Oral cancer is a substantial, though often unrecognized issue globally, with close to 300,000 new cases reported annually (18). The disease presents a management conundrum. It occurs at a site that is easily accessible for examination, yet, regrettably, is often diagnosed at an advanced stage when functional impairment due to treatment and mortality rates are high. Late stage identification is associated with high mortality with 5-year survival rates ranging from 30- 60%, depending on the global locale (64; 76). It is also associated with high rates of second oral malignancies, with up to a third of such patients suffering a recurrence of the tumor or the development of a second primary despite intensive follow-up (11; 36; 56). These figures have shown little improvement over the past few decades.
Despite these statistics, there is hope for change in the not-too-distant future. This hope is fueled by recent strides being made in our understanding of cancer and in the emergence of promising technologies that could aid clinicians in detection, risk assessment and management of this disease. Several alternative detection and diagnostic aids for examining the oral cavity are commercially available or in development. The current challenge is to recognize that these devices are still evolving, yet in many cases they already are moving into the clinical arena. There is a need for the clinician to better understand these new innovations: the biology underlying their function, their strengths and weaknesses, and the scenarios under which they may demonstrate clinical utility.
The intent of this paper is to highlight parameters to be considered when utilizing such technology and to indicate where we are in the development and validation of such approaches and in their incorporation into clinical practice. The paper will explore more recent concepts with respect to tissue change that underlies evident disease, briefly summarize difficulties with the current clinicopathological approaches for detection and diagnostic management of disease and then present an overview of current and developing diagnostic and screening methods. There have been several recent reviews of some of these devices (16; 32; 33; 40; 41). Our intent is not to duplicate this activity but rather to supplement it by building a framework for technology assessment that integrates data summarized in these papers.
A new paradigm for evaluating tissue change
Oral cancer screening and diagnostic strategies have at their core the conventional head, neck and oral soft tissue examination with its systematic inspection and palpation, both extraorally and intraorally. Mucosal lesions identified in this process are assessed for specific characteristics such as size, color, texture and outline as well as lesion history (74). Suspicious mucosal lesions are biopsied to confirm the presence and degree of the dysplasia (23). This combination of clinical and histological evaluations is likely to remain the gold standard for screening and diagnosis of oral premalignant and malignant conditions for the foreseeable future; however, it is becoming increasingly apparent that the conventional approach is somewhat limited when viewed against our present knowledge of cancer development and progression.
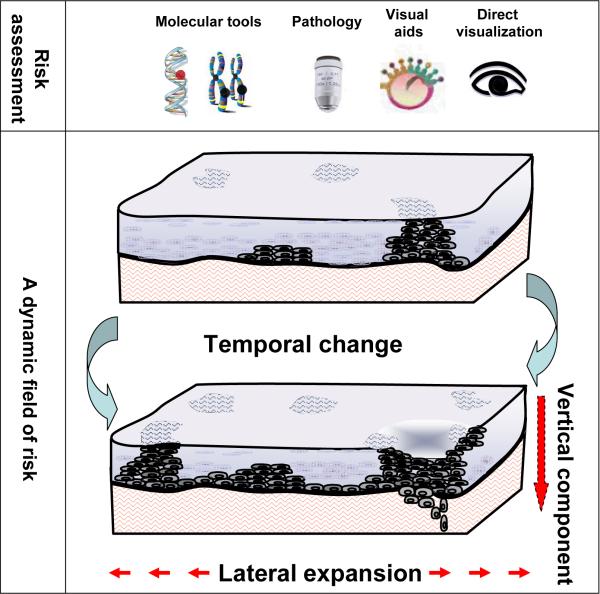
Underlying the clinical and histological change is a complex and dynamic process of mutation and expansion of altered cells within the tissue. Genetic change commences in histologically normal epithelium and accumulates with progression of the disease. Outgrowth of molecularly altered cells leads to abnormal clones or patches of cells that can be extensive and multifocal, scattered throughout the affected epithelium. Such clones are sometimes clinically and histologically apparent and at other times not (Figure 1). These clinically, histologically and molecularly apparent fields of change alter with time, with changes extending over decades. In addition to such temporal change, there are lateral and vertical components of these fields that are also clinically significant. Alterations can occur as discrete patches or show wide lateral spread or “skip” across the epithelium with normal tissue sandwiched between multiple foci. Change may be observed at the surface, be restricted to the lower layers of the epithelium, or extend to the underlying stroma.
Figure 1.
Assessment of a dynamic field at risk
Much of the technology that will enrich future oral mucosal assessments is still in early stages of development and validation. However, tissue optics and contrast agents are already impacting on knowledge generation of clinical fields, suggesting potential noninvasive approaches to the characterization of morphological and biochemical change of these disease processes as they develop in situ. As another “viewing window”, computer imaging and processing technology is being harnessed to describe and objectively quantify combinations of subtle alterations to histological and cytological features that associate with detection, diagnosis and outcome prediction. These technologies will integrate with developing molecular approaches to provide a new paradigm for detection, risk assessment and management of this disease.
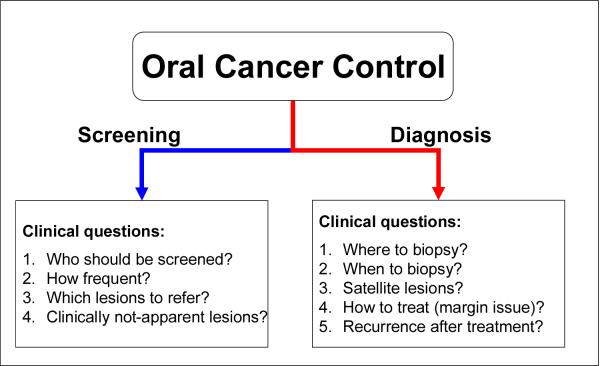
The power of such developments is yet to be ascertained. It will lie in the ability to determine which adjunctive tools best complement the conventional visual and manual head, neck and oral examination to facilitate clinical decision making. Such decision-making is dependent upon both the setting in which the patient is being examined - screening or diagnostic - and the clinical questions being addressed (Figure 2). The following sections indicate the broad spectrum of practical clinical questions that such technology could be focused towards.
Figure 2.
Clinical questions in oral cancer control in screening and diagnosis
What do we really want to know - the “end game” and how do we get there
Screening
There has been much discourse over the years on the value of the standard method for oral cancer screening - the conventional oral exam. It is well known that treatment of early stage oral cancers achieves less treatment morbidity and higher survival rates; however, although the potential benefit of identification of early disease is readily apparent, the actual effectiveness of such activity remains controversial.
The first clear evidence to support the efficacy of an oral cancer screening program was published in 2005 (54). The study involved a large-sized prospective randomized trial of visual screening for oral cancer in India, in which more than 95,000 people were offered oral visual inspection by community health workers, with a similar number of people not offered screening, and followed for up to 12 years, monitoring for mortality. That study demonstrated that screening in high-risk communities, i.e., among users of tobacco or alcohol, results in significant improvement of mortality rates. These data have revitalized the call to action for screening throughout the world and have led to a re-examination of data available in other settings for screening. Several recent systematic reviews have reported on other measures of effectiveness such as acceptance of screening, yield of abnormalities, shifts towards earlier stage cancers and survival data for subjects in which cancer was detected (8; 12; 32; 40; 60). Such reviews conclude that while there is no strong direct evidence of benefit, screening by a general dental practitioner in an opportunistic fashion (as part of regular clinic activity), especially in high-risk subjects, might be cost-effective (60).
There are several perceived barriers to the detection of abnormal clinical changes in subjects in community settings. Of primary concern is the low frequency of oral premalignant lesions and oral cancers in the general population as compared to the prevalence of potential confounders. Early detection relies heavily on the clinician's ability to discriminate sometimes subtle alterations associated with premalignant lesions and cancers from reactive and inflammatory conditions that represent the majority of mucosal abnormalities. The training and experience of the health providers in making such judgment is crucial.
Downer et al. (12) have recently published results of a meta-analysis of eight prospective studies in which the performance of the conventional oral exam was compared between screeners who were either general dentists or trained health workers and, as a “soft” gold standard, the oral specialist. A range of values were observed for sensitivity (59 to 97%) and specificity (75 to 99%) with overall sensitivity and specificity from the meta-analysis being 85% and 97% respectively. This wide variation in values may be associated with different levels of skill sets and experience among community screeners that could be overcome with training.
A more difficult issue lies within the definition of those lesions that should be referred forward for further assessment. Conventional exams target red or white lesions or persistent ulcers that cannot be diagnosed as any other condition. However, the vast majority of such lesions will be clinically and histologically benign. The ability of new devices to filter out those lesions that have a high probability of malignant transformation, excluding low-risk lesions, would greatly improve the effectiveness of the screening process. To be effective, such devices should be able to detect not only high-grade lesions (severe dysplasia and carcinoma in situ) but also those lesions with little or no dysplasia that have a high likelihood of progression.
A final worry is that some lesions may not be readily seen by the unaided eye (e.g., clinically occult lesions). This possibility is supported by data obtained in referral settings and mainly among patients with invasive and preinvasive cancers (46; 47). Whether occult disease is also characteristic of subjects in community settings is unknown.
Diagnosis: the unanswered questions
The assessment and management of oral mucosal fields includes many deliberations that have a potential high impact on long-term patient morbidity and survival. The addition of new parameters that facilitate decision-making at such points could have great benefit.
A key issue is the decision of where and when to biopsy such fields. This is particularly important when lesions are ill-defined, diffuse or clinically occult or if the field is multifocal (with satellites) or spread widely across the mucosa. Each of these situations can be major challenges even for experienced professionals. Moreover, although biopsy provides some assessment of risk for premalignant lesions, for example, for high-grade dysplasia (23), it is apparent that histology itself can be “masked”, i.e., a lesion can appear histologically benign and yet have significant risk of malignant progression (53). Identifying such aggressive lesions, particularly those that are “fast progressors”, can be a pressing concern. Molecular profiles may provide a method of predicting such behavior and this is an area of much research (5; 24; 36; 59); however, the development and implementation of such profiles is dependent upon the initial decision to procure samples from specific areas within the field. Hence, devices that provide an indication of field areas of concern are critical.
Given that an area of risk is identified in a field, another further area of concern involves the decision on appropriate management. Will surgery be effective or is a combination of radiation and chemotherapy required? Again, molecular profiling may partially address this question. However, the spread of high-risk fields both laterally and temporally is an aspect of management that involves other technology, such as visualization optics. If surgery is elected, how does one better guide the choice of surgical margins in the operating room to ensure capture of all high-risk tissue? In recognition of the spread of cancer into tissue surrounding the clinically apparent lesion, current practice is to arbitrarily remove a 10-mm normal-looking oral mucosa border around the clinical cancerous lesion, if anatomically possible. The frequent occurrence of histologically-positive surgical margins and the high rate of recurrence of carcinomas at the primary site (10-30% of cases) (4; 31; 36; 39; 65; 66) indicate the inadequacy of this approach.
Finally, follow-up of patients after treatment is difficult. Clinical examinations are often confounded by the development of treatment-associated reactive white and red lesions at the previous cancer site that are not readily differentiated from (pre-)malignant changes. Repeated comparative biopsies are impractical for such patients. Given the high risk of recurrence of disease and of second primaries, there is a need for technology that can be used to identify areas of concern as early as possible where field changes indicate a potential for disease recurrence.
Criteria used for technology assessment
The need for technology to be evaluated in a systematic fashion is a core concept that has received much attention over the years (20; 34; 48). This is particularly true for emerging technologies since such a framework will provide a mechanism by which new technology can be optimized to fill specific clinical niches.
Littenberg (34) has proposed five levels of technology assessment that can be applied to both emerging and existing technologies. They include biological plausibility, technical feasibility, intermediate effects, patient outcomes, and societal outcomes.
Biological plausibility involves a basic understanding of how the device works and how strongly it is connected to our understanding of the biology and pathology of the lesion in question. Information on plausibility comes from a wide range of sources such as animal models, ex vivo biochemical assessment in human tissues and disease modeling.
Technical feasibility involves the determination of whether a device can reliably and reproducibly measure the biological phenomena upon which it is built and whether multiple devices can be built that have equivalent performance. Much of this assessment involves the determination of whether a device meets its performance specifications.
Intermediate effects include an assessment of the efficacy of the technology as compared to existing approaches in order to determine if there is an added value. For new technology, such information quite often appears in the form of non-randomized clinical studies or pivotal trials. Although informative, such information can have significant bias (see the following section). Randomized clinical trials provide the best evidence of efficacy (71). However, they are costly. Data from earlier non-randomized studies is often needed to support both the feasibility of a trial and to guide its design. The commonly used statistical parameters used to evaluate efficacy include: sensitivity (proportion of individuals with the disease who test positive), specificity (proportion without the disease who test negative), positive predictive value (proportion of individuals with positive results that have the disease) and negative predictive value (those with negative results who do not have the disease).
The remaining levels of technology assessment are often measured once the efficacy of a device becomes apparent. However, elements of this determination can be acquired in earlier feasibility studies or through focus groups in different settings. These aspects of a device's utility are clearly an important consideration early in its assessment. The evaluation of patient outcome involves a determination of impact on the physical and functional well-being of the patient, quality of life, psychological stress and patient satisfaction. Assessment of societal outcomes revolves around issues of the cost and ethical implications of the technology and the long-term impact on morbidity and mortality and can be used to find the optimal clinical niche for the technology.
Methodological standards in evaluation of technology
It is important for clinicians to be aware of potential biases in design that affect the quality of studies on new technology and impact on the assessment of efficacy. This information can be used in development of future studies and to help standardize the collection of data. It also provides a framework for evaluating current literature. Several well-established general standards for assessing technology have been presented by Reid and co-workers (48) and are expanded upon below.
A chief factor to be considered in studies evaluating the accuracy (e.g., sensitivity/specificity) of a device is the spectrum composition of test subjects. For example, the development and evaluation of new technology for oral cancer management frequently takes place within secondary care settings where tested patients are substantially symptomatic with severe disease. The application of these test results to patients with predominantly mild or asymptomatic disease can be misleading. This problem becomes even more apparent when devices developed within diagnostic settings are transferred to communities for population screening. The disease spectrum shifts to asymptomatic disease with a greater proportion of oral anomalies being benign and reactive lesions and the utility of the device needs to be re-assessed. In all instances, the choice of test subjects places a critical limit on the clinical applicability of the data.
Even if the spectrum of evaluated subjects is appropriate, the assessment of accuracy may vary widely depending upon the composition of subjects within a study. This is a particular problem when samples sizes are small and where heterogeneity of patients is more likely to have impact. The device may perform well among certain subgroups and not others. Thus, concise and detailed description of test subjects is critical. Key descriptors include demographics (age/gender), risk habits (smoking/alcohol), medical history, and clinical and histological characteristics. If possible, the information should be provided for each case in association with the performance of the technology, not just given as a summary group statement. This will allow for a later pooling of results across studies in a meta-analysis framework. For premalignant disease, key information includes the degree of dysplasia, whether the dysplasia occurs in patients with former oral cancers, as part of a cancer or as a primary dysplasia without history of dysplasia.
Work-up bias occurs when patients with positive (or negative) results are preferentially referred to receive verification by the gold standard procedure. This bias can distort sensitivity/specificity assessments. For example, if patients with positive rather than negative results are preferentially referred for biopsy, this can lead to inflation of the true-positive and false-positive numbers. This bias becomes an important issue in the assessment of technology to detect lesions requiring follow-up in community settings when the focus is on lesions of low risk and where biopsy of all lesions is not likely.
Review bias occurs when either the diagnostic test or gold standard procedure is determined without precautions to be objective in their sequential interpretation. For example, prior knowledge of patient diagnosis (e.g., cancer, dysplasia) could potentially impact on the interpretation of the device results.
Sample size biases can occur when too few patients are evaluated. The impact of such numerical instability on determination of efficacy is usually indicated statistically as confidence intervals (CIs), which generally narrow as the sample size increases.
Two final issues include the presentation of indeterminate results and test reproducibility. With new technology and inexperienced users, tests do not always have a clear-cut result, i.e., they may be equivocal. The frequency of unclear results and how such data is handled is critical. Finally, reproducibility between device users is an important issue and should be ascertained. This is particularly important when a device shifts from use among experienced specialists to primary care providers; for example, when devices developed in referral centers are transferred into community settings.
The following sections will describe some of the current and future technology in light of the above considerations. We will describe 2 such technologies, optical contrast agents and visualization systems and high resolution computer imaging systems, in the following sections. The discussion is given in broad strokes rather than a detailed assessment of strength of study design for individual papers.
Optical contrast agents and visualization systems
Optical imaging encompasses a collection of techniques that are capable of detecting and visualizing key biological processes within living systems, enabling the study of disease processes as they develop in situ. The field has two main components. One revolves around the development of exogenously administered contrast agents that can be “painted” on the tissue surface to enhance detection of changes. The other involves the application of optical devices to specifically explore those morphologic and biochemical alterations beyond the naked eye that affect the optical properties of a tissue.
Toluidine blue: an early contrast agent
Contrast agents are optically active agents which are targeted towards the specific cells of interest (e.g., cancer). When applied to the tissue, the agents offer an opportunity to increase our ability to distinguish normal structures from abnormal structures. An example of such an agent, discussed below, is the well-known vital dye, toluidine blue.
Toluidine blue (TB) is a metachromatic vital dye that has been used for over 40 years as a method of detecting mucosal abnormalities in the oral cavity. The dye is used either as a mouthrinse or applied to the oral mucosal surface using a swab. Retention of the stain by tissue is evaluated in comparison with adjacent normal mucosa.
There are numerous reports on the use of this dye. Most are convenience sample studies, usually conducted within secondary care settings where there is a high prevalence rate of oral neoplasia by specialists and often with lesions with known diagnoses. Hence its utility for screening in primary care settings is unresolved.
There is a general consensus that the dye has a fairly high sensitivity for the detection of oral cancers. One recent report gave a range of 0.79 to 1.00 for its sensitivity with a range of 0.31 to 1.0 for specificity (21). The lower level of specificity is most often associated with non-specific binding to areas of inflammation and trauma. A second follow-up of a lesion suspected to be toluidine blue positive is recommended prior to biopsy, to eliminate possible local causes of tissue irritation and improve specificity.
The literature on the use of the dye has undergone several systematic reviews (21; 40; 41; 52). Overall, the strongest support for clinical utility of the dye appears to lie in its use as a diagnostic adjunct. There is some indication of its use to identify oral cancers or dysplasia that would not otherwise have been diagnosed (2; 72). One such indication is for the assessment of the oral mucosa prior to surgery. Another is for surveillance of high-risk individuals, patients with high-grade dysplasia, or those with a past history of oral cancer, at risk for disease recurrence. Examples of such uses are given in several recent publications (15; 17; 32; 40; 41).
The ability of TB to detect oral premalignant lesions is controversial. A significant portion of dysplasia do not stain with the dye (37; 38; 72). However, recent results from an ongoing longitudinal study of 100 patients with primary oral premalignant lesions suggest that TB may preferentially stain lesions at elevated risk of malignant progression (78). That study reported a strong correlation between dye retention and risk of progression, seen as an association of staining with the presence of clinicopathogical risk factors, high-risk molecular patterns and with outcome. Histologically, TB stained 16 of 17 cases of severe dysplasia. Although the dye was positive in only 26% (5 of 19) of nondysplastic oral premalignant lesions and 23% (15 of 64) of lesions with low-grade (mild/moderate) dysplasia, it was strongly correlated with lesions in these groups that had high-risk molecular profiles. Overall, there was a six-fold elevation in cancer risk for positive lesions, with 12 of 15 progressing lesions staining positive for the dye. Perhaps the most important point to come out of this study was the demonstration of the need to study devices within longitudinal frameworks when assessing impact on premalignant lesions.
Evolution of contrast agents: a move towards molecular paints
In the future, molecular findings will be integrated into the development of contrast agents to engineer markers that label specific molecular alterations known to be involved in the neoplastic process. Examples could include agents targeted to overexpression of Epidermal Growth Factor Receptor, Matrix metalloproteins or cytokeratins, expression of proliferation-associated molecules such as cyclin D1 or alteration of DNA check-point sentinels such as p53. These novel optical markers are being produced through the conjugation of gold and silver nanoparticles, gold nano-rods, carbon nano-tubes and various fluorescent markers and beacons with specific chemical structures that bind to the known molecular markers (see recent review by Pierce et al. (44).
Initial use of such optical contrast agents will be mostly limited to high resolution optical techniques (e.g., in vivo confocal microscopes) which are capable of detecting labeling of alterations present as a low number of particles per cell. However, as molecular alterations involving highly expressed proteins are identified, it may be possible to attach physiologically large numbers of optical contrast agent particles per cell such that the bulk optical properties of the tissue may be altered allowing for wide field optical technologies (such as visual to the naked eye) to record the presence or absence of the molecular targeted signals. Alternatively, the strength of the optical marker could be improved sufficiently so that even a few particles can be discernable visually. For example, keratin is a highly expressed protein within cells; labeling keratin would result in many signals in the cell. In contrast, p53, a molecular switch, is expressed at such low levels in the cell that even if every molecule was labeled, the strength of the signal would be below that detected by the naked eye unless the optical contrast agent generated a very strong signal.
The first of such molecular paints are close to getting FDA approval for investigational use. One of the hurdles is the effective delivery of the optical paint to the target tissue. These paints can be large molecules that have difficulty penetrating multiple cell layers. One of the advantages of the oral cavity is that this can be done topically as opposed to systemically. Additional challenges are toxicity of the paint and the direction of its activity, both of which favor topical applications over systemic. Thus it is likely that molecular paints will find utility for epithelial tissues first. The oral cavity is currently one the most active sites for development of molecular paints.
Fluorescence Visualization
Optical devices have as their basis the interaction of light with tissue. We can optimize the physical characteristics and arrangement of illumination of light onto tissue (e.g., wavelength, duration of illumination, polarization, location of illumination relative to detection, angles of illumination). The light interacts with tissue in such fashion that it is affected by the morphology, the chemistry and the structure of the tissue (e.g., blood vessels, collagen matrix, nuclear/cytoplasmic ratios, cellular density, and epithelial thickness).
The fundamental ways in which light interacts with tissue are through scattering, absorption and absorption with re-admission. The penetration depth of visible light in tissue is generally fairly small because of the strong interaction of the light with the tissue. While this is a disadvantage for extracting information from deep within the body, it is an advantage when trying to interrogate changes predominantly located in the thin layer of epithelial tissue at risk of malignant transformation. The following sections will focus on the evolution of one such approach, autofluorescence visualization, for detection and diagnosis of oral cancer and premalignancy.
Tissue fluorescence results from the absorption of a high energy photon (short wavelength light) by a molecule within the tissue such that the light is re-emitted as a lower energy photon (longer wavelength light) leaving behind some energy, which is turned into heat. An example of this process would be blue light being absorbed by collagen cross-links and being re-emitted as green autoflourescent light. This is in contrast to conventional white light examination which makes use of reflectance imaging where the wavelengths of the light illuminating the tissue is the same as that detected from the tissue. In other words, fluorescence is detecting wavelength-shifted light whereas reflectance is not. This article will first describe work with fluorescence with extension to reflectance imaging in the subsequent section.
The Wood's lamp which makes use of tissue autofluorescence (AF) has been used in dermatology for many decades for the identification of a host of skin diseases and bacterial and fungal infections (75). AF technology has been developed for use in different sites to localize disease and facilitate detection of lesions requiring biopsy. AF is now an established clinical technique in the lung for detecting cancer and premalignant disease with device development proceeding for the cervix, esophagus and colon as well as the oral cavity (9; 10; 26; 43; 56).
The biology underlying tissue fluorescence visualization (FV) is based on the combination of tissue morphology and native fluorescence. The intrinsic fluorescence is produced by naturally occurring fluorophores in the epithelium and stroma that become excited when specific wavelengths of light are absorbed, re-emitting light of a different wavelength. This fluorescence is modified during carcinogenesis through direct alterations to the fluorophores themselves or indirectly by changes in tissue morphology that affect light absorption and scattering.
The endogenous fluorophores most relevant to optical screening and diagnosis of precancer and cancer are those that excite in the violet-blue part of the visible spectrum (400-450 nm) through the ultraviolet A (UV-A, 315-400 nm) and have properties that have been spectroscopically correlated with disease progression (13; 14; 42; 49). For visible light, the majority of fluorescence originates from collagen cross-links that bind collagen fibrils together to form fibers in the stroma (collagen matrix). A small fraction originates from the reduced form of nicotinamide adenine dinucleotide (NADH) and the oxidized form of flavin adenine dinucleotide (FAD), important fluorophores that excite at these wavelengths in the cells of the epithelium.
Among biochemical changes associated with alteration to fluorescence during cancer development is the reduction in AF from the collagen cross-links, possibly due to the breakdown of the extracellular matrix. This change has been hypothesized to be due to collagen remodeling associated with alterations to matrix metalloproteinases (MMP) expression in host stromal cells as well as stromal remodeling associated with angiogenesis (27; 67). Although collagen change is thought to be a primary biochemical cause of AF alteration, other fluorophores also change. For example, alterations to metabolic activity with dysplasia are accompanied by changes to NADH and FAD levels in the electron transport chain. FAD florescence intensity decreases with dysplastic progression. Finally, an increase in micro-vasculature in the stroma during carcinogenesis can also result in a reduction in AF since hemoglobin strongly absorbs the violet-blue excitation light, with less of it reaching the fluorophores.
In addition to the reduction of the intrinsic sources of tissue fluorescence, changes of epithelium thickness and nuclear morphology at the cellular level during the disease progression have a significant impact on fluorescence via scattering of the excitation and emission light. For example, a thicker epithelium composed of more cells with increased nuclear scattering means that less excitation light will reach the stroma where the bulk of the fluorescence is generated; consequently, a reduction of the intensity of autofluorescence will be observed.
Potential clinical utility of FV
The first commercially available AF imaging device approved for use in the oral cavity is the VELscope® (LED Dental Inc., White Rock, BC, Canada). This simple handheld device uses a blue/violet light (400-460 nm) to illuminate the oral tissue. A selective long-pass filter in the eyepiece allows the viewer to directly visualize the pale green autofluorecence that is given off by normal tissue (30). Abnormal or suspicious tissue shows decreased levels of normal AF, appearing a dark brown to black region by comparison to the surrounding healthy tissue.
The first reported use of this device involved a small study of 44 patients within the Oral Cancer Prediction Longitudinal Study in Vancouver, BC. This included 33 patients with invasive SCC and 11 with severe dysplasia/CIS. Six normal cases were used as controls. All were biopsy-confirmed. Data were promising with a 98% sensitivity and a 100% specificity for discriminating dysplasia and cancers from normal mucosa (30).
Since that time, we have continued to use the device on patients in the ongoing longitudinal study, periodically reporting on the performance of the device in different clinical scenarios (46). The following is a summary of our observations, based on abstracts presented on interim analyses. A full analysis of consecutive cases within this database is on-going.
With respect to detection of disease, FV has correctly identified ~ 95% of the 120 invasive SCCs in the study that have FV analysis. Nearly all cases of severe dysplasia/carcinoma in situ also show loss: 82 of 83 such cases had loss of AF as well as 59 of 76 low-grade (mild/moderate) dysplasia. The significance of loss in these lesions has yet to be established. An early analysis suggests the possibility of an association between loss of AF in low-grade dysplasia and the presence of a high-risk molecular profile (i.e., loss of heterozygosity profile) (77). This suggests the possibility that FV is able to mark some of the tissue alteration that is associated with risk of malignant transformation. However, follow-up time is very short for these cases (~24 months). Our experience with progression rates for low-grade dysplasias based on molecular profiles is that approximately 50% of the lesions progress within 5 years.
An intriguing early observation in the use of AF for diagnostic examination in the oral and cervical cavities has been the discovery that lesions were identified with AF that were clinically occult under white light inspection (30; 51). This capacity is yet to be fully explored; however, it suggests three potential clinical venues for use of the device: 1) initial diagnosis of cancers and premalignant lesions that are clinically occult; 2) early detection of recurrent disease, either recurrent at treated lesion site or as a second primary tumor elsewhere within the oral cavity; and 3) better delineation of the surgical margin of cancers. An article published in 2007 reports examples of cases in which each of these potential uses was apparent (46).
Support for the potential use of FV to provide real-time guidance in direct intraoperative use comes from a study in which FV was used to detect surgical tumor margins for oral cancer in the operating room (47). The study examined 20 consecutive patients undergoing surgical excision, documenting histological and molecular changes within areas showing loss of AF in tumor margins. Nineteen of the 20 cancers demonstrated loss of AF that extended beyond the clinically visible tumor boundary, varying in extent (from 4 to 25 mm) with the extension being unevenly distributed around the clinical clinically apparent perimeter. Eighty-nine percent (32 of 36) of margin biopsies from these areas showed either cancer (7 SCC) or dysplasia (10 severe dysplasia/carcinoma in situ, 15 mild/moderate dysplasia). Molecular analysis of AF margins with low-grade or no dysplasia in this series suggests that FV identifies histologically low-grade margins that contain high-risk molecular clones. Sixty-three percent (12/19) of such margins displayed high-risk loss of heterozygosity (LOH) patterns. These data are very promising and results are intriguing. However, larger sample sets and eventual movement to a clinical trial is necessary to confirm utility of the approach within this setting.
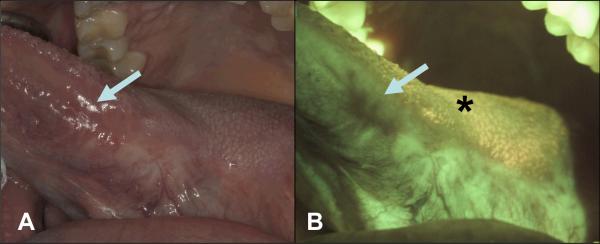
Results of this study suggest a potential utility for FV to identify lesions that cannot be seen by clinical examination alone. This possibility is being explored within the ongoing longitudinal study in Vancouver among cancer patients in follow-up for recurrence, looking for the reappearance of a clinical lesion at the treatment site that may or may not show AF loss and the appearance of such loss without clinical change. In the latter case, determinants being explored are the persistence of areas showing loss of fluorescence (called “FVL” for fluorescence visualization loss) over time and subsequent development of clinical lesions and changes to the size and intensity of FVL fields (see example in Figure 3).
Figure 3. The use of FV during follow-up for detection of cancer recurrence.
A 42-year-old male nonsmoker was examined 4 years after surgery and radiation therapy for Stage III squamous cell carcinoma involving the left lateral tongue and left cervical lymph nodes. A. White light image of a well-healed scar at left lateral tongue with no clinically visible lesion (arrow); B. The same area (arrow) under FV showing a dark brown FVL. The comparative biopsy from the FVL area 4-and-half years after initial treatment showed severe epithelial dysplasia. The area posterior to the FVL showed FVR representing a well-healed scar (star).
In a recent interim analysis of patients begin followed in the longitudinal study, we identified 41 FVL sites that persisted after surgical treatment. Thirty-four of these sites later developed clinical lesions. To date, 21 have been biopsied with 16 (76%) showing high-grade and 3 (14%) low-grade dysplasia, and 2 being nondysplastic. All 7 of the cases without clinical lesion development have been biopsied yielding 3 high-grade and 3 low-grade dysplasia and 1 nondysplastic lesion.
We are also focusing our efforts on looking at the entire oral mucosa of cancer patients after treatment, to determine whether FV will detect new anatomically separate lesions, possibly second primaries. Figure 4 shows such a case, in which a patient had an excisional biopsy of a carcinoma in situ with development of a new lesion more than 8 cm from the original site. FVL detected both recurrent and new lesions supporting its usage in individuals with very unstable, widespread disease.
Figure 4. The use of FV to identify a distant lesion during follow-up.
A 68-year-old female smoker presented with a carcinoma in situ on the left anterior ventral tongue after completion of an excisional biopsy with margin positive for mild epithelial dysplasia. At 3 months post surgery, she was referred for the assessment of the surgical site. A. White light image showing a scar without clinically visible lesion on left anterior ventral tongue (arrow); B. FV image showing some FVL area (arrow). C. White light image showing an ill-defined mildly erythematous area at left posterior soft palate and FV image showing a well-defined dark brown FVL area (D, arrow) 8 cm distant to the initial cancer site. The biopsy showed carcinoma in situ.
Further evolution of optical devices
Data to date have been restricted to referral settings and to use by oral specialists and have not addressed the sensitivity and specificity of the device for the typical spectrum of oral pathologies seen in community settings. From this standpoint, confounding due to the more common benign inflammatory conditions, which are often accompanied by an increase in absorbing hemoglobin content, is likely to be one of the more significant issues that need to be addressed, as it can lead to false positives. Several approaches are being taken in an attempt to improve the ability of the device to discriminate such confounding. At the same time, other strategies are focused on the extraction of additional disease-specific information for optical devices, to improve diagnostic sensitivity.
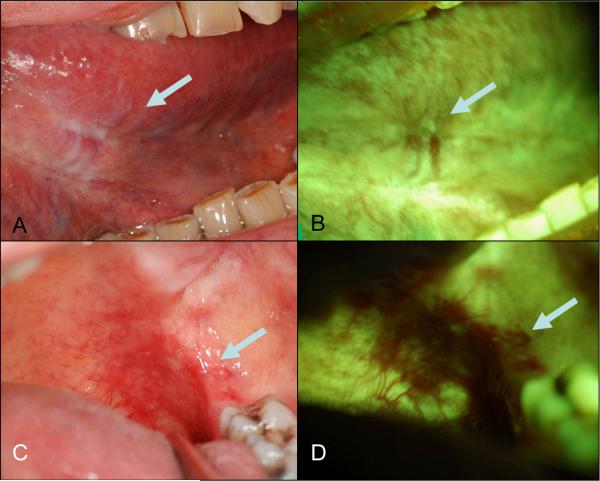
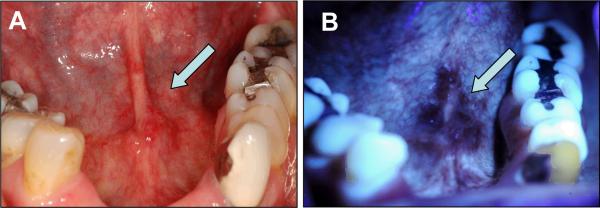
The initial clinical FV systems described above made use of 440 nm excitation (blue) and green and red emission. More recent FV devices are starting to use shorter wavelength illuminations such as 380 nm (UV) or 405 nm (violet) for excitation with blue, green and red emission to possibly improve disease detection and delineation. For example, in a recent development, the Identifi™ 3000 (Trimera™) uses 405 nm (violet) excitation for FV. An example of images taken of a severe dysplasia at anterior floor of mouth using this wavelength is shown in Figure 5. While intriguing, this technological development is very recent and the impact on both sensitivity and specificity of the FV is as yet unknown.
Figure 5. Demarcation of dysplastic lesion with 405 nm excitation.
A 55-year-old female former smoker presented with a pathology-proven severe epithelial dysplasia at the anterior of floor of mouth extending to the lingual frenum. A. White light image showing an ill-defined, slightly erythematous lesion (arrow) at anterior of mouth; B. FV image showing a well-demarcated area of FVL area (arrow).
In addition, several groups are adding reflectance imaging (broad band and narrow band) to improve the discrimination between tissue at risk and tissue which may mimic at-risk changes (50; 57). Narrow band imaging uses a very limited range of color (575 ± 15 nm) to target specific tissue components (such as blood vessels), whereas broad band reflectance imaging generally makes use of white light (e.g., 400 to 650 nm) to visualize all the structures in the tissue at the same time (50).
A further exciting area of study involves computer manipulation of the fluorescence/reflectance image data to generate probability maps. This involves processing the image data to highlight areas in which the measurement characteristics are consistent with at-risk tissue. A recent study used such an approach on images collected from 56 patients with oral lesions and 11 normal volunteers (50). The investigators designed a simple classification algorithm to discriminate between neoplastic and non-neoplastic areas. This algorithm was then applied to patient images to create visual disease-probability maps across the field of view. These early results were quite promising with sensitivities and specificities of training and validation sets in the mid-90s.
A final area of active research is the use of narrow field of view optical technologies such as confocal in vivo microscopy or point spectroscopy for improved discrimination between neoplastic and non-neoplastic tissue without the removal of tissue. These point technologies sacrifice field of view for detail of interrogation. Confocal in vivo microscopy enables the collection of pathological level images from the tissue for disease detection. In contrast, point spectroscopy makes use of subtle spectrum changes for disease detection. An additional refinement of this can be seen in depth resolved spectroscopy in which the angle of the illumination and detection optics allow for the measurement of spectral changes from specific depths within the epithelial layer and underlying stroma (57). As a further evolution of such approaches, some groups are exploring a combination of sensitive wide field detection with point spectroscopy, to allow the examiner to use point spectrum analysis to review areas of abnormality identified during a wide field exam.
In summary, optical technology represents a rapidly evolving technology likely to impact on several clinical arenas. A key feature of this evolution is the degree to which devices are optimized for specific needs. To do this, these targeted devices need to be both developed and validated within the appropriate spectrum of disease and by the proposed end users.
High resolution computer imaging systems
The history of automated cytometry and histology
The difference between microscopy and quantitative microscopy lies in the capacity to quantify characteristics of cells and their arrangement in a microscope field. The human observer is very good at recognizing cells, clusters of cells and structures, but poor at quantifying differences in size, texture or alterations to cellular or tissue structure. For example, humans have difficulty distinguishing two balls side by side that differ in size by less than 20%; if not side by side, the difference can be 50% or more and they still can't recognize the larger of the two objects. Further, although humans can identify changes in individual cells, they have difficulty recognizing shifts in populations of cell characteristics. For example, if a cell population has twice the variance in its size compared with another population, this characteristic will be not be visible to the human observer with the exception of the rare individual whose gestalt impression can encompass these types of changes. Quantitative microscopy has been successfully used for more than 3 decades to make such assessments. The Achilles' heel of such technology, however, is the difficulty of training the system to automatically recognize and segment cells. This is even more difficult when quantitative microscopy is applied to overlapping clusters of cells or to tissues.
The utilization of quantitative microscopy for cancer detection began in the mid-1960s (69; 73) with a focus on development of automated cervical screening devices. This effort culminated in the late 1990s in several automated imaging systems which sought and received approval for automated cervical cancer screening (1; 3; 6; 29; 62; 68; 70). These systems basically employed two fundamental approaches. One approach made use of the conventional Pap smear and tried to duplicate in the device `the pathologist's capabilities'. This was done by measuring the morphology and texture of nuclei and cytoplasm in Papanicolaou-stained cells in a fully automated fashion (19; 28). Cell classification was based upon these measured features and image characteristics used in decision trees or neural nets. These systems would automatically scan the slide, segment objects, recognize the difference between cells and debris and then recognize the difference between normal cells and abnormal cells. The systems were generally not accurate enough to function without some human assessment so they essentially triaged all the “abnormal-like” objects for human review.
The other approach made use of stoikiometric DNA specific stains to enable the measurement of additional information, alone or in conjunction with counterstains (1; 3; 6; 22; 35). A primary determinant of such systems was the evaluation of ploidy, which is known to be associated with cancer risk. An additional benefit of stoikiometric stains was that the measurement of the texture within cell nuclei was more quantitative than that of samples which use Haemotoxylin as the nuclear stain.
The success of any of these devices depends on their ability to correctly recognize and segment the cells on the slide. One could argue that the most successful of these devices combine the two approaches.
Variants of these technologies are still in use today and for detection of oral cancers, for example, the PAPNET device originally developed for assisting cervical screening has evolved for use in oral screening in the OralCDx® BrushTest® (OralCDx Laboratories, Inc., New York). The quantitative approach has found use in cervical screening in China (63) and for oral cancer screening as OralAdvance™ (Perceptronix Medical Inc., Canada).
The move to oral cancer is a relatively new move for OralAdvance™. Like the majority of the automated cervical screening systems, it makes use of pathologist's interpretation of the samples with non-normal cells. The technology is biologically plausible since it makes use of DNA ploidy, a well recognized marker associated with cancer development. However, its technical efficacy has yet to be published.
In contrast, Oral CDx has been marketed for some time now with literature available on its performance, albeit mostly in referral settings.
Oral CDx
The Oral CDx method was developed as an adjunct tool for use in primary care settings for guiding the determination of which clinical lesions with innocuous clinical features require biopsy. This would include those lesions that would likely be ignored or undergo prolonged periods of observation. It was not proposed for use with clinically suspicious lesions that should go to immediate incisional biopsy. Moreover, the approach was to be a triage method, followed by the scalpel biopsy of all oral lesions with abnormal CDx.
Results with this approach have been encouraging. Reports from referral centres and among oral specialists have shown fairly strong associations between a positive or atypical result with the Oral CDx method and the presence of cancer or dysplasia at biopsy when the test was applied to clinically suspicious lesions, i.e., to test potential oral cancer or premalignant lesions (45; 55; 58). Unfortunately, its utility in its targeted setting, with innocuous lesions and in dental care settings, is as yet unresolved. This is largely due to a work-up bias in most studies with referral to biopsy primarily occurring among positive cases with most negative samples not receiving a confirmatory biopsy for histological status. Thus, although dysplasia and cancer have been uncovered in innocuous lesions that might otherwise have gone unsampled (7), supporting its potential, there is still substantial debate over the accuracy (sensitivity/specificity) of the approach for the targeted lesion. There is a need for these studies to be extended into community settings using primary care screeners where the spectrum of the disease is appropriate to its targeted use. Issues of work-up bias in such settings also need to be addressed.
The future: targeting evolution of high resolution computer imaging systems to clinical need
We have taken advantage of the ongoing longitudinal study in Vancouver to develop two high resolution computer imaging systems targeted towards specific needs: a quantitative histology system for tissue analysis, primarily for prediction of malignant progression of low-grade dysplasia, and a quantitative cytometry system for use with exfoliated cell brushings, in this case to facilitate referral decisions made by clinicians in community settings.
These systems semi-automatically detect and quantify very subtle alterations to the nuclear phenotype, targeting features that are associated with disease detection and with outcome. The systems examine ~110 nuclear features in cell nuclei in each of the hundreds of cells of both tissue and cytology specimens and archive these analyses. Some features assess the size, shape and the amount of DNA within the nucleus. The majority, however, describe the distribution of DNA, unraveling very complex features into quantifiable algorithms, for example, the distribution of DNA around the edge of the nucleus or clustered in the center, whether the nucleus is dark with light areas or light with dark areas, whether the increased chromatin is evenly distributed (euchromatin, active gene transcription) or clumped locally (heterochromatin, hypermethylated and/or acetylated), and whether the chromatin clumps are large or small (23; 46).
The quantitative cytometry system is intended to be used with both conventional visual assessment and with sensitive field of view FV-assisted screening technologies. The objective is to use this device to facilitate screening activities in community settings by less experienced users where prevalence of the disease is low compared with confounders and benign lesions. As mentioned above, current FV devices are generally sensitive for the detection of suspect oral cancers and premalignant lesions, but can highlight areas where actual characteristics may be masked by inflammation/ulceration and other conditions. Although the system is intended to be used with brushings targeted to discrete foci or lesions, it has been trained to be tolerant to near misses, i.e., with brushings that include exfoliated cells from the targeted lesion and surrounding area (~ 1 cm outside of clinically apparent lesion).
Automated image cytometry measures individual cell ploidy as well as morphological characteristics. These features are used to recognize cells versus debris and then to differentiate between cells with frankly abnormal ploidy values (such as more than 2 and a half times the range of normal DNA). To maximize the sensitivity of the system towards subtle ploidy alterations as opposed to frank ploidy alterations, the system is trained to differentiate between normal cycling cells and abnormal stem cell populations in the following fashion.
For this study we collected 538 cytological samples using targeted brushing of select areas in the oral cavity from individuals with squamous cell carcinoma (SCC), carcinoma in situ (CIS), severe dysplasia (n = 125), no areas of abnormality (n = 316) and subjects with areas of inflammation/infection (n = 97). All of these samples were spun down onto slides and the DNA quantitatively labeled with a modified Feulgen-Thionin stain and the slides automatically scanned. Approximately 90% of the 316 samples from known normal areas and 125 samples from (SCC, CIS and severe dysplasia) were used as the training set to determine the characteristics of normal cells in and out of cycle versus genetically altered stem cell populations found in the abnormal samples. The frequency of these two classes of cells were recorded from the training sets and the appropriate thresholds for the frequency of cells displaying characteristics associated with cancer detection were defined. Using these thresholds the system correctly identified 84% of the abnormal cases and 97% of the normal cases in the training samples. In the ~10% hold out test set using these thresholds the system correctly identified 75% of the abnormal cases and 100% of the normal cases in the test samples. When tested on the 97 inflammation/infection cases the system correctly identified 98% of the samples as non OPLs. These pilot results indicate that image cytometry may have a role to play in oral cancer screening in the community. However, the device performance needs to be further assessed in an actual community setting.
DNA ploidy can also be performed on disaggregated biopsy samples using the Hedley procedure (25). A recent retrospective study used this approach to determine whether DNA ploidy analysis by image cytometry was able to distinguish between those dysplasia at high-risk of malignant progression. Although the dataset was small (86 lesions, 42 of which progressed to oral SCC), the results are encouraging. The sensitivity, specificity, positive and negative predictive values (0.33, 0.88, 0.74 and 0.58 respectively) suggest that this approach requires some future refinement before clinical utility can be achieved. The authors concluded that oral dysplastic lesions with aneuploidy have a high risk of progression and that DNA image cytometry might identify those lesions most at risk. The Hedley procedure represents a half-way method between cytology and histology, using a cytological methodology but starting with histological samples. Unfortunately, this approach is labor intensive and has a low yield for intact cells that are required for DNA image cytometry.
Our second platform (quantitative histology) is an alternate approach to the Hedley method in which assessments are made on quantitatively stained sectioned material. This system makes use of conventional 4 micron thick sections from formalin-fixed paraffin-embedded tissue that are stained with thionine. As such, one cannot calculate ploidy information on these samples (the nuclei are truncated by the sectioning); however, this is compensated by the system's ability to measure morphological and texture information on hundreds of diagnostic cells in situ.
A recent retrospective study evaluated the use of this computer-driven microscope imaging system as an adjunct tool to assist pathologists in judging the progression risk of oral premalignant lesions with no or low-grade (mild/moderate) dysplasia (23). As a training set, data was collected from 4,027 “normal” nuclei (selected from 29 normal oral biopsies) and from 4, 298 “abnormal” nuclei (selected from 30 SCC biopsies). Five of the system features that best differentiated between these 2 types of cells were combined to create a discriminant function that was called the Nuclear Phenotype Score (NPS). This NPS was then determined for a test set of 69 oral premalignant lesions. Elevated NPS was strongly associated with the both the presence of high-risk loss of heterozygosity (LOH) patterns and with risk of malignant progression, with a 10-fold increase in progression for dysplasias with high NPS. In the multivariate Cox model, LOH and NPS together were the strongest predictors for progression. Although promising, these results need to be confirmed on larger datasets and with prospectively collected samples.
Conclusions
It is recognized that we are at the first steps in the development and integration of new ways of measuring tissue change and that such tools will continue to evolve as we understand more about the biology underlying the disease. However, a new paradigm is developing for evaluation of tissue change of clinical significance. To build this paradigm effectively, it is critical that new technology be developed and evaluated appropriately. This involves an early determination of a best fit with respect to clinical niche and the evaluation of the technology in a manner that can be used to guide its further development in optimizing it for specific roles.
The advent of new technology has stimulated a renewed interest by clinicians in exploring new ways of both detecting and managing oral cancers and premalignant disease. This enthusiasm is being further fueled by a growing awareness of the disease through both news releases around this technology and campaigns focused on education of both clinicians and the general public (61). The challenge will be to best use this increased activity to begin to make a difference to patient outcome.
Acknowledgements
Supported by grants from the Institute of Dental and Craniofacial Research grants R01DE13124 and R01 DE017013. CFP is supported by Canadian Institutes of Health Research and Michael Smith Foundation for Health Research (MSFHR) and DML by MSFHR and BC Cancer Foundation.
References
- 1.Banda-Gamboa H, Ricketts I, Cairns A, Hussein K, Tucker JH, Husain N. Automation in cervical cytology: an overview. Anal Cell Pathol. 1992;4:25–48. [PubMed] [Google Scholar]
- 2.Barrellier P, Babin E, Louis MY, Meunier-Guttin A. The use of toluidine blue in the diagnosis of neoplastic lesions of the oral cavity. Rev Stomatol Chir Maxillofac. 1993;94:51–54. [PubMed] [Google Scholar]
- 3.Bocking A, Nguyen VQ. Diagnostic and prognostic use of DNA image cytometry in cervical squamous intraepithelial lesions and invasive carcinoma. Cancer. 2004;102:41–54. doi: 10.1002/cncr.11889. [DOI] [PubMed] [Google Scholar]
- 4.Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 5.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 6.Carothers A, McGoogan E, Vooijs P, Bird C, Colquhoun M, Eason P, McKie M, Nieuwenhuis F, Pitt P, Rutovitz D. A collaborative trial of a semi-automatic system for slide preparation and screening in cervical cytopathology. Anal Cell Pathol. 1994;7:261–274. [PubMed] [Google Scholar]
- 7.Christian DC. Computer-assisted analysis of oral brush biopsies at an oral cancer screening program. J Am Dent Assoc. 2002;133:357–362. doi: 10.14219/jada.archive.2002.0175. [DOI] [PubMed] [Google Scholar]
- 8.Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, Taylor R. The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation. Health Technol Assess. 2003;7:iii–v. 1–127. doi: 10.3310/hta7070. [DOI] [PubMed] [Google Scholar]
- 9.de Veld DC, Skurichina M, Witjes MJ, Duin RP, Sterenborg HJ, Roodenburg JL. Clinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopy. J Biomed Opt. 2004;9:940–950. doi: 10.1117/1.1782611. [DOI] [PubMed] [Google Scholar]
- 10.de Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–131. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Dhooge IJ, De Vos M, Van Cauwenberge PB. Multiple primary malignant tumors in patients with head and neck cancer: results of a prospective study and future perspectives. Laryngoscope. 1998;108:250–256. doi: 10.1097/00005537-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Downer MC, Moles DR, Palmer S, Speight PM. A systematic review of measures of effectiveness in screening for oral cancer and precancer. Oral Oncol. 2006;42:551–560. doi: 10.1016/j.oraloncology.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Drezek R, Brookner C, Pavlova I, Boiko I, Malpica A, Lotan R, Follen M, Richards-Kortum R. Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochem Photobiol. 2001;73:636–641. doi: 10.1562/0031-8655(2001)073<0636:AMOFCT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Drezek R, Sokolov K, Utzinger U, Boiko I, Malpica A, Follen M, Richards-Kortum R. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements, and implications. J Biomed Opt. 2001;6:385–396. doi: 10.1117/1.1413209. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JB, Feldman R, Dolor RJ, Porter SR. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck. 2003;25:911–921. doi: 10.1002/hed.10309. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JB, Gorsky M, Fischer D, Gupta A, Epstein M, Elad S. A survey of the current approaches to diagnosis and management of oral premalignant lesions. J Am Dent Assoc. 2007;138:1555–1562. doi: 10.14219/jada.archive.2007.0104. quiz 1614. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JB, Silverman S, Jr., Epstein JD, Lonky SA, Bride MA. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44:538–544. doi: 10.1016/j.oraloncology.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. IARC Press; Lyon: 2004. [Google Scholar]
- 19.Fetterman B, Pawlick G, Koo H, Hartinger J, Gilbert C, Connell S. Determining the utility and effectiveness of the NeoPath AutoPap 300 QC System used routinely. Acta Cytol. 1999;43:13–22. doi: 10.1159/000330862. [DOI] [PubMed] [Google Scholar]
- 20.Frazier HS, Mosteller F. Medicine Worth Paying for: Assessing Medical Innovations. Havard University Press; Cambridge, MA: 1995. [Google Scholar]
- 21.Gray M, Gold L, Elley A, Bursla A. The Effectiveness of Toluidine Blue Dye as Adjunct to Oral Cancer Screening in General Dental Practice. University of Birmingham Department of Public Health and Epidemiology; 2000. [Google Scholar]
- 22.Guillaud M, Benedet JL, Cantor SB, Staerkel G, Follen M, MacAulay C. DNA ploidy compared with human papilloma virus testing (Hybrid Capture II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmation. Cancer. 2006;107:309–318. doi: 10.1002/cncr.21993. [DOI] [PubMed] [Google Scholar]
- 23.Guillaud M, Zhang L, Poh C, Rosin MP, MacAulay C. Potential use of quantitative tissue phenotype to predict malignant risk for oral premalignant lesions. Cancer Res. 2008;68:3099–3107. doi: 10.1158/0008-5472.CAN-07-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 25.Hedley DW. DNA analysis from paraffin-embedded blocks. Methods Cell Biol. 1994;41:231–240. doi: 10.1016/s0091-679x(08)61721-5. [DOI] [PubMed] [Google Scholar]
- 26.Heintzelman DL, Utzinger U, Fuchs H, Zuluaga A, Gossage K, Gillenwater AM, Jacob R, Kemp B, Richards-Kortum RR. Optimal excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy. Photochem Photobiol. 2000;72:103–113. doi: 10.1562/0031-8655(2000)072<0103:OEWFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 28.Irwig L, Macaskill P, Farnsworth A, Wright RG, McCool J, Barratt A, Simpson JM. A randomized crossover trial of PAPNET for primary cervical screening. J Clin Epidemiol. 2004;57:75–81. doi: 10.1016/S0895-4356(03)00259-2. [DOI] [PubMed] [Google Scholar]
- 29.Isenstein LM, Zahniser DJ, Hutchinson ML. Combined malignancy associated change and contextual analysis for computerized classification of cervical cell monolayers. Anal Cell Pathol. 1995;9:83–93. [PubMed] [Google Scholar]
- 30.Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, Williams PM, Zhang L, Rosin MP, MacAulay CE. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11:024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 31.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Lingen MW. Improving translational research for oral cancer screening aids: putting my "money" where my mouth is. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:301–302. doi: 10.1016/j.tripleo.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littenberg B. Technology assessment in medicine. Acad Med. 1992;67:424–428. doi: 10.1097/00001888-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzato M, Bory JP, Cucherousset J, Nou JM, Bouttens D, Thil C, Dez F, Evrard G, Quereux C, Birembaut P, Clavel C. Usefulness of DNA ploidy measurement on liquid-based smears showing conflicting results between cytology and high-risk human papillomavirus typing. Am J Clin Pathol. 2002;118:708–713. doi: 10.1309/6NXC-V9XD-YM87-8FAE. [DOI] [PubMed] [Google Scholar]
- 36.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 37.Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:444–446. doi: 10.1016/s1079-2104(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 38.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:535–540. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 39.Partridge M, Emilion G, Pateromichelakis S, A'Hern R, Phillips E, Langdon J. Allelic imbalance at chromosomal loci implicated in the pathogenesis of oral precancer, cumulative loss and its relationship with progression to cancer. Oral Oncol. 1998;34:77–83. doi: 10.1016/s1368-8375(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 40.Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol. 2003;39:708–723. doi: 10.1016/s1368-8375(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 41.Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008;139:896–905. doi: 10.14219/jada.archive.2008.0276. quiz 993-894. [DOI] [PubMed] [Google Scholar]
- 42.Pavlova I, Sokolov K, Drezek R, Malpica A, Follen M, Richards-Kortum R. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem Photobiol. 2003;77:550–555. doi: 10.1562/0031-8655(2003)077<0550:maboon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Pavlova I, Williams M, El-Naggar A, Richards-Kortum R, Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Clin Cancer Res. 2008;14:2396–2404. doi: 10.1158/1078-0432.CCR-07-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer. 2008;123:1979–1990. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poate TW, Buchanan JA, Hodgson TA, Speight PM, Barrett AW, Moles DR, Scully C, Porter SR. An audit of the efficacy of the oral brush biopsy technique in a specialist Oral Medicine unit. Oral Oncol. 2004;40:829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Poh CF, Ng SP, Williams PM, Zhang L, Laronde DM, Lane P, Macaulay C, Rosin MP. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29:71–76. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 47.Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, Berean KW, Ng S, Tseng OL, MacAulay C, Rosin MP. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 48.Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. Jama. 1995;274:645–651. [PubMed] [Google Scholar]
- 49.Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- 50.Roblyer D, Kurachi C, Stepanek V, Williams MD, El-Naggar AK, Lee JJ, Gillenwater AM, Richards-Kortum R. Objective Detection and Delineation of Oral Neoplasia Using Autofluorescence Imaging. Cancer Prevention Research. 2009 doi: 10.1158/1940-6207.CAPR-08-0229. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roblyer D, Richards-Kortum R, Sokolov K, El-Naggar AK, Williams MD, Kurachi C, Gillenwater AM. Multispectral optical imaging device for in vivo detection of oral neoplasia. J Biomed Opt. 2008;13:024019. doi: 10.1117/1.2904658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol. 1989;67:621–627. doi: 10.1016/0030-4220(89)90286-7. [DOI] [PubMed] [Google Scholar]
- 53.Rosin MP, Lam WL, Poh C, Le ND, Li RJ, Zeng T, Priddy R, Zhang L. 3p14 and 9p21 loss is a simple tool for predicting second oral malignancy at previously treated oral cancer sites. Cancer Res. 2002;62:6447–6450. [PubMed] [Google Scholar]
- 54.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, Rajan B. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 55.Scheifele C, Schmidt-Westhausen AM, Dietrich T, Reichart PA. The sensitivity and specificity of the OralCDx technique: evaluation of 103 cases. Oral Oncol. 2004;40:824–828. doi: 10.1016/j.oraloncology.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz RA, Gao W, Daye D, Williams MD, Richards-Kortum R, Gillenwater AM. Autofluorescence and diffuse reflectance spectroscopy of oral epithelial tissue using a depth-sensitive fiber-optic probe. Appl Opt. 2008;47:825–834. doi: 10.1364/ao.47.000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz RA, Gao W, Redden Weber C, Kurachi C, Lee JJ, El-Naggar AK, Richards-Kortum R, Gillenwater AM. Noninvasive evaluation of oral lesions using depth-sensitive optical spectroscopy. Cancer. 2009 doi: 10.1002/cncr.24177. 2009 Jan 23. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sciubba JJ. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J Am Dent Assoc. 1999;130:1445–1457. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 59.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 60.Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, Augustovski F. The cost-effectiveness of screening for oral cancer in primary care. Health Technol Assess. 2006;10:1–144. iii–iv. doi: 10.3310/hta10140. [DOI] [PubMed] [Google Scholar]
- 61.Stahl S, Meskin LH, Brown LJ. The American Dental Association's oral cancer campaign: the impact on consumers and dentists. J Am Dent Assoc. 2004;135:1261–1267. doi: 10.14219/jada.archive.2004.0401. [DOI] [PubMed] [Google Scholar]
- 62.Stenkvist B, Bergstrom R, Brinne U, Hesselius I, Kiviranta A, Nordgren H, Schnurer L, Stendahl U, Stenson S, Soderstrom J. Automatic analysis of Papanicolaou smears by digital image processing. Gynecol Oncol. 1987;27:1–14. doi: 10.1016/0090-8258(87)90225-3. [DOI] [PubMed] [Google Scholar]
- 63.Sun XR, Wang J, Garner D, Palcic B. Detection of cervical cancer and high grade neoplastic lesions by a combination of liquid-based sampling preparation and DNA measurements using automated image cytometry. Cell Oncol. 2005;27:33–41. doi: 10.1155/2005/981612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surveillance E, End Results (SEER) Program, National Cancer Institute Surveillance Research Program . Oral cancer 5-year survival rates by race, gender, and stage of diagnosis. Vol. 2008. US National Institutes of Health; 2006. [Google Scholar]
- 65.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Kummer JA, Leemans CR, Braakhuis BJ. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin Cancer Res. 2004;10:3607–3613. doi: 10.1158/1078-0432.CCR-03-0632. [DOI] [PubMed] [Google Scholar]
- 66.Tabor MP, Brakenhoff RH, van Houten VM, Kummer JA, Snel MH, Snijders PJ, Snow GB, Leemans CR, Braakhuis BJ. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523–1532. [PubMed] [Google Scholar]
- 67.Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35:227–233. doi: 10.1016/s1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 68.Tolles WE, Bostrom RC. Automatic screening of cytological smears for cancer: the instrumentation. Ann N Y Acad Sci. 1956;63:1211–1218. doi: 10.1111/j.1749-6632.1956.tb32131.x. [DOI] [PubMed] [Google Scholar]
- 69.Tolles WE, Horvath WJ, Bostrom RC. A study of the quantitative characteristics of exfoliated cells from the female genital tract. I. Measurement methods and results. Cancer. 1961;14:437–454. doi: 10.1002/1097-0142(199005/06)14:3<437::aid-cncr2820140302>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 70.Tucker J, Stenkvist B. Whatever happened to cervical cytology automation? Anal Cell Pathol. 1990;2:259–266. [PubMed] [Google Scholar]
- 71.US Preventive Service Task Force . Guide to Clinical Preventive Services: An Assessment of the Effectiveness of 169 Interventions. 1989. [Google Scholar]
- 72.Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScan (R) toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97–103. doi: 10.1111/j.1600-0714.1996.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 73.Wied GL, Bartels PH, Bahr GF, Oldfield DG. Taxonomic intracellular analytic system (TICAS) for cell identification. Acta Cytol. 1968;12:180–204. [PubMed] [Google Scholar]
- 74.Williams PM, Poh CF, Hovan AJ, Ng SP, Rosin MP. Evaluation of a suspecious oral lesion. JCDA. 2008;74:275–280. [PubMed] [Google Scholar]
- 75.Wood R. Secret communications concerning light rays. J Physiol. 1919;5e [Google Scholar]
- 76.Yeole BB, Ramanakumar AV, Sankaranarayanan R. Survival from oral cancer in Mumbai (Bombay), India. Cancer Causes Control. 2003;14:945–952. doi: 10.1023/b:caco.0000007965.61579.b2. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Ng S, Poh CF, Williams PM, Laronde DM, MacAulay C, Rosin MP. Fluorescence visualization identifies primary oral premalignant lesions (OPL) with high-risk molecular patterns. Paper presented at the 99th Annual Meeting of the American Association for Cancer Research; San Diego. 12-16 April, 2008.2008. [Google Scholar]
- 78.Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, Durham S, Nakamura H, Berean K, Hovan A, Le ND, Hislop G, Priddy R, Hay J, Lam WL, Rosin MP. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–8021. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]