Abstract
Protein phosphatase 2A (PP2A) is a serine and threonine protein phosphatase that regulates cell cycle progression and apoptosis. PP2A is composed of various subunits. Among these subunits, subunit B plays an important role in the modulation of PP2A function in the brain. This study investigated PP2A subunit B expression levels after neuronal cell injury. Middle cerebral artery occlusions (MCAO) were surgically induced in adult male rats to induce focal cerebral ischemic injury, and brain tissues were collected 24 h after MCAO. A proteomic approach revealed reduction of PP2A subunit B protein spots in MCAO-operated animals in comparison to sham-operated animals. Western blot analysis confirmed that MCAO induces reductions in PP2A subunit B levels. Moreover, glutamate exposure induces neuronal cell death and leads to reductions of PP2A subunit B levels in a hippocampal-derived cell line. This study demonstrated the decrease of PP2A subunit B in ischemic neuronal cell injury. These results suggest that the decrease of PP2A subunit B after ischemic brain injury can mediate neuronal cell death.
Keywords: Glutamate, middle cerebral artery occlusion, PP2A subunit B
Stroke is a major cause of death from cerebral ischemia. Cerebral ischemia leads to serious neuronal cell damage and induces disruption of neuronal function. Middle cerebral artery occlusion (MCAO) is used as a representative animal model of ischemic brain injury by inducing serious infarct lesions and neuronal cell damage (Longa et al, 1989; Ferrer and Planas, 2003; Koh, 2010). A previous study showed that focal cerebral ischemic injury caused by MCAO leads to up- and down-regulation of proteins that mediate neuronal cell death (Koh, 2010).
Phosphorylation and dephosphorylation mechanisms of signal transduction related proteins are crucial for cell survival (Siesjö, 1988). Protein phosphatase 2A (PP2A) is an important serine and threonine phosphatase and a critical enzyme within various signal transduction pathways, as it regulates essential cellular activities such as cell cycle progression, cell division, apoptosis and development (Milward, et al, 1999; Zolnierowicz, 2000; Janssens and Goris, 2001; Sontag, 2001). PP2A is a heterotrimeric phosphatase that is composed of scaffolding/structural A, regulatory/targeting B, and catalytic C subunits (Stone et al, 1987). A and C subunits of PP2A are ubiquitously expressed, while B subunit is highly enriched in the brain (Strack et al, 1998; Janssens and Goris, 2001). The A and C subunits contribute to form a core, while the regulatory B subunit confers to the PP2A holoenzymes and facilitates various functions of PP2A. PP2A subunit B plays special roles in the nervous system (Strack et al, 1998), but little information is available regarding PP2A subunit B expression levels after ischemic brain injury. In this study, a proteomic approach revealed decreases of PPA2 proteins in an animal model of focal cerebral ischemia. We investigated PP2A subunit B expression levels in focal cerebral ischemic brain injury and in glutamate-exposed hippocampal neurons.
All animal experiments were followed a protocol approved by the Committee for Animal Experimentation at the Gyeongsang National University. Adult male Sprague-Dawley rats (210-220 g, n=20) were provided from Samtako Animal Breeding Center (Osan, Korea) and were randomly divided into two groups, sham-operated group and MCAO-operated group (n=10 per group). Animals were housed in temperature (25℃) and lighting-controlled (14L:10D) environment, and allowed free access to food and water.
Focal cerebral ischemic injury was induced by MCAO operation (Longa et al, 1989). Rats were anesthetized with sodium pentobarbital (100 mg/kg). A 4/0 nylon suture with its tip rounded by heating was introduced into the common carotid artery and advanced into the right internal carotid artery to the base of the right middle cerebral artery. Animals were scarified 24 h after MCAO for the investigation in late stage of apoptosis. The right cerebral cortex was eliminated. The tissues were homogenized in lysis buffer (8 M urea, 4% CHAPS, ampholytes, and 40 mM Tris-HCl) and centrifuged at 16,000 g for 20 min at 4℃. The total protein concentration was determined using the Bradford method (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol.
A proteomic analysis was performed as previously described method (Koh, 2010). The first dimensional gel electrophoresis was carried out with the following steps. The IPG strips (IPG, 17 cm, Bio-Rad) were re-hydrated in rehydration buffer [8 M urea, 2% CHAPS, 20 mM dithiothreitol (DTT), 0.5% IPG buffer, bromophenol blue] at room temperature that contained lysed proteins for 13 h. The isoelectric focusing (Protean IEF Cell, Bio-Rad) for pH 4-7 and pH 6-9 was performed as followed steps: 250 V (15 min), 10,000 V (3 h), and then 10,000 V to 50,000 V. For the second dimension, strips were equilibrated in 50 mM Tris, 6 M urea, 30% glycerol, 2% sodium dodesyl sulfate (SDS) and 1% DTT for 10 min. 2D gel electrophoresis (Protein-II XI, Bio-Rad) was carried out using gradient gels (7.5-17.5%) at constant 10 mA/gel when the dye reached end of the gels. The gels were fixed in a solution (12% acetic acid, 50% methanol). The fixed gels finally stained with a sliver solution (0.2% silver nitrate, 0.75 mL/L formaldehyde). The images of stained gels were scanned by Agfar ARCUS 1200™ (Agfar-Gevaert, Mortsel, BEL). The protein spots were detected and their intensities were analyzed using PDQuest software (Bio-Rad). Differentially expressed proteins were identified in sham-operated and MCAO-operated animals. The differentially expressed protein spots were cut and distained. The gel pieces were reacted with reduction solution (20 mM DTT in 0.1 M NH4HCO3) and in alkylation solution (55 mM idoacetamide in 0.1 M NH4HCO3) for 10 min. After dehydration by acetonitrile, the proteins in gel particles were cleaved with trypsin-containing digestion buffer (Promegea, Madison, WI, USA). The extract peptides were used for MALDI-TOF mass spectrometry (Voyager-DE™ STR biospectrometry workstation, Applied Biosystem, Forster City, CA, USA). The spectra for MALDI-TOF were analyzed using MS-Fit and ProFound program for identification. SWISS-PROT and NCBI were used as the protein sequence databases.
A mouse hippocampal cell line (HT22) was grown in Dulbecco's modified Eagle's medium (DMEM, without L-glutamine) with 10% fetal bovine serum (FBS), streptomycin (100 µg/mL), and penicillin (100 unit/mL) (Gibco BRL, Gaithersburg, MD, USA). HT22 cells were incubated at 37℃ in a humidified incubator with 5% CO2 and 95% air. The cells were seeded on 60-mm culture dishes at 100,000 cells per dish. After 24 h, cell density was closely monitored to prevent excessive growth and was maintained 70% or less confluence as described previously (Maher and Davis, 1996; Koh, 2007). The medium was changed and cells were treated with 5 mM glutamate (Sigma; St. Louis, MO, USA) for 24 h. Ethanol was used at a final concentration of 0.1% as a vehicle control. This concentration of ethanol had no effect on cell viability or glutamate toxicity. The cells were collected for the Western blot analysis.
For the Western blot analysis, protein samples (30 µg) per lane were loaded to 10% SDS-polyacrylamide gels and separated by electrophoresis. Proteins were transferred to a poly-vinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk and washed in Tris-buffered saline containing 0.1% Tween-20 (TBST). The blots were incubated with the following antibodies: anti-PP2A subunit B (diluted 1:1,000, Cell Signaling Technology, Beverly, MA, USA) and anti-actin antibodies (diluted 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibodies. The membranes were sequentially reacted with secondary antibody (1:5,000; Pierce, Rockford, IL, USA). The immunoblots were visualized on X-ray film using the ECL Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The intensity analysis was carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA). All data are expressed as mean±SEM. The results in each group were compared by one-way analysis of variance (ANOVA) followed by Student's t-test.
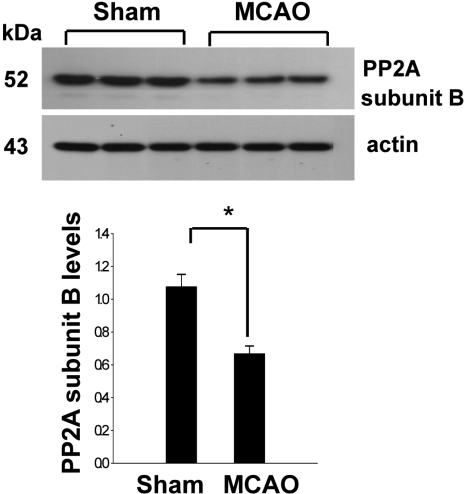
In a proteomic approach, PP2A subunit B protein spots were identified as protein spots with more than two-fold changes in intensity of the cerebral cortices in MCAO-operated and sham-operated rats (Figure 1). The peptide mass of PP2A subunit B is 9/56 and the sequence of this protein is 29%. PP2A subunit B protein levels are decreased in MCAO-operated animals compared to sham-operated animals. Western blot analysis clearly showed that MCAO injury induces decreases in PP2A subunit B levels (Figure 2). The levels of PP2A subunit B were 1.05±0.06 and 0.64±0.04 in the cerebral cortices of sham-operated and MCAO-operated animals, respectively. Glutamate toxicity induces oxidative stress and leads to neuronal cell death in HT22 cells (Koh, 2007). In Figure 3, glutamate exposure is observed to induce reductions of PP2A subunit B levels in HT22 cells. The levels of PP2A subunit B were 0.94±0.03 in the vehicle-treated group, and 0.38±0.02 in the glutamate-treated group, respectively (Figure 3).
Figure 1.
Protein phosphatase 2A (PP2A) subunit B protein spots identified by MALDI-TOF. Arrows indicate the protein spots. The intensity of spots was measured using PDQuest software. The ratio of intensity is described as spots intensity of middle cerebral artery-occluded (MCAO) animal to spots intensity of sham-operated animal. Data are shown as mean±SEM. *P<0.05 (vs. Sham).
Figure 2.
Western blot analysis of protein phosphatase 2A (PP2A) subunit B in the cerebral cortex from sham-operated and middle cerebral artery-occluded (MCAO) animals. Each lane represents an individual experimental animal. Densitometric analysis of PP2A levels is represented as intensity of PP2A to intensity of actin. Data are shown as mean±SEM. *P<0.05 (vs. Sham).
Figure 3.
Western blot analysis of protein phosphatase 2A (PP2A) subunit B in HT22 cells. Glutamate (5 mM) or vehicle was exposed to HT22 cells for 24 h. Each lane represents an individual experimental animal. Densitometric analysis of PP2A levels is represented as intensity of PP2A to intensity of actin. Data are shown as mean±SEM. *P<0.05 (vs. Vehicle).
MCAO is well accepted as a general method to induce focal cerebral cortex ischemia in experimental animal models (Longa et al, 1989; Ferrer and Planas, 2003; Koh, 2010). Cerebral ischemia by MCAO leads to neuronal cell death through various signaling pathways (Li et al, 1997; Ferrer and Planas, 2003). This study demonstrates the down-regulation of PP2A subunit B protein in an animal model of MCAO-induced ischemic brain injury.
In this study, a decrease of PP2A subunit B proteins was observed after MCAO-induced brain injury using a proteomic approach. Western blot analysis confirmed that ischemic brain injury significantly decreases PP2A subunit B levels. A previous study demonstrated that the activity of PP2A is down-regulated in Alzheimer's disease and that the disruption of PP2A is involved in neurodegenerative disease (Gong et al, 2000). Moreover, decreased PP2A activity in the brain is involved in intracellular neurofibirillary tangle formation and neurodegeneration (Liu and Wang, 2009). PP2A binds and dephosphorylates the neuronal microtubule-associated protein tau (Sontag et al, 1996). Tau promotes tubulin polymerization and stabilizes microtubules, while hyperphosphorylated tau induces axonal degeneration in Alzeheimer's disease and leads to central nervous system disorders (Liu and Wang, 2009). A regulatory B subunit confers cell specificity to the PP2A holoenzyme in the brain and regulates PP2A activity (Janssens and Goris, 2001). The results of this study indicate that decrease of PP2A subunit B proteins during MCAO injury induces the phosphorylation of tau and leads to neuronal cell death and damage. An in vitro study indicated that glutamate toxicity induces oxidative stress and leads to neuronal cell death. Oxidative stress impedes redox homeostasis and consequently causes cell death. Previous studies demonstrated that glutamate exposure leads to ischemic conditions in HT22 cells and results in neuronal cell death (Maher and Davis, 1996; Koh, 2007). The present study found that glutamate exposure induces decreases in PP2A subunit B levels in HT22 cells. A balance between protein kinase and phosphatase activity is necessary to maintain normal cellular function, and therefore phosphatases are as tightly controlled as kinases. The results of this study suggest that decreased PP2A activity after ischemic brain injury disturbs the balance of protein kinase and phosphatase activity and leads to neurodegenerative conditions. Although further studies are necessary to disclose the role of PP2A subunit B in brain injury, the results of the present study demonstrate that decreases of PP2A subunit B levels in MCAO-induced injury and in glutamate-exposed HT22 cells regulate neuronal cell death. Thus, these findings suggest that decreases in PP2A subunit B can lead to neuronal cell death during ischemic brain injury.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0007881).
References
- 1.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle inthe penumbra. J Neuropathol Exp Neurol. 2003;62(4):329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 2.Gong CK, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275(8):5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 3.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(Pt 3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh PO. 17Beta-estradiol prevents the glutamate-induced decrease of Akt and its downstream targets in HT22 cells. J Vet Med Sci. 2007;69(3):285–288. doi: 10.1292/jvms.69.285. [DOI] [PubMed] [Google Scholar]
- 5.Koh PO. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010;72(2):181–185. doi: 10.1292/jvms.09-0364. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997;765(2):301–312. doi: 10.1016/s0006-8993(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Wang JZ. Protein phosphatase 2A in Alzheimer's disease. Pathophysiology. 2009;16(4):273–277. doi: 10.1016/j.pathophys.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 9.Maher P, Davis JB. The role of monoamine metabolism inoxidative glutamate toxicity. J Neurosci. 1996;16(20):6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24(5):186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 11.Siesjö BK. Mechanisms of ischemic brain damage. Crit Care Med. 1988;16(10):954–963. doi: 10.1097/00003246-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17(6):1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 13.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13(1):7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 14.Stone SR, Hofsteenge J, Hemmings BA. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit ofprotein phosphatase 2A. Biochemistry. 1987;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- 15.Strack S, Zaucha JA, Ebner FF, Colbran JR, Wadzinski BE. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392(4):515–527. [PubMed] [Google Scholar]
- 16.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60(8):1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]