Abstract
Osteonecrosis of the femoral head is an idiopathic, debilitating and progressive disease. A number of traumatic or non-traumatic animal models have been reported for research on osteonecrosis. This study was performed to compare the efficacy of femoral head osteonecrosis in rabbits by traumatic and non-traumatic methods. Twenty-seven New Zealand White rabbits were divided into three experimental groups, nine heads each. Two groups were surgically induced into osteonecrosis; a steel cerclage wire was ligated tightly around the neck of the right femoral head (Group W), and the femoral neck was tied with a cerclage wire in the same way as in the W group, and burned by attachment of an electrode tip to the wire and then the wire was removed (Group B). The other group was induced into osteonecrosis with a single intra-muscular injection of 20 mg/kg methyl-prednisolone acetate single injection (Group M). In the control group, the left femoral head of animals in group W and B was used. After two weeks, rabbits were sacrificed and the femoral head and neck were collected. Osteonecrosis of the femoral head was evaluated by radiography, histology and immunohistology methods. Osteonecrosis lesions in the femoral head were identified in traumatic models of groups W and B. Cartilage degeneration in the superficial layer and TUNEL positive cells in the femoral head were detected more in Group B than in Group W. These findings revealed that short-term induced osteonecrosis of the femoral head was effectively achieved by cautery around the femoral neck.
Keywords: animal model, bone, cartilage, osteonecrosis, rabbit
Osteonecrosis of the femoral head is an idiopathic, debilitating and progressive disease, with various causes including disruption of the blood supply to the femoral head and results in increased intraosseous pressure. Etiologies include alcoholism, blood disorders, radiation therapy, trauma, corticosteroid administration and dysbaria [1]. It is generally accepted that osteonecrosis of the femoral head includes the pathway of ischemia with trabeculae, marrow-cell and osteocyte necrosis [2].
A number of traumatic models have been implemented in experimental animals. In rabbits, traumatic modelings include use of ligamentum teres resection, in conjunction with a steel cerclage wire tightened around the femoral neck [3] and ligamentum teres excision with the femoral neck osteotomy [4]. Ligamentum teres resection and nylon ligature tightly around the femoral neck was used [5] and chromic ligature around the femoral neck outside of the capsule to interrupt blood flow to the femoral head in rabbits was introduced [6]. Recently, the periosteum and blood vessels covering the femoral neck were cauterized circumferentially and a 3 mm unicortical drill hole was made; the marrow cavity was then cauterized in order to interrupt the intramedullary blood supply to the femoral head [7]. In rats, the femoral neck was burned using electro-cautery to disrupt the blood supply to the femoral head [8,9]. Incising the periosteum at the cervical base of the femoral head was used in order to lead coagulation necrosis of the femoral head epiphysis in rats [10].
Non-traumatic osteonecrosis of the femoral head may be caused by different conditions; however, the most common is corticosteroid administration. Osteonecrosis of the femoral head was found to have a serious complication associated with corticosteroid treatment. Association of thromboembolism, fat embolism, blood flow impairment, increased intraosseous pressure, and intramedullary hemorrhage with corticosteroid-induced osteonecrosis has been reported [1]. Recent studies have demonstrated that, in rabbits, methylprednisolone was able to cause corticosteroid-induced osteonecrosis [11].
Since the late 1960s, various techniques have been used for treatment of osteonecrosis of the femoral head; however, it remains difficult to treat [12]. If left untreated, the disease progresses and leads to joint pain, loss of function and collapse of the femoral head and hip joint destruction [13]. On this score, confirmation of the most effective osteonecrosis modeling is necessary from among the various modeling methods for prevention and treatment of the disease.
In this study, we compared the efficacy of osteonecrosis modeling of the femoral head between cerclage wiring around the femoral head, burning the femoral neck by contact of the wire with monopolar electro-cautery and a single high-dose injection of methylprednisolone acetate. The effectiveness of the above procedure was confirmed by radiography, histology such as haematoxylin and eosin and safranin O, and immunohistology such as TUNEL methods.
Materials and Methods
Experimental animals
Twenty-seven New Zealand White rabbits (16 weeks, male, 2.6 to 3.1 kg) were used for the experiments after an acclimation period of about a week. Rabbits were housed in single cages at the Laboratory Animal Research Center of Chungbuk National University and maintained on a 12/12-hour light/dark cycle, standard laboratory diet and water, 23±2℃ temperature, 55±10% humidity, 12 times/hr ventilation, 150-300 luces illumination. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC), the Laboratory Animal Research Center of Chungbuk National University.
Osteonecrosis induction
Twenty-seven rabbits were divided into three groups, nine heads each. Animals were anesthetized and a proximal longitudinal skin incision centered over the cranial border of the greater trochanter was made after preparation of the surgical site. The abductor muscle was split from the greater trochanter to the third trochanter. The gluteus superficialis muscle was reflected caudally in order to visualize the greater trochanter. Osteotomy of the greater trochanter was performed using a high-speed drill and an oscillating saw. After incision of the joint capsule, the ligamentum teres was cut and the femoral head was subluxated. The circulation of the femoral head was obstructed surgically in two ways. In the wiring induced (W) group, the femoral neck was tied with a wire (diameter 0.4 mm), and in the burning induced (B) group, the area was tied with a wire in the same way as in the W group, and burned by attachment of the electrode tip to the wire for 5 seconds. Wire was removed after burning. After the femoral head was brought back to the original position, the greater trochanter was fixed with an intramedullary pin and the incised muscle and skin were sutured. We used the left femoral head of groups A and B for the control group. Nine rabbits receved a single intramuscular injection with 20 mg/kg methyprednisolone acetate (M group). Rabbits were sacrificed 2 weeks after procedures for detection of short-term induced osteonecrosis.
Radiographic study
Radiographs of bone tissue were taken with DXG-525RF (Dong-A X-ray co. Ltd., Korea). The radiological images were digitalized by means of Directview CR500 (Kodak, Japan), and enlarged ×100 to be analyzed for sphericity. The picture was adjusted using the eFilm Workstation® 2.1 (Merge Healthcare, Milwaukee, USA). In this study, the radiological features of the femoral head were evaluated in 3 types; round type (no sign of deformation or fracture), flat type (deformation of femoral head without bone fracture), and crushed type (deformation and bone fracture of femoral head) [14].
Histological examinations
All bone samples were fixed in 10% formalin and decalcified. After cutting along the axial plane, they were embedded in paraffin. The samples were cut to 4 mm thick, and stained with hematoxylin and eosin and safranin O. For evaluation of necrotic bone and cartilage degeneration, we used the method of Levin et al. [15]. Histological analyses were performed for observation of osteonecrotic change and cartilage degeneration in the femoral heads. Evidenced by optically empty osteocytic lacunae [16], the extent of bone necrosis was assigned a score of 1+ when less than one-third of the femoral head is affected, 2+ when one-to two-thirds thereof is affected, and 3+ when over two-thirds thereof is affected, respectively. Articular cartilage degeneration was marked 1+ where loss of metrical basophil and safraninophilia in the femoral head. In 2+ scored instances, the cartilage was thinned, chondrocytes were randomly distributed, some chondrocytic clones were evident, and a filmy fibrous pannus covered the surface. 3+ indicates that the cartilage was fibrillated, clefted, and focally acellular covered by a proteinaceous layer.
TUNEL Assays
To evaluate cell fragmentation of DNA, which is known to be associated with apoptotic cell death, an Apoptag® S7101 kit (Chemicon International Inc, Temecula, USA) was used.
Statistical analysis
All of the measured values of necrotic change and cartilage degeneration are expressed in average±standard errors. Significant differences were determined using one-way ANOVA with the TUKEY post hoc test. A value of P<0.05 was considered significant.
Results
Radiological analysis
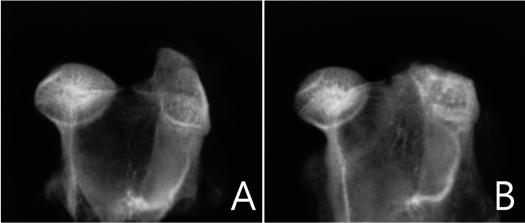
No significant differences in osteonecrotic changes of the femoral head were observed between each group in radiographic evaluation. The radiographic morphology of the femoral heads of rabbits was round in all groups. No collapse or changes such as flat and crushed types were identified in the femoral head in all groups (Figure 1).
Figure 1.
Digital radiograghic appearances of the normal (A) and osteonecrotic (B) femoral head. Radiodensity is increased in the osteonecrotic femoral head. No collapse or change was observed in femoral head sphericity.
Histological analysis
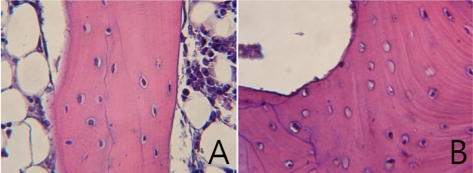
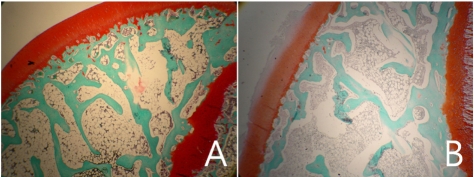
Normal femoral heads of the control group showed osteocyte lacunae containing well-stained nuclei of osteocytes, intratrabecular chondrocytes and epiphyseal bone marrow cells (Figure 2A). Normal femoral heads had normal articular cartilage and physis (Figure 3A). To the contrary, in the osteonecrosis-induced group, trabecular bone exhibited an accumulation of bone marrow cell debris and the appearance of empty osteocyte lacunae. The bone marrow space was filled with dead cells and debris and condensed nucleus (Figure 2B). Cartilage degenerations including disappearances of basophil and safraninophilia were observed in the superficial layer of the femoral head in the osteonecrosis-induced group. On the cartilage degeneration surface of the femoral head, weakly stained necrotic change was observed due to decrease in the proteoglycan ingredient (Figure 3B).
Figure 2.
Histology of the epiphyseal region in the femoral head (H&E stain). Magnification ×400. A. Normal femoral head in the control group shows well stained nuclei of osteocytes and hematopoietic tissue, and normal trabecular bone and bone marrow. B. Osteonecrotic lesions in the femoral head of group B. The osteonecrotic lucanae are empty. Necrotic cells were observed in intertrabecular spaces.
Figure 3.
Histology of the epiphyseal cartilage region in the femoral head (Safranin O stain). Magnification ×400. A. Articular cartilage of the normal femoral head. B. Degenerated articular cartilage with fibrillation and loss of glycosaminoglycans of the femoral head in group B.
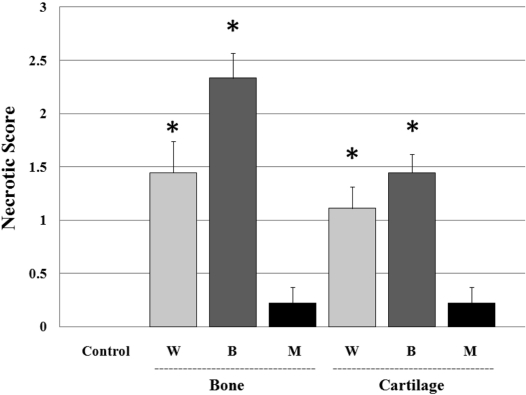
Average bone necrotic scores by group were as follows: the control group: 0; the W group: 1.4; B group: 2.3; M group: 0.2 and the average of the cartilage necrotic scores by group were as: the control group: 0; the W group: 1.1; B group: 1.4, M group: 0.2; where the W and B groups showed a significant extent of necrosis (P<0.05) and the B group showed necrotic lesions, which were increased compared with the W group (Figure 4).
Figure 4.
The average necrotic score of each group in trabecular bone and cartilage of the femoral heads. W group: wiring induced group, B group: burning induced group, M group: steroid induced group. Necrotic bone and cartilage changes were found to be significantly high in the W group and B group, compared with the control and M group (P<0.05).
Detection of apoptosis by TUNEL
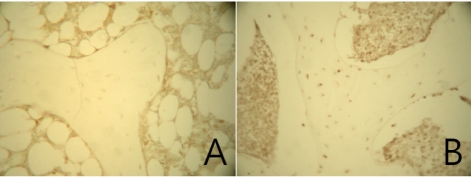
To clarify the effects by each experimental group, the incidence of apoptosis among femoral heads was examined using TUNEL assays (Figure 5). In the experimental groups, TUNEL-positive reactions were shown clearly with yellow to brown colors. On the contrary, in the control group, no TUNEL-positive cells were observed. The TUNEL-positive cells were confirmed in epiphyseal lesions of the femoral head where osteonecrosis was manifest from histological analysis.
Figure 5.
TUNEL labeling of the epiphyseal trabecular bone in the femoral head. Magnification ×400. A. No TUNEL positive cells or bone marrow cells were observed in the normal femoral head of the control group. B. TUNEL positive cells were observed. The TUNEL reaction shows apoptotic bodies in osteocytes and bone marrow cells.
Discussion
Osteonecrosis of the femoral head is a common disorder in a relatively young patient population, with an average age of mid-thirties. It is estimated to afflict approximately 15,000 new human patients per year in the United States [17]. Traumatic and non-traumatic osteonecrosis of the femoral head is assumed to have an ischaemic origin and blood supply disorder although the pathomechanism of osteonecrosis is still not clear. However, it is impressed that the pathology is quiet similar even with variable etiological factors [18]. Osteonecrosis of the femoral head progresses and leads to joint pain, loss of function, collapse of the femoral head and hip joint destruction [13]. To date, many studies have reported on prevention and treatment of femoral head osteonecrosis using animal models. Although differences of osteonecrosis development mechanism exist between humans and animals, there may be several common pathways which lead to osteonecrosis development [19]. Successful prevention and treatment of osteonecrosis of the femoral head is hampered by the lack of an ideal experimental animal model of the disease.
In this study, we compared the efficacy of osteonecrosis modeling of the femoral head by traumatic methods, including wiring, burning and non-traumatic methods including methylprednisolone injection in immature New Zealand White rabbits.
In group W, placement of steel cerclage wire ligature was used to interrupt the blood supply using previous described methods [3,5,6]. In group B, burning around the femoral neck was used, similar to the methods of Hofstaetter et al. [7]. The method of burning around the femoral neck using bipolar electro-cautery in rats has been reported [8]. However, there are some distinctions between our method and the above methods. We did not make the drill hole in the femoral neck and burn the marrow cavity. Another difference is as follows, we used steel cerclage wire ligature before burning the femoral neck using monopolar electro-cautery. We placed the wire tightly around the femoral neck and burned the femoral neck by contact of the wire with electro-cautery. It is supposed that the effect of electro-cautery was very transmissible all around the femoral. In group M with injection of methylprednisolone acetate, 20 mg/kg, was used according to a previous described method. A previous study in rabbit, demonstrated histologically that a single dose of methylprednisolone 20 mg/kg could induce osteonecrosis [11].
In the present study, osteonecrosis lesions in the femoral head were identified in traumatic models of wiring and burning methods. According to histological analysis, it seems that methylprednisolone acetate injection was not effective for induction of early stage osteonecrosis. Wiring and burning methods appear to be very effective for induction of bone necrosis (P<0.05). Although no significant difference was observed between the wiring and burning method, the burning method appeared to be more effective according to the necrotic bone score. In articular cartilage change, which is a later stage of osteonecrosis than bone necrosis, we confirmed that the burning method was more effective for induction of progressive osteonecrosis (P<0.05). Regulation of the contacting and burning time can induce various degrees of osteonecrosis.
These results suggested that osteonecrosis of the femoral head in rabbits was made effectively by short-term induced trauma by cautery around the femoral head. It is expected that these results will be helpful in study on prevention and treatment of osteonecrosis in the femoral head using effective and ideal animal models.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00656).
References
- 1.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br. 1957;39-B:358–394. doi: 10.1302/0301-620X.39B2.358. [DOI] [PubMed] [Google Scholar]
- 3.Rokkanen P, Slatis P. Effect of compression on the healing of subcapital osteotomies of the femoral neck and on the avascularized femoral head. An experimental study on adult rabbits. Acta Orthop Scand. 1967;38:163–173. doi: 10.3109/17453676708989630. [DOI] [PubMed] [Google Scholar]
- 4.Kenzora JE, Steele RE, Yosipovitch ZH. Experimental osteonecrosis of the femoral head in adult rabbits. Clin Orthop. 1978;130:8–46. [PubMed] [Google Scholar]
- 5.Rösingh GE, James J. Early phases of avascular necrosis of the femoral head in rabbits. J Bone Joint Surg Br. 1969;51:165–174. [PubMed] [Google Scholar]
- 6.Robichon J, Desjardins JP, Koch M, Hooper CE. The femoral neck in Legg-Perthes' disease. Its relationship to epiphysial change and its importance in early prognosis. J Bone Joint Surg Br. 1974;56:62–68. [PubMed] [Google Scholar]
- 7.Hofstaetter JG, Wang J, Yan J, Glimcher MJ. Changes in bone microarchitecture and bone mineral density following experimental oteonecrosis of the hip in rabbits. Cells Tissues Organs. 2006;184:138–147. doi: 10.1159/000099620. [DOI] [PubMed] [Google Scholar]
- 8.Yamako G, Tokunaga K, Takano R, Endo N, Hara T. Morphological and mechanical evaluation of the cancellous bone in the rat femoral head after traumatic osteonecrosis. J Biomech Sci Eng. 2006;1:195–203. [Google Scholar]
- 9.Kim J, Park J, Choi SH, Kim G. A comparison of surgical methods of inducing femoral head osteonecrosis in rats. J Vet Clin. 2010;27:240–245. [Google Scholar]
- 10.Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. Int J Exp Pathol. 1998;79:173–181. doi: 10.1046/j.1365-2613.1998.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effect of pulse methylprednisolone on bone and marrow tissue: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. doi: 10.1002/art.1780401119. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Wu X, Mei R, Yang C, Li J, Xu W, Ye S. Biomaterial-loaded allograft threaded cage for the treatment of femoral head osteonecrosis in a goat model. Biotechnol Bioeng. 2008;100:560–566. doi: 10.1002/bit.21792. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-osteonecrotic lesions of the hip. J Bone Joint Surg. 2004;86:2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama T, Nishida K, Yorimitsu M, Doi H, Miyazawa S, Kitamura A, Yoshida A, Nasu Y, Abe N, Ozaki T. Decreased levels of insulin-like growth Factor-1 and vascular endothelial growth factor relevant to the ossification disturbance in femoral heads spontaneous hypertensive rats. Acta Med Okayama. 2006;60:141–148. doi: 10.18926/AMO/30749. [DOI] [PubMed] [Google Scholar]
- 15.Levin D, Norman D, Zinman C, Rubinstein L, Sabo E, Misselevich I, Reis D, Boss JH. Treatment of experimental avascular necrosis of the femoral head with hyperbaric oxygen in rats. Exp Mol Pathol. 1999;67:99–108. doi: 10.1006/exmp.1999.2273. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka K, Yoshioka T, Hosoya T, Ono K, Takase T. The repair process in experimentally induced avascular necrosis of the femoral heads in dogs. Arch Orthop Trauma Surg. 1981;99:109–115. doi: 10.1007/BF00389746. [DOI] [PubMed] [Google Scholar]
- 17.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 18.Arlet J. Nontraumatic avascular necrosis of the femoral head: past, present and future. Clin Orthop. 1992;277:12–21. [PubMed] [Google Scholar]
- 19.Kabata T, Kubo T, Matsumoto T, Hirata T, Fujioka M, Takahashi KA, Yagishita S, Kobayashi M, Tomita K. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology. 2005;44:1233–1237. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]