Abstract
Objective
Research is increasingly linking autism spectrum disorders and other neurodevelopmental disorders to synaptic abnormalities (“synaptopathies”). PSD-95 (postsynaptic density-95, DLG4) orchestrates protein-protein interactions at excitatory synapses and is a major functional bridge interconnecting a neurexin-neuroligin-SHANK pathway implicated in autism spectrum disorders.
Method
The authors characterized behavioral, dendritic, and molecular phenotypic abnormalities relevant to autism spectrum disorders in mice with PSD-95 deletion (Dlg4−/−). The data from mice led to the identification of single-nucleotide polymorphisms (SNPs) in human DLG4 and the examination of associations between these variants and neural signatures of Williams’ syndrome in a normal population, using functional and structural neuroimaging.
Results
Dlg4−/− showed increased repetitive behaviors, abnormal communication and social behaviors, impaired motor coordination, and increased stress reactivity and anxiety-related responses. Dlg4−/− had subtle dysmorphology of amygdala dendritic spines and altered forebrain expression of various synaptic genes, including Cyln2, which regulates cytoskeletal dynamics and is a candidate gene for Williams’ syndrome. A significant association was observed between variations in two human DLG4 SNPs and reduced intraparietal sulcus volume and abnormal cortico-amygdala coupling, both of which characterize Williams’ syndrome.
Conclusions
These findings demonstrate that Dlg4 gene disruption in mice produces a complex range of behavioral and molecular abnormalities relevant to autism spectrum disorders and Williams’ syndrome. The study provides an initial link between human DLG4 gene variation and key neural endophenotypes of Williams’ syndrome and perhaps cortico-amygdala regulation of emotional and social processes more generally.
Neurodevelopmental disorders such as fragile X syndrome and autism spectrum disorders (hereafter referred to as “autism”) (1–3) are increasingly linked to synaptic abnormalities (“synaptopathies”). Recent evidence indicates that copy number variants in synaptic genes tend to be overrepresented in neurodevelopmental disorders (4–6). Postsynaptic function is controlled by a highly complex, interconnected network of molecules (7). However, a specific synaptic pathway comprising the predominantly presynaptic neurexins (NRXN) and their postsynaptic binding partners, neuroligins (NLGN) and SHANK (8), has been implicated in autism. Human loss-of-function variants in NLGN3, NLGN4, NRXN1, and SHANK3 are associated with autism (3). In mice, Nlgn3 and Nlgn4 null mutation have been found in some studies to cause autism-like traits (for example, references 9, 10–12), while Shank1 deletion has been found to alter dendritic spine size and learning (13).
PSD-95 (postsynaptic density-95, DLG4), a member of the membrane-associated guanylate kinase family of synaptic molecules, serves as a major functional bridge interconnecting the neurexin-neuroligin-SHANK pathway (7) and orchestrates protein-protein interactions and receptor stabilization at excitatory synapses (14). Gene variation and gene deletion of another family member, SAP102 (DLG3), is associated with X-linked mental retardation in humans (15) and autism-like abnormalities in mice (16), respectively. Microdeletion of chromosomal region 3q29 containing the membrane-associated guanylate kinase SAP-97 (DLG1) is also associated with autistic traits (17). However, a possible role for PSD-95 in autism and other neurodevelopmental disorders has not been studied.
In this study, we characterized mice with complete functional null mutation of PSD-95 (Dlg4−/−) for behavioral, dendritic, and molecular phenotypic abnormalities relevant to neurodevelopmental disorders (18, 19). Our results then led us to examine associations between human DLG4 gene variation and neural signatures of the neurodevelopmental disorder Williams’ syndrome.
Method
For a full methodological description, see the data supplement that accompanies the online edition of this article.
Mouse Phenotyping
Dlg4−/− were generated as described previously (20) and back-crossed to ~95% C57BL/6J congenicity. Homozygous Dlg4 deletion adversely affected survival (only ~13% progeny were Dlg4−/− at weaning), but surviving mice showed no obvious neurological or sensory abnormalities, including in tests for startle, sensorimotor gating, pain, and olfaction (see Table S1 and Figure S1 in the online data supplement).
Mice were tested for autism-related phenotypes in three major behavioral domains: repetitive and social, locomotor and motor, and anxiety-related (19, 21). To minimize potential carryover effects, multiple cohorts of age- and sex-matched Dlg4−/− and Dlg4+/+ littermates were tested, with more stressful tests later in sequences. (See figure footnotes for number of mice tested.)
Repetitive behaviors were examined through a discrete-trial T-maze, grooming, and marble burying. Social behaviors in males were assayed through ultrasonic vocalizations to a proestrous female, free social interaction, and social approach. Given evidence implicating metabotropic glutamate receptor 5 in mouse fragile X syndrome and autism-related abnormalities (1, 22), effects of receptor antagonism with 2-methyl-6-phenylethynylpyridine (MPEP) (10 mg/kg) were tested on social approach. As a control task, the social approach procedure was replicated with nonsocial objects.
Anxiety-related behaviors and stress reactivity were measured using light/dark exploration, elevated plus-maze (starting location either facing an open or closed arm), stress-induced hyperthermia, and restraint stress-induced corticosterone. Corticolimbic neuronal dendritic spine density/morphology was measured using biolistic labeling of basolateral amygdala and anterior cingulate cortex neurons.
Locomotor activity and motor coordination were examined using novel open field, accelerating rotarod, balance beam, inverted wire-hang, and homecage activity. Effects of MPEP on novel open-field behavior were tested. Nonquantitative histological analysis of the cerebellum was performed using immunostaining of the major cerebellar cell types. Whole brain volume and morphology were measured with structural MRI.
Microarray analysis was conducted on forebrain tissue using Affymetrix GeneChip. mRNA changes in a subset of genes identified by microarray were confirmed by quantitative reverse transcription polymerase chain reaction. Protein levels of Cyln2, a major candidate for Williams’ syndrome (23), were analyzed by Western blot.
Data were analyzed using Student’s t tests, analysis of variance, Fisher’s least-significant-difference post hoc tests, and Kolmogorov-Smirnov tests (spine analysis). Alpha was set at 0.05.
Human Neuroimaging
Participants from a multicenter study on intermediate neuroimaging phenotypes (24) underwent genome-wide single-nucleotide polymorphism (SNP) analysis using Illumina Human610-Quad BeadChip (Illumina, Inc., San Diego). On 3-T scanners, 115 participants completed an imaging genetics protocol comprising several functional magnetic imaging (fMRI) measurements, including an fMRI face matching task to engage the amygdala (25). Connectivity between the amygdala and the subgenual anterior cingulate cortex was analyzed using a seed region approach (24). Structural MRI was conducted on 93 participants. Voxel-based morphometry (26) focused on the occipital/intraparietal cortex.
Four DLG4 SNPs were available on the Illumina chip, of which three (rs17203281, rs3826408, rs390200) met quality control criteria. Given high linkage disequilibrium (r=0.8) between rs17203281 and rs3826408, only rs17203281 and rs390200 were analyzed. Gentype distributions were in Hardy-Weinberg equilibrium for both SNPs. Because of the small number of homozygote rs17203281 A-allele carriers (N=15), AA and AG were combined for comparison with G allele homozygotes. Genotype effects on neural activation and subgenual anterior cingulate cortex-amygdala connectivity were analyzed using the general linear model in a second-level random-effects analysis, with genotype as the covariate of interest and scanner site as a nuisance covariate. Results were corrected for multiple comparisons using false discovery rate within prespecified regions of interest.
Results
Repetitive and Social Behaviors
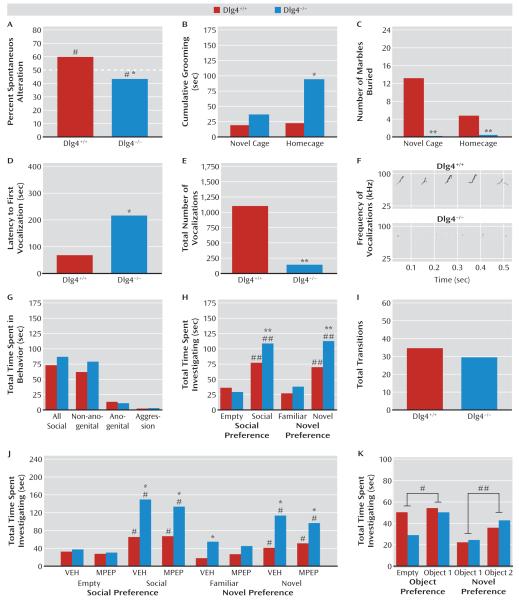
We phenotyped Dlg4−/− for various measures of repetitive and stereotypical behaviors. On a T-maze spontaneous alternation test, Dlg4+/+ alternated significantly more than would be expected due to chance (t=2.40, df=8, p<0.05), while Dlg4−/− alternated significantly less than would be expected due to chance (t=2.65, df=7, p<0.05) (Figure 1A). When observed for grooming behavior, Dlg4−/− groomed significantly more than did Dlg4+/+ in the homecage (t=2.64, df=11, p<0.05) but not in the novel cage (Figure 1B). Lastly, in both a novel cage (t=3.50, df=12, p<0.01) and homecage (t=3.05, df=12, p<0.01), Dlg4−/− buried significantly fewer marbles than did Dlg4+/+ (Figure 1C).
FIGURE 1. Repetitive and Social Behaviors in Dlg4+/+ and Dlg4−/− Micea.
a In panel A, Dlg4+/+ showed spontaneous alternation in a T-maze, while Dlg4−/− repeatedly investigated the same arm (#p<0.05 compared with chance in same genotype; *p<0.05 compared with Dlg4+/+) (N=8–9). Panel B, Dlg4−/− groomed more in the homecage but not in the novel cage (*p<0.05 compared with Dlg4+/+) (N=6–7). Panel C, Dlg4−/− buried fewer marbles in novel and homecage (**p<0.01 compared with Dlg4+/+) (N=6–8). Panels D, E, and F, Dlg4−/− were slower to first ultrasonic vocalization to a proestrous female and made fewer ultrasonic vocalizations overall (**p<0.01, *p<0.05 compared with Dlg4+/+) (N=6–7). Panel G, genotypes did not differ in social behavior in a free dyadic interaction (N=18). Panel H, both genotypes investigated a mouse more than an empty cage, and a novel mouse over a familiar one, but Dlg4−/− investigated the mouse and then the novel mouse more than did Dlg4+/+ (##p<0.01 compared with empty/familiar in same genotype, **p<0.01 compared with Dlg4+/+) (N=17–26). Panel I, genotypes did not differ in transitions between stimuli. Panel J, genotype differences described in panel H were unaffected by 2-methyl-6-phenylethynylpyridine (MPEP) treatment (#p<0.05 compared with empty/familiar in same genotype, *p<0.05 compared with Dlg4+/+) (N=5–6). VEH=vehicle (control condition). Panel K, both genotypes investigated an object more than an empty cage, and a novel object over a familiar one (#p<0.05 compared with empty cage, ##p<0.01 compared with object 1) (N=10–11). Data are mean values.
We next examined Dlg4−/− for communicative and social phenotypes. Dlg4−/− had a significantly longer latency to first vocalization (t=2.36, df=11, p<0.05) and made significantly fewer vocalizations overall than Dlg4+/+ (Figure 1D–F). During a free dyadic encounter, genotypes did not differ significantly in social behaviors directed toward the stimulus mouse (Figure 1G). In a choice-based social approach test, both genotypes investigated a mouse significantly more than they did an empty cage, but investigation of the mouse was significantly greater in Dlg4−/− than in Dlg4+/+ (genotype-by-stimulus interaction: F=10.87, df=1, 41, p<0.01) (Figure 1H). Both genotypes investigated a novel mouse significantly more than they did a familiar mouse, but novel investigation was again significantly greater in Dlg4−/− than in Dlg4+/+ (genotype-by-stimulus interaction: F=4.47, df=1, 41, p<0.05) (Figure 1H). Total transitions between stimuli did not differ between genotypes (Figure 1I). Following MPEP treatment, a separate cohort of Dlg4−/− again showed relatively more social (compared with empty) and novel-social behavior (compared with familiar), regardless of treatment (Figure 1J). Finally, in a replication of the social approach test procedure, using nonsocial stimuli, both genotypes equivalently investigated an object significantly more than an empty cage (stimulus effect: F=4.42, df=1, 19, p<0.05) and a novel object over a familiar one (stimulus effect: F=11.25, df=1, 19, p<0.01) (Figure 1K).
Motor Functions and Cerebellar Morphology
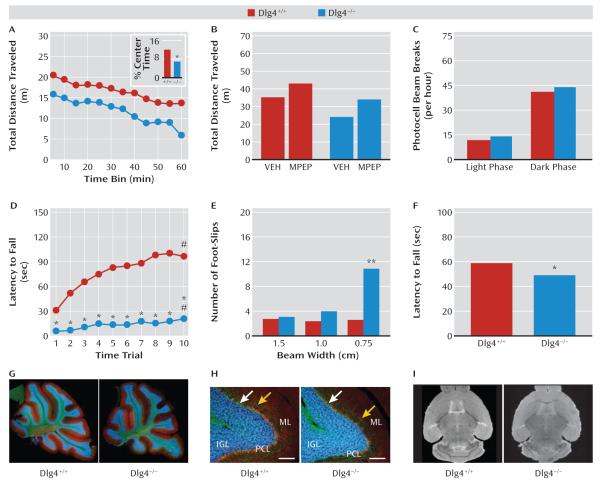
Given the prevalence of motor deficits in autism and other neurodevelopmental disorders, we conducted extensive analysis of Dlg4−/− in this domain. In a novel open-field test, Dlg4−/− traveled significantly less (genotype main effect: F=8.89, df=1, 33, p<0.01) and avoided the center more (t=2.39, df=33, p<0.05) than did Dlg4+/+ (Figure 2A). Following treatment with MPEP, novel open-field locomotion was significantly increased in both genotypes relative to vehicle (control condition) (drug effect: F=7.38, df=1, 25, p<0.05), such that MPEP-treated Dlg4−/− were no different from vehicle-treated Dlg4+/+ (genotype effect: F=11.37, df=1, 25, p<0.01) (Figure 2B). In contrast to reduced locomotion in a novel open-field setting, locomotor activity in the familiar homecage environment did not differ between genotypes (Figure 2C).
FIGURE 2. Motor Functions and Cerebellar Morphology in Dlg4+/+ and Dlg4−/− Micea.
a In panel A, Dlg4−/− traveled less far and spent less time in the center (inset) in a novel open field (*p<0.05 compared with Dlg4+/+) (N=13–22). Panel B, 2-methyl-6-phenylethynyl-pyridine (MPEP) increased open-field activity in both genotypes, normalizing Dlg4−/− activity to vehicle-treated (VEH; control condition) Dlg4+/+ levels (N=7–8). Panel C, genotypes did not differ in homecage locomotor activity (N=9–11). Panel D, Dlg4−/− had lower accelerating rotarod latencies (#p<0.05 compared with trial 1, *p<0.05 compared with Dlg4+/+) (N=21–26). Panel E, Dlg4−/− made more foot-slips on a narrow balance beam (**p<0.01 compared with Dlg4+/+) (N=11–12). Panel F, Dlg4−/− had lower wire-hang latency (*p<0.05 compared with Dlg4+/+) (N=13–22). Panels G and H, genotypes did not differ in gross cerebellar morphology. Parasagittal sections immunostained for calbindin (red=Purkinje cells) and neurofilaments (green=Purkinje cell axons, basket cell axons around Purkinje cell somata [white arrow], and molecular layer [ML] interneuron axons [yellow arrow]) and stained with DAPI (blue=granule cell nuclei in internal granule layer [IGL], and sparse basket and stellate cells in ML) (scale bar=100 μm). PCL=Purkinje cell layer. Panel I, structural MRI found no genotype differences in gross brain morphology or volume. Data are mean values.
On the accelerating rotarod assay for motor coordination, Dlg4−/− had significantly lower latencies to fall than did Dlg4+/+ on all trials, and both genotypes improved over trials (genotype-by-trial interaction: F=9.52, df=9, 405, p<0.01) (Figure 2D). Dlg4−/− also made significantly more foot-slips than did Dlg4+/+ on a balance beam test, but only on the narrowest beam (genotype-by-beam width interaction: F=20.67, df=2, 42, p<0.01) (Figure 2E). Finally, Dlg4−/− had a significantly lower inverted hang latency than did Dlg4+/+ on an inverted cage test for hypotonia (t=2.54, df=33, p<0.05) (Figure 2F).
Despite these behavioral abnormalities, there were no obvious genotype differences in cerebellar morphology, as measured from parasagittal sections immunostained for calbindin (Purkinje cells) and neurofilaments (Purkinje cell axons, basket cell axons, and molecular layer interneuron axons) and stained with DAPI (granule cell nuclei) (Figure 2G,H). Structural MRI also revealed no genotype difference in gross morphology or total brain volume (Figure 2I).
Anxiety- and Stress-Related Phenotypes
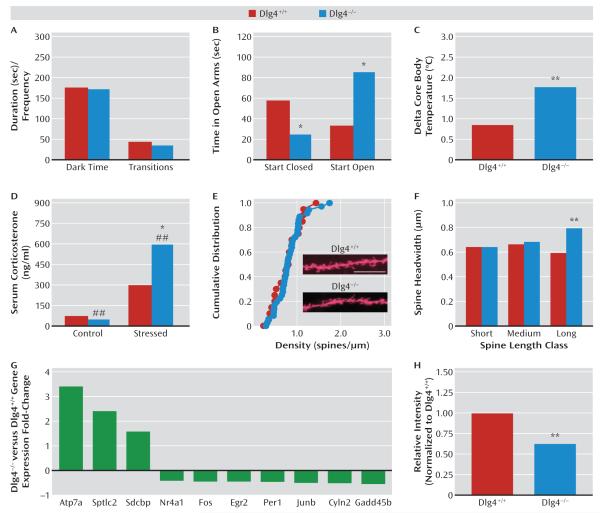
The final behavioral domain we examined was anxiety- and stress-related phenotypes. On the light/dark exploration test, genotypes did not significantly differ either in dark time or light/dark transitions (Figure 3A). However, Dlg4−/− spent less time (t=2.29, df=23, p<0.05) and made fewer entries (not shown) into the open arms of the elevated plus-maze test than did Dlg4+/+ when started facing a closed arm (Figure 3B). By contrast, when started facing an open arm, a separate cohort of Dlg4−/− spent more time (t=4.03, df=40, p<0.01) and made more entries (not shown) into the plus-maze open arms than did Dlg4+/+ (Figure 3B). On a non-exploration-based assay for anxiety-related behavior and stress reactivity, Dlg4−/− showed significantly greater stress-induced hyperthermia than did Dlg4+/+ (t=3.44, df=14, p<0.01) (Figure 3C). Similarly, Dlg4−/− showed a significantly greater stress-induced serum corticosterone response to restraint stress than did Dlg4+/+ (genotype-by-stress interaction: F=9.67, df=1, 17, p<0.01) (Figure 3D).
FIGURE 3. Anxiety- and Stress-Related Phenotypes, Amygdala Spine Morphology, and Forebrain Gene Expression in Dlg4+/+ and Dlg4−/− Micea.
a In panel A, genotypes did not differ in dark time or light/dark transitions in the light/dark test (N=9–22). Panel B, Dlg4−/− spent less time in the elevated plus-maze open arms when started facing a closed arm (N=16–26) and more open time when started facing an open arm (*p<0.05 compared with Dlg4+/+) (N=11–14). Panel C, Dlg4−/− showed greater stress-induced hyperthermia (**p<0.01 compared with Dlg4+/+) (N=7–9). Panel D, Dlg4−/− had greater corticosterone response to restraint stress (##p<0.01 compared with control, *p<0.05 compared with Dlg4+/+) (N=4–7). Panels E and F, Dlg4−/− showed larger headwidth of relatively long dendritic spines in basolateral amygdala neurons but normal spine density distribution (**p<0.01 compared with Dlg4+/+) (scale bar=10 μm) (N=4–6/genotype, 9–10 neurons, 21–37 dendrites). Panel G, Dlg4−/− differed in forebrain expression of 10 synaptic and plasticity-related genes (N=3). Panel H, Dlg4−/− had reduced forebrain protein levels of Cyln2 (**p<0.01 compared with Dlg4+/+) (N=4). Data are mean values.
To uncover possible neural correlates of these anxiety- and stress-related abnormalities, we examined spine density and morphology of neurons in the anterior cingulate cortex and the basolateral amygdala. Dlg4−/− had significantly larger spine headwidth than did Dlg4+/+ in relatively long dendritic spines in basolateral amygdala neurons (D=0.42, p<0.01). Genotypes did not differ in spine density distribution (Figure 3E,F). Neither spine headwidth nor density in anterior cingulate cortex neurons differed between genotypes (see Figure S2 in the online data supplement).
Finally, we performed microarray analysis of forebrain tissue as a preliminary effort to elucidate molecule disturbances in Dlg4−/−. Atp7a, Sdcbp, and Sptlc2 were significantly up-regulated, and Nr4a1, Fos, Egr2, Per1, Junb, Cyln2, and Gadd45b were significantly down-regulated in Dlg4−/− (Figure 3G). A subset of these genes were confirmed by quantitative reverse transcription polymerase chain reaction (see Table S2 in the online data supplement). Using Western blot, we confirmed significantly lower protein levels of Cyln2 in Dlg4−/− than in Dlg4+/+ (t=4.63, df=6, p<0.05) (Figure 3H).
Human Neuroimaging
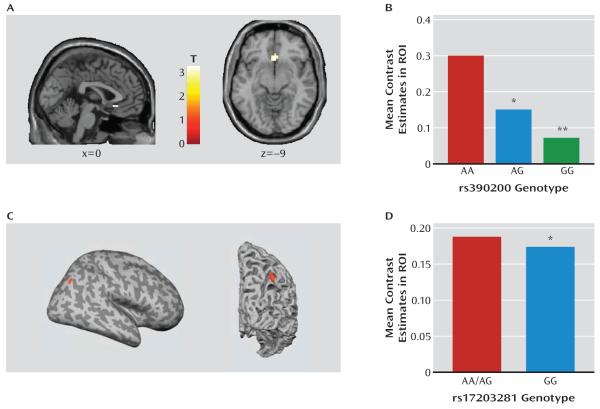
While some of our data in Dlg4−/− supported our hypothesis that a null mutation of this key synaptic molecule would lead to autism-like behavioral deficits, the pattern of at least some of the behavioral phenotypes observed (see the Discussion section), coupled with the reductions in the candidate gene, Cyln2, suggested that Dlg4−/− may produce abnormalities related to Williams’ syndrome. This led us to ask whether human DLG4 variation could contribute to neural endophenotypes of Williams’ syndrome in unaffected individuals. We found that G allele carriers of one of the SNPs we identified, rs390200, showed significantly lesser subgenual anterior cingulate cortex-amygdala connectivity than did homozygous A allele carriers (peak voxel: x=0, y=24, z=−9; t=3.30, df=110, p<0.05) (Figure 4A,B). In addition, rs17203281 homozygote G allele carriers had significantly lesser volume near the right intraparietal sulcus relative to A allele carriers (peak voxel: x=43, y=−81, z=43; t=4.57, df=87, p<0.05) (Figure 4C,D).
FIGURE 4. Human Variation in Cortico-Amygdala Connectivity and Gray Matter Volume Observed by Neuroimaging in DLG4 SNPsa.
a In panels A and B, DLG4 SNP rs390200 G allele carriers showed lesser subgenual anterior cingulate cortex-amygdala connectivity than did homozygous A allele carriers during a threatening face task (**p<0.01, *p<0.05 compared with AA) (N=115). Panels C and D, homozygous DLG4 SNP rs17203281 G allele carriers had lower gray matter volume near the intraparietal sulcus than did A allele carriers (*p<0.05 compared with AA/AG) (N=93). Data are mean values. ROI=region of interest.
Discussion
Our a priori hypothesis was that Dlg4 deletion would produce phenotypic abnormalities relevant to neurodevelopmental disorders, particularly autism. We found that Dlg4−/− exhibited a set of behavioral, dendritic spine, and molecular abnormalities, some of which were autism-like, but others that are more related to other neurodevelopmental disorders, notably Williams’ syndrome. Furthermore, we found that DLG4 variation was associated with signature neural endophenotypes of Williams’ syndrome in a normal human population.
Autism is characterized by repetitive and stereotypic behaviors (19, 21). On a T-maze test, which measures the tendency of rodents to spontaneously alternate exploration of two goal arms, Dlg4−/− exhibited modest but statistically significant repetitive exploration of the same arm. Dlg4−/− also displayed excessive grooming behavior (as also found in putative mouse autism models [27–29]). Notably, this was seen only in a homecage setting and not in a novel context, possibly because of response competition with the suppression of behavior in novel environments we observed in the mutants (see below). This could also possibly account for the decrease, rather than repetitive-like increase, in marble burying in Dlg4−/−, although that was seen in both novel and homecage environments. Nevertheless, these data show that Dlg4 deletion produced some autism-like repetitive, stereotypical behaviors in both the cognitive and noncognitive realms.
Abnormal communicative and social behavior are cardinal features of autism. Male Dlg4−/− vocalized less in the presence of a proestrous female—a phenotype thought to be salient to communication deficits in autism (19). Dlg4 deletion did not, however, produce an autism-like reduction in social behavior, either during a free dyadic encounter or in a social approach test (19). Unexpectedly, Dlg4−/− actually spent more time investigating the social and novel-social stimulus than Dlg4+/+. Demonstrating that this increased social investigation was not confounded by Dlg4−/− simply moving less in this test context (as they do in novel, nonsocial contexts—see below), genotypes did not differ in the number of transitions between stimuli. Furthermore, when we replaced social stimuli with inanimate objects in the social approach procedure, Dlg4−/− did not show increased investigation of nonsocial objects, indicating that a general preference for novelty per se did not drive the social phenotype in the mutants. Thus, these data point to an unanticipated increase in certain social behaviors in Dlg4−/−. Although previous studies have shown that treatment with MPEP can prevent seizures and sensorimotor gating deficits in a mutant mouse model of fragile X syndrome (1), we found that MPEP treatment did not decrease social behavior in Dlg4−/− to Dlg4+/+ levels. Recent work has shown that social abnormalities in a model of autism are similarly insensitive to this treatment (22), and additional studies are needed to identify pharmacological and behavioral manipulations that effectively normalize such disturbances.
In addition to repetitive motor actions, autism typically presents with various motor deficits, including clumsiness; poor balance and coordination; unusual gait patterns, such as toe-stepping; hypotonia; and either locomotor hyperactivity or motor retardation (21). A recent meta-analysis concluded that motor incoordination should be considered a cardinal feature of autism (30). Consistent with such problems in motor coordination, Dlg4−/− displayed impaired performance on the accelerating rotarod test, as well as poor balance and gait on the narrowest balance beam and mild hypotonia on an inverted hang test. Similar deficits are seen in various glutamate signaling molecule mutants, such as lurcher and Grid2−/−, due to cerebellar developmental malformation (31). However, our nonquantitative histological analysis did not reveal gross abnormalities in cerebellar lamination or foliation in Dlg4−/−. Gross brain morphology and volume, as measured by structural MRI, also appeared normal. While these data clearly demonstrate that Dlg4−/− phenocopy another major feature of autism, this appears to reflect yet-to-be-determined subcellular or physiological anomalies in the cerebellum or other brain regions controlling motor functions (e.g., striatum, motor cortex) rather than any major anatomical aberration.
Also within the motor domain, Dlg4−/− had reduced locomotor activity in a novel open field. This was associated with increased center avoidance, suggesting that the hypoactivity could reflect anxiety-driven suppression of exploration rather than a more generalized locomotor retardation that might be related to the motor incoordination. Affirming this interpretation, Dlg4−/− showed normal levels of activity in the nonstressful homecage environment and normal behavior in another locomotion-based but putatively less stressful anxiety-related test: light/dark exploration. Moreover, treatment with the anxiolytic drug MPEP (32) was sufficient to normalize open-field hypoactivity in Dlg4−/− to levels of untreated Dlg4+/+—an effect that would be difficult to explain if the mutants were simply motor compromised. We also tested Dlg4−/− in the elevated plus-maze, but behavior here was difficult to interpret because of a strong influence of start location. However, physiologic and neuroendocrine responses to stress were exaggerated in Dlg4−/−. Taken together, this profile points to exaggerated stress reactivity and a task-dependent increase in anxiety-like behavior in Dlg4−/− and provides a further parallel with the emotional dysregulation, oftentimes increased anxiety (21), found in most neurodevelopmental disorders.
While the neural basis of these behavioral abnormalities remains to be elucidated, we found that Dlg4−/− had a subtle and specific alteration in the morphology of dendritic spines on basolateral amygdala neurons—a key neural locus mediating emotional and social behavior. Specifically, the heads of a subset of relatively long synaptic spines on basolateral amygdala neurons were enlarged in Dlg4−/−. This change was not associated with an alteration in spine density, nor in the spine density or morphology of spines in a prefrontal region that interacts with the basolateral amygdala to regulate emotion, the anterior cingulate cortex (33). It would be premature at this time to draw clear links between these changes and the behavioral abnormalities exhibited by Dlg4−/−. It is an intriguing observation, however, considering that dendritic spine dysmorphology is increasingly linked to neurodevelopmental disorders (34) and given evidence that Dlg4 regulates microtubule dynamics and motor proteins, such as dynein, that support the cytoskeletal machinery modulating dendritic spine morphology (14, 35). It is also worth noting that spine head size correlates with synaptic strengthening (36), particularly in the context of previous work demonstrating that Dlg4−/− have enhanced long-term potentiation at hippocampal and corticostriatal glutamate synapses, coupled with impaired spatial reference memory and psychostimulant sensitization (20, 37).
Providing some initial insight into molecular factors that might contribute to the spine morphology and/or behavioral disturbances caused by Dlg4 deletion, gene expression analysis revealed forebrain expression in a suite of synaptic genes, including genes associated with autism (GADD45B, Per1) and Menkes’ disease (Atp7a) (38, 39). Another striking change was in Cyln2 (cytoplasmic linker protein of 115 kDa, CLIP-115), which was decreased almost 50% at both the mRNA and the protein level. The human CYLN2 gene is hemideleted within a microdeletion at chromosome 7q11.23 in Williams’ syndrome (23, 40–42). In fact, along with LIMK1 and GTF2I, CYLN2 loss is a principal candidate for two unusual features of this disorder—hypersociability coupled with heightened anxiety to nonsocial stimuli (23). While we do not claim that loss of Cyln2 renders Dlg4−/− a model of Williams’ syndrome, there are interesting parallels with the increased social and anxiety-related phenotype we observed in these mice. In another parallel, Williams’ syndrome typically presents with motor incoordination manifested as abnormal gait and impaired stair stepping.
DLG4 is not located within the Williams’ syndrome region or the orthologous region on mouse chromosome 5. Previous studies have found that targeted heterozygous deletion of Cyln2 impaired motor and fear-related behaviors (43), while deletion of Cyln2 along with proximally neighboring Williams’ syndrome genes produced hypersociability (in choice, not free interaction tests), heightened anxiety-like behavior (in the novel open-field test, not in the light/dark test), and motor coordination deficits (44). These similarities to the Dlg4−/− profile suggest a heuristic model in which Dlg4−/− deletion, via trans-acting epistatic or molecular interaction, alters Cyln2 expression to drive, at least in part, the behavioral and possibly the morphological disturbances displayed by Dlg4−/−. Since Cyln2 is thought to support proper synaptic clustering of proteins by bridging microtubules and protein complexes (43), functional interactions with Dlg4 would not be unexpected. However, to our knowledge, this has not been directly demonstrated and remains a major question.
Extending these basic research findings, we provide preliminary evidence that variation in the human DLG4 gene is associated with key neural Williams’ syndrome endophenotypes. First, in a population of unaffected individuals, those carrying the G allele of rs390200 had lesser subgenual anterior cingulate cortex-amygdala connectivity than homozygous A allele carriers during a threatening faces task (25), mimicking the profile associated with the anxiety and hypersocial phenotype of Williams’ syndrome (23). Reduced cingulate-amygdala connectivity has also been linked to harm avoidance, an anxiety-related personality variable, in healthy individuals studied with the same task as employed here (45). Second, homozygote carriers of the rs17203281 G allele had lower volume near the right intraparietal sulcus than A allele carriers, phenocopying the best-replicated structural abnormality in Williams’ syndrome (23). Although the functionality of these two SNPs remains to be determined, these data establish a link between DLG4 variation and both functional and structural intermediate neural Williams’ syndrome phenotypes. This finding raises some interesting questions for future work, such as whether these variants are present in Williams’ syndrome patients and interact with the microdeletion to affect the clinical phenotype.
Conclusions
Our study demonstrates that Dlg4 deletion in mice leads to a complex phenotypic profile of increased repetitive behaviors on some measures, abnormal communication and social behaviors, impaired motor coordination, and increased stress- and anxiety-related responses. These behavioral disturbances were associated with dysmorphology of amygdala dendritic spines and altered forebrain expression of a set of synaptic genes that included the Williams’ syndrome gene Cyln2. We also established an initial link between variation in two human DLG4 SNPs and the Williams’ syndrome neural endophenotypes of reduced intraparietal sulcus volume and abnormal cortico-amygdala coupling. Collectively, our findings offer novel evidence of a contribution of DLG4 to certain behavioral abnormalities found in neurodevelopmental disorders, such as autism and Williams’ syndrome, and the neural and molecular mechanisms that underlie these disorders and perhaps normal variation in social and emotional traits. More generally, given the position of Dlg4 as a major orchestrator of synaptic function, our data provide further support for the conceptualization of neurodevelopmental disorders as “synaptopathies.”
Supplementary Material
Acknowledgments
Dr. Meyer-Lindenberg has received funding from AstraZeneca, Hoffmann LaRoche, Janssen-Cilag, Pfizer, and Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz and receives compensation from Thieme for serving as an editor of Pharmacopsychiatry.
Supported by the NIAAA Intramural Research Program (Dr. Holmes), Bundesministerium für Bildung und Forschung (Nationales Genomforschungsnetz plus MooDs) (Dr. Meyer-Lindenberg), Deutsche Forschungsgemeinschaft (SFB 636-B7) (Dr. Meyer-Lindenberg), and the Wellcome Trust Genes to Cognition program (Dr. Grant).
The authors thank Raimund Specht of Avisoft Bioacoustics for the loan of the ultrasonic recording equipment, Yoshihiro Kashiwaya for use of the confocal microscope, and Jonathan Brigman for support with behavioral testing.
Footnotes
All other authors report no competing interests.
References
- 1.Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33:84–87. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, Summers A, Gibbons CA, Teebi A, Chitayat D, Weksberg R, Thompson A, Vardy C, Crosbie V, Luscombe S, Baatjes R, Zwaigenbaum L, Roberts W, Fernandez B, Szatmari P, Scherer SW. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autism Genome Project Consortium. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coba MP, Pocklington AJ, Collins MO, Kopanitsa MV, Uren RT, Swamy S, Croning MD, Choudhary JS, Grant SG. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal. 2009;2(68):ra19. doi: 10.1126/scisignal.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 15.Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, Cox J, Davies H, Edkins S, Holden S, Korny A, Mallya U, Moon J, O’Meara S, Parker A, Stephens P, Stevens C, Teague J, Donnelly A, Mangelsdorf M, Mulley J, Partington M, Turner G, Stevenson R, Schwartz C, Young I, Easton D, Bobrow M, Futreal PA, Stratton MR, Gecz J, Wooster R, Raymond FL. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willatt L, Green AJ, Trump D. Satellites on the terminal short arm of chromosome 12 (12ps), inherited through several generations in three families: a new variant without phenotypic effect. J Med Genet. 2001;38:723–727. doi: 10.1136/jmg.38.10.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 20.Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 22.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- 24.Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, von Boberfeld C Opitz, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 25.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 26.Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- 27.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 28.Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. doi: 10.1007/s10803-010-0981-3. (Epub ahead of print, Mar 2, 2010) [DOI] [PubMed] [Google Scholar]
- 31.Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neuro-chemistry. Brain Res. 2007;1140:51–74. doi: 10.1016/j.brainres.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Spooren W, Gasparini F. mGlu5 receptor antagonists: a novel class of anxiolytics? Drug News Perspect. 2004;17:251–257. doi: 10.1358/dnp.2004.17.4.829052. [DOI] [PubMed] [Google Scholar]
- 33.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 37.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 38.Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholas B, Rudrasingham V, Nash S, Kirov G, Owen MJ, Wimpory DC. Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Mol Psychiatry. 2007;12:581–592. doi: 10.1038/sj.mp.4001953. [DOI] [PubMed] [Google Scholar]
- 40.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- 41.Jarvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, Korenberg JR. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Dev Psychopathol. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- 43.Hoogenraad CC, Akhmanova A, Galjart N, De Zeeuw CI. LIMK1 and CLIP-115: linking cytoskeletal defects to Williams syndrome. Bioessays. 2004;26:141–150. doi: 10.1002/bies.10402. [DOI] [PubMed] [Google Scholar]
- 44.Li HH, Roy M, Kuscuoglu U, Spencer CM, Halm B, Harrison KC, Bayle JH, Splendore A, Ding F, Meltzer LA, Wright E, Paylor R, Deisseroth K, Francke U. Induced chromosome deletions cause hypersociability and other features of Williams-Beuren syndrome in mice. EMBO Mol Med. 2009;1:50–65. doi: 10.1002/emmm.200900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.