity, have a greater incidence of orthostatic intolerance compared to younger people.
Acknowledgments
We are grateful to Christopher Johnson for his technical assistance and to Shelly Roberts, Jean Knutson, Karen Krucker, Shirley Kingsley-Berg, Jessica Sawyer, Casey Hines, Pam Engrav and Nancy Meyer for their assistance in the conduct of the studies. Finally, we thank the subjects for their participation. This study was supported by NIH HL083947 (MJJ, BGW, NC) and AHA 070036Z (ECH). This projected was also supported by grant 1 UL1 RR024150 from the National Center for Research Resources (NCRR) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Additional support came from the Mayo Foundation including a philanthropic gift from the Caywood family and the Mayo Clinic Department of Anesthesia.
Glossary
Abbreviations
- BRS
baroreflex sensitivity
- DBP
diastolic blood pressure
- MAP
mean arterial pressure
- MSNA
muscle sympathetic nerve activity
- SBP
systolic blood pressure
Author contributions
E.C.H.; conception, design and completion of study. Data analysis and manuscript write-up. B.G.W.; conception and design of study. Manuscript revision. T.B.C.; completion of study and manuscript revision. M.J.J.; conception, design and completion of study. Manuscript revision and mentoring throughout study. T.K.; data analysis and design of data analysis software. N.C.; conception, design and completion of study. Co-writer of manuscript and mentoring throughout study.
References
- Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol. 2005a;289:H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005b;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi M, Zidichouski JA, Kombian SB, Saleh TM. 17β-Estradiol attenuates excitatory neurotransmission and enhances the excitability of rat parabrachial neurons in vitro. J Neurosci Res. 2006;84:666–674. doi: 10.1002/jnr.20959. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Kim A, Young CN, Fadel PJ. Carotid baroreflex control of arterial blood pressure at rest and during dynamic exercise in aging humans. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1241–R1247. doi: 10.1152/ajpregu.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009a;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009b;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2010;298:H816–H822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kawada T, Shimizu S, Sugimachi M. Closed-loop spontaneous baroreflex transfer function is inappropriate for system identification of neural arc but partially appropriate for peripheral arc: a predictability analysis. J Physiol. 2011;589:1769–1790. doi: 10.1113/jphysiol.2011.203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Elam M. Prolonged surges of baroreflex-resistant muscle sympathetic drive during periodic breathing. Clin Auton Res. 2002;12:165–169. doi: 10.1007/s10286-002-0032-z. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Auton Nerv Syst. 1995;53:137–147. doi: 10.1016/0165-1838(94)00173-h. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AZ, Shupnik MA, Hoffman GE. Androgen and estrogen (alpha) receptor distribution in the periaqueductal gray of the male rat. Horm Behav. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne P, Montano N, Hering D, Kara T, Somers VK. Sympathetic neural outflow and chemoreflex sensitivity are related to spontaneous breathing rate in normal men. Hypertension. 2006;47:51–55. doi: 10.1161/01.HYP.0000197613.47649.02. [DOI] [PubMed] [Google Scholar]
- Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. doi: 10.1016/s0002-9343(99)00425-8. [DOI] [PubMed] [Google Scholar]
- Rea RF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. Am J Physiol Regul Integr Comp Physiol. 1987;253:R929–R934. doi: 10.1152/ajpregu.1987.253.6.R929. [DOI] [PubMed] [Google Scholar]
- Rothlisberger BW, Badra LJ, Hoag JB, Cooke WH, Kuusela TA, Tahvanainen KU, Eckberg DL. Spontaneous ‘baroreflex sequences’ occur as deterministic functions of breathing phase. Clin Physiol Funct Imaging. 2003;23:307–313. doi: 10.1046/j.1475-0961.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120:975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat. 2009;38:185–196. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–2057. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Hart EC, Wehrwein EA, Charkoudian N, Joyner MJ. Relationship between breathing and cardiovascular function at rest: sex-related differences. Acta Physiol (Oxf) 2010;200:193–200. doi: 10.1111/j.1748-1716.2010.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Joyner MJ, Hart EC, Wallin BG, Karlsson T, Charkoudian N. Blood pressure regulation in humans: calculation of an ‘error signal’ in control of sympathetic nerve activity. Hypertension. 2010;55:264–269. doi: 10.1161/HYPERTENSIONAHA.109.141739. [DOI] [PMC free article] [PubMed] [Google Scholar]


