Abstract
Aims
Despite their ability to cause septic shock and myocardial dysfunction, components of Gram-negative bacterial cell walls, like lipopolysaccharide, have been shown in numerous studies to induce myocardial protection during ischaemia–reperfusion injury. Muramyl dipeptide (MDP) is another such component recognized by an intracellular receptor, nucleotide-binding oligomerization domain 2. Receptor activation leads to intracellular signals through receptor interacting protein-2 (RIP2) and tumour growth factor-β-activated kinase-1 (TAK1). However, little is known about the RIP2/TAK1 pathway in the heart. The aim of this study was to determine whether the RIP2/TAK1 pathway has a cardioprotective role in a mouse model of myocardial infarction.
Methods and results
We isolated and subjected wild-type (WT) and RIP2−/− mouse hearts to 30 min of global ischaemia and 120 min of reperfusion with or without perfusion of MDP (10 µg/mL) before or after the ischaemic period and determined the infarct size. We examined activation of the TAK1/nuclear factor κB (NFκB) signalling pathway. The effect of TAK1 inhibition on MDP-induced cardioprotection was also evaluated. Exposure to MDP during reperfusion significantly reduced infarct size in WT hearts (from 51.7 ± 5.6% in control to 38.1 ± 6.7%, P < 0.05), but not in RIP2−/− hearts or in WT hearts with coincident pharmacological inhibition of TAK1. MDP treatment significantly increased the levels of p-TAK1 and p-JNK (Jun N-terminal kinase) and led to NFκB activation via phosphorylation and degradation of IkappaB in the WT, but not in the RIP2−/−, myocardium.
Conclusion
These results indicate that MDP at reperfusion induced cardioprotection through an RIP2/TAK1-dependent mechanism.
KEYWORDS: Postconditioning, TAK1, RIP2, MDP, Ischaemia–reperfusion
1. Introduction
Besides mechanical interventions like gradual or interrupted reperfusion, several studies have demonstrated that proteins including adipocytokines, insulin, and erythropoietin,1,2 when administered at the time of reperfusion, confer powerful cardioprotection. These effects could be attributable, in part, to the modulation of several kinases [phosphoinositide-3 kinase (PI3K), mitogen-activated protein (MAP) kinases, …]3 which result in the recruitment of downstream antiapoptotic pathways promoting cell survival. These include the phosphorylation and inactivation of proapoptotic proteins.4
Bacteria-derived ligands have paradoxical effects in biological systems. Despite their ability to cause septic shock and myocardial dysfunction,5 numerous studies have shown that the Gram-negative bacterial cell wall component, lipopolysaccharide (LPS), induces myocardial protection during ischaemia–reperfusion injury.6–8 This beneficial effect is mediated through pro-survival protein kinases, such as the PI3K/Akt-system,9 and is not unique to LPS, since lipoteichoic acid, a cell wall component of Gram-positive bacteria, also reduces infarct size when administered before ischaemia. The effect of lipoteichoic acid is mediated through the Toll-like receptor pathway.10 Muramyl dipeptide (MDP) is also a component of bacterial cell walls recognized by an intracellular receptor, nucleotide-binding oligomerization domain (NOD)-2. NOD2 activation led to intracellular signals through receptor interacting protein-2 (RIP2) and tumour growth factor-β-activated kinase-1 (TAK1) in non-cardiac tissue which modulated the nuclear factor κB (NFκB) pathway and promoted pro-inflammatory cytokine [interleukin-1β and tumour necrosis factor (TNF)-α] production.11 However, little is known about the RIP2/TAK1 pathway in the heart. TAK1 is activated after acute myocardial infarction12 and may play an important role in ventricular hypertrophy and heart failure.13 RIP2 does not seem to influence myocardial sensitivity to infarction.14 Moreover, the NOD2 receptor is present in the heart15 and, like LPS, MDP contributes to septic shock,16 which is in part the result of depressed cardiac contractility through RIP2 and p38 activation.14
Thus, there is conflicting evidence on the potential beneficial role of MDP in different tissues and models. The aim of this study was to determine whether MDP has a cardioprotective role in a mouse model of myocardial infarction. Therefore, we perfused mouse hearts with MDP for 15 min before or after the ischaemic period and measured haemodynamic recovery and infarct size. We found that only pharmacological postconditioning with MDP is able to protect the heart against ischaemia–reperfusion injury and that this occurs via the RIP2/TAK1 pathway.
2. Methods
2.1. Products
MDP was from Invivogen, San Diego, CA, USA (catalog no. tlrl-mdp), and 5Z-7-oxozeanol (TAK1 inhibitor) was from Analyticon Discovery, Potsdam, Germany (catalog no. NP-009245).
2.2. Animals
All experiments were performed in accordance with United Kingdom Home Office Guidance on the Operation of Animals (Scientific Procedures) Act 1986, published by Her Majesty’s Stationary Office, London, and with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.3. Perfusion of isolated murine hearts
Male RIP2 null (RIP2−/−)17 and wild-type (WT) C57BL6 mice were anaesthetized with pentobarbital (300 mg/kg) and heparin (150 U) intraperitoneally. Hearts were rapidly isolated, mounted onto a Langendorff apparatus, and retrogradely perfused at a constant pressure of 80 mmHg with Krebs–Henseleit buffer (in mmol/L: 118.5 NaCl, 25 NaHCO3, 4.75 KCl, 0.18 KH2PO4, 1.19 MgSO4, 11.0 d-glucose, and 1.41 CaCl2) equilibrated with 95% O2 and 5% CO2 at 37°C. A fluid-filled balloon inserted into the left ventricular cavity monitored contractile function. The balloon was gradually inflated until the left ventricular end-diastolic pressure was between 2 and 8 mmHg. Atrial pacing was performed at 580 bpm and coronary flow (CF) was measured by timed collection of perfusate.
2.4. Experimental protocol for isolated murine myocardial infarction studies
Hearts were stabilized for 30 min after initiation of retrograde perfusion. For inclusion, all hearts had to fulfil the following criteria: CF between 1.5 and 4.5 mL/min, initial heart rate >300 bpm (unpaced), left ventricular developed pressure >55 mmHg, time from thoracotomy to aortic cannulation <3 min, and no persistent dysrhythmias during the stabilization period. All hearts underwent 30 min of global ischaemia followed by 2 h of reperfusion. At the end of reperfusion, hearts were perfused for 1 min with 5 mL of 1% triphenyl tetrazolium chloride (TTC) in PBS and then placed in an identical solution at 37°C for 10 min. The atria were then removed, and the hearts were blotted dry, weighed, and stored at −20°C. Hearts were subsequently thawed, placed in 2.5% glutaraldehyde for 1 min and set in 5% agarose. The agarose heart blocks were then sectioned from apex to base in 0.75 mm slices using a vibratome (Agar Scientific). Sections were compressed between glass plates and scan-imaged (Epson model G850A). After magnification, planimetry was carried out using image analysis software (Adobe Photoshop 7.0). Risk and infarct areas were calculated from surface area analysis of whole myocardium and TTC-negative myocardium, respectively. Infarct analysis was performed in all cases by an investigator blinded to the group assignments.18,19
2.5. Protocol
Our first objective was to assess the potential beneficial effect of pharmaceutical preconditioning with MDP to limit infarct size in WT mice.
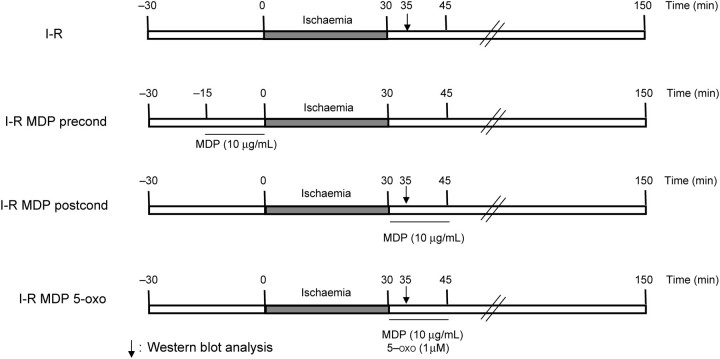
The control group was perfused with Krebs buffer during the pre- and post-ischaemic phase. The preconditioning group was perfused with MDP (10 µg/mL) 15 min before ischaemia. All the hearts were stabilized for 30 min after initiation of retrograde perfusion and subjected to 30 min of ischaemia followed by 2 h of reperfusion (Figure 1).
Figure 1.
Experimental protocols for ex vivo murine infarction studies in C57BL6 (WT) and RIP2−/−. All hearts were subjected to 30 min of stabilization, 30 min of global ischaemia, followed by 120 min of reperfusion at the end of which the infarct size was determined by triphenyl tetrazolium chloride staining. For the first protocol, muramyl dipeptide (MDP) was perfused 15 min before ischaemia (I-R MDP precond). In a second protocol, MDP was perfused immediately at the onset of reperfusion in the presence or absence of the TAK1 inhibitor (5Z-7-oxozeanol, 1 µmol/L).
Our second objective was to assess the potential beneficial effect of pharmaceutical postconditioning with MDP to limit infarct size in WT mice. WT and RIP2−/− mice were divided into three study groups, as shown in Figure 1. In the control group (I-R), normal Krebs buffer was perfused during the pre- and post-ischaemic phase. In the MDP group (I-R MDP postcond), MDP (10 µg/mL) was perfused immediately at the onset of reperfusion for 15 min, followed by Krebs buffer alone for the remaining 105 min. The final group (I-R MDP 5-oxo) comprised the TAK1 inhibitor (5Z-7-oxozeanol, 1 µmol/L) with and without coincident MDP to determine the role of TAK1 in the postconditioning effect of MDP. The 5Z-7-oxozeanol (1 µmol/L) was perfused immediately at the onset of reperfusion for 15 min, followed by Krebs buffer alone for the remaining 105 min (Figure 1).
For protein biochemistry, WT and RIP2−/− hearts were instantly frozen in liquid nitrogen after 5 min of reperfusion with or without MDP or 5Z-7-oxozeanol and stored at −80ºC before use. WT and RIP2−/− mouse hearts perfused for 65 min were used as a control group.
2.6. Immunoblotting
Samples were thawed and homogenized in protein extraction buffer (500 mmol/L NaCl, 50 mmol/L Tris–HCl, pH 7.5, 1 mmol/L EDTA, 1% (v/v) Triton X-100, 0.1% β- mercaptoethanol, 0.1% SDS, 5 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, and protease inhibitor tablets (Complete®, Roche Applied Science) (1 Tab/50 mL of buffer). One millilitre of extraction buffer was used per 100 mg of frozen tissue. Homogenates were centrifuged at 4°C for 10 min at 13 000 rpm (15 600 g) and the clear supernatant kept for western blot analysis. Proteins were electrophoresed on a 10% polyacrylamide gel and then blotted onto a PVDF membrane. After blocking with 5% non-fat milk, the membranes were incubated with the following primary antibodies at dilutions of 1:1000 or 1:500 recognizing total p38 MAPK (catalog no. 9212), total TAK1 (catalog no. 4505), phospho-Thr187 of TAK1 (catalog no. 4536), Phospho-Ser271/Thr275 of MKK7 (catalog no. 4171), phospho- thr183/tyr185 of SAPK/JNK (catalog no. 9251), Phospho-Ser32 of IκBα (catalog no. 2859), and total IκBα (catalog no. 9242) from Cell Signaling Technology, Danvers, MA, USA.
2.7. Statistical analyses
All data are presented as means ± SEM. Comparisons between groups were assessed for significance by analysis of variance (ANOVA) or repeated measures ANOVA, as appropriate. When significant differences were detected, individual mean values were compared by Bonferroni’s post hoc test which allowed for multiple comparisons. P-values <0.05 were considered significant.
3. Results
3.1. MDP treatment is cardioprotective at reperfusion but not before the ischaemic period
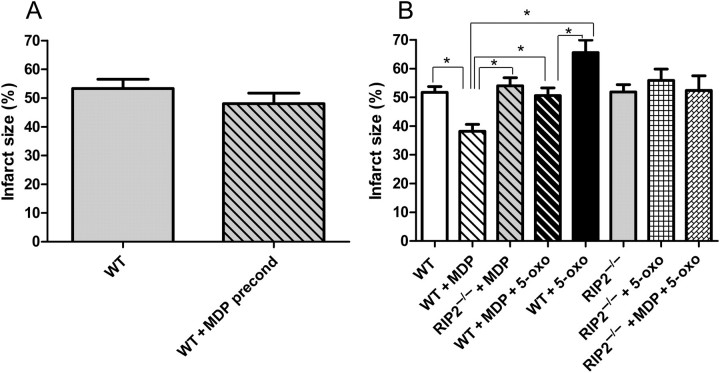
To determine whether MDP could have a cardioprotective effect in common with other components of the bacterial wall, we perfused mouse hearts with MDP for 15 min before the ischaemic period and measured haemodynamic recovery (Table 1) and infarct size (Figure 2A). Baseline coronary flow (CF) and contractile parameters were similar at the end of the stabilization period. The haemodynamic parameters remained the same before and after the 15 min perfusion of MDP (10 µg/mL). MDP treatment did not change the haemodynamic parameters compared with the control group, and the infarct size after 2 h was the same in control and MDP pre-ischaemia groups.
Table 1.
Summary of haemodynamic parameters in wild-type mouse hearts perfused with or without muramyl dipeptide for 15 min before the ischaemic period
| Treatment | n | Body weight (g) | Heart weight (mg) | LVDP (mmHg) |
LVEDP (mmHg) |
CF (mL/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | ||||
| WT | 5 | 27.1 ± 1.4 | 141 ± 5 | 65.2 ± 1.4 | 10.1 ± 2.1 | 10.4 ± 1.6 | 4.6 ± 1.1 | 53.5 ± 1.7 | 49.4 ± 2.3 | 2.5 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.1 |
| WT + MDP | 5 | 26.8 ± 1.5 | 135 ± 4 | 65.2 ± 2.8 | 8.4 ± 1.6 | 10.4 ± 2.0 | 5.0 ± 1.1 | 65.2 ± 6.5 | 58.2 ± 8.8 | 2.6 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.2 |
Values are means ± SEM. Left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP), and coronary flow (CF) were measured after 30 min of stabilization (baseline), and at 60 min (Rep 60 min) and 120 min (Rep 120 min) after the onset of reperfusion.
Figure 2.
Infarct size study. (A) Pharmacological preconditioning study. Infarct size was measured in wild-type (WT) hearts with or without muramyl dipeptide (MDP) before ischaemia. (B) Pharmacological postconditioning study. Infarct size was measured in WT and RIP2−/− hearts exposed to MDP (10 µg/mL) at the time of myocardial reperfusion, in the presence or absence of the TAK1 inhibitor (5Z-7-oxozeanol, 1 µmol/L). MDP is shown to reduce myocardial infarct size significantly only when administrated during reperfusion, and this cardioprotective effect is abolished in the RIP2−/− mice and in the presence of the TAK1 inhibitor. *P < 0.05 (n = 5–8 in each group).
Preconditioning and postconditioning have been widely studied and there is evidence that there are common and specific signalling pathways involved in these cardioprotective mechanisms. So we looked at the role of MDP at reperfusion as a postconditioning agent. MDP was administered for 15 min at the onset of reperfusion, and haemodynamic recovery (Table 2) and infarct size (Figure 2B) were measured. Baseline CF and contractile parameters were similar at the end of the stabilization period. However, when MDP was administered after the ischaemic period, the developed pressure after 2 h of reperfusion had increased by 50.2% (P < 0.05) over the control group. This recovery was reflected in the infarct size in MDP-exposed hearts, which was reduced from 51.7 ± 5.6% in control to 38.1 ± 6.7%, P < 0.05 (Figure 2B).
Table 2.
Summary of haemodynamic parameters in wild-type and RIP2−/− mouse hearts perfused with or without muramyl dipeptide for 15 min after the ischaemic period, associated or not with a TAK1 inhibitor (5Z-7-oxozeanol)
| Treatment | n | Body weight (g) | Heart weight (mg) | LVDP (mmHg) |
LVEDP (mmHg) |
CF (mL/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | Baseline | 60 min Rep | 120 min Rep | ||||
| WT | 8 | 25.1 ± 1.5 | 135.5 ± 4.9 | 68.6 ± 2.2 | 7.0 ± 1.8 | 10.0 ± 2.4 | 4.4 ± 0.9 | 56.0 ± 1.3 | 45.3 ± 1.1 | 2.7 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 |
| WT + MDP | 8 | 24.8 ± 1.2 | 134.5 ± 3.4 | 68.0 ± 1.8 | 15.6 ± 3.8* | 19.9 ± 3.3* | 6.1 ± 0.5 | 43.0 ± 4.7* | 31.9 ± 3.3* | 2.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| RIP2−/− | 6 | 26.1 ± 1.8 | 135.5 ± 6.2 | 74.5 ± 1.1 | 4.3 ± 2 | 7.2 ± 2.1 | 3.7 ± 0.8 | 58.7 ± 6.0 | 46.2 ± 6.9 | 2.7 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 |
| RIP2−/− + MDP | 6 | 25.9 ± 1.4 | 139.4 ± 5.2 | 74.0 ± 1.6 | 9.0 ± 4$ | 7.8 ± 3.8 | 5.0 ± 1.1 | 58.8 ± 4.4 | 49.2 ± 5.3 | 2.7 ± 0.1 | 1.4 ± 0.2 | 1.1 ± 0.1 |
| WT + 5-oxo | 5 | 25.9 ± 2.3 | 133.3 ± 5.4 | 69.2 ± 1.5 | 4.6 ± 1.3 | 8.6 ± 1.7 | 3.4 ± 0.6 | 54.2 ± 1.7 | 52.0 ± 7.3 | 2.3 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| WT + 5-oxo + MDP | 5 | 26.5 ± 2.1 | 136.4 ± 6.3 | 73.6 ± 2.8 | 3.6 ± 1.7# | 9.2 ± 2.3# | 2.4 ± 0.4 | 50.8 ± 3.0 | 46.0 ± 4.1 | 2.4 ± 0.4 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| RIP−/− + 5-oxo | 6 | 26.9 ± 1.5 | 142.1 ± 9.4 | 79.0 ± 3.7 | 5.5 ± 1.4 | 5.3 ± 1.7 | 6.3 ± 1.0 | 62.0 ± 2.7 | 58.2 ± 4.0 | 2.9 ± 0.3 | 1.6 ± 0.2 | 1.3 ± 0.2 |
| RIP2−/− + 5-oxo + MDP | 6 | 26.8 ± 1.2 | 136.2 ± 10.4 | 77.2 ± 2.8 | 7.2 ± 0.9 | 6.2 ± 1.1 | 5.8 ± 0.6 | 60.6 ± 5.1 | 55.6 ± 4.3 | 2.9 ± 0.2 | 1.7 ± 0.3 | 1.4 ± 0.2 |
Values are means ± SEM. Left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP), and coronary flow (CF) were measured after 30 min of stabilization (baseline), and at 60 min (Rep 60 min) and 120 min (Rep 120 min) after the onset of reperfusion.
*P < 0.01 WT vs. WT + MDP.
$P < 0.01 WT + MDP vs. RIP2−/− + MDP.
#P < 0.01 WT + MDP vs. WT + MDP + 5-oxo.
3.2. MDP is acting through RIP2
MDP has been described as a ligand of the NOD2 receptor and acts via the recruitment of RIP2 protein. To identify whether RIP2 is implicated in the cardioprotective effect of MDP, we perfused RIP2−/− mice. In mice lacking RIP2, the protective effects of MDP are absent (Table 2 and Figure 2B). Thus, RIP2 is necessary for cardioprotection with MDP.
3.3. TAK1 activity is cardioprotective
Upon activation, RIP2 has been described to form a complex with other proteins including TAK1 and TNF receptor-associated factor 6. To determine the relevance of this axis to our observation, we perfused mouse hearts with the TAK1 inhibitor 5Z-7-oxozeanol at the onset of reperfusion.
5Z-7-Oxozeanol abolished the decrease in the infarct size observed in the MDP-treated hearts (38.1 ± 6.7% in MDP vs. 50.6 ± 6.1% with MDP + 5Z-7-oxozeanol, P < 0.05; Figure 2B), indicating that TAK1 is required for MDP’s cardioprotective effect. It is of interest to note that the administration of 5Z-7-oxozeanol alone significantly increased infarct size (65.6 ± 9.6% vs. 51.7 ± 5.6%, P < 0.05), suggesting a potential role for TAK1 in the modulation of infarction in the absence of MDP. To rule out any non-specific toxic effect of the 5Z-7-oxozeanol, we perfused the TAK1 inhibitor alone for 15 min and we did not notice any alteration of contractility (data not shown).
3.4. Biochemical analysis of the cardioprotective effect
To better understand the mechanisms responsible for the cardioprotective effect of MDP during reperfusion, we examined the activation of the known proteins downstream of RIP2: TAK1, JNK, and MKK.
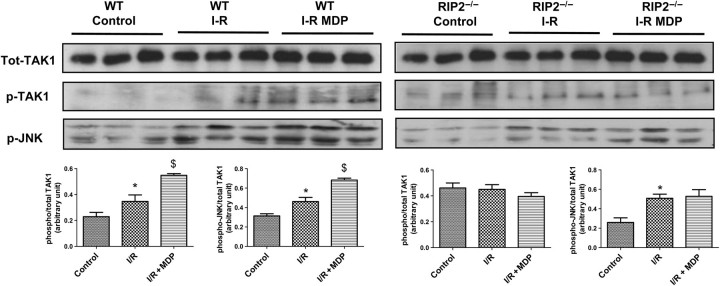
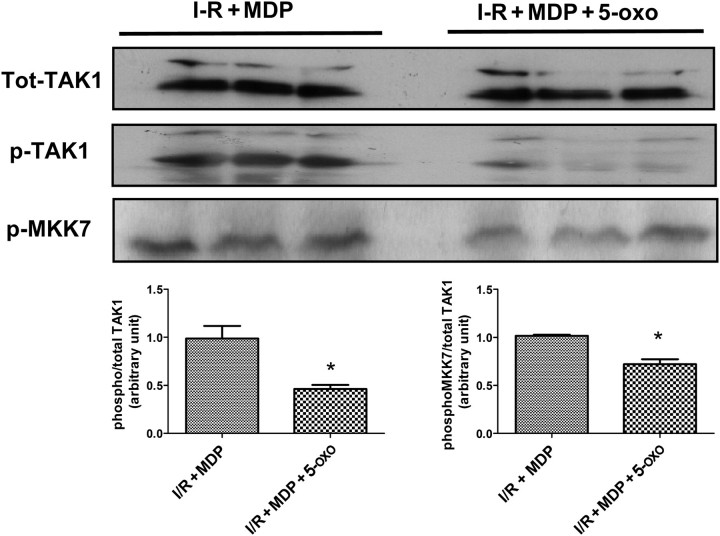
As previously published, ischaemia–reperfusion in WT mice triggers the activation of many kinases including TAK1 and JNK (Figure 3, left panel). MDP exposure at reperfusion was able to increase the activating phosphorylation of TAK1 and JNK (Figure 3). In RIP2−/− mice (Figure 3, right panel), TAK1 phosphorylation states were similar with or without ischaemia; however, JNK was more phosphorylated during the reperfusion phase. MDP was not able to modify TAK1 or JNK phosphorylation in the RIP2−/− mice. To be sure that the perfusion of TAK1 inhibitor 5Z-7-oxozeanol was effective, we probed for the autophosphorylation site of TAK1 (Thr187) (Figure 4), the diminution of phosphorylation confirms pharmacological inhibition of TAK1 and MKK7 during reperfusion.
Figure 3.
Effects of muramyl dipeptide (MDP) at reperfusion on the activation of mitogen-activated protein kinase pathway in isolated mouse heart. Hearts from wild-type (n = 3) and RIP2-deficient (RIP2−/−; n = 3) mice were subjected to 30 min of aerobic perfusion, 30 min of global ischaemia, and 5 min reperfusion with Krebs buffer (I-R) or with MDP (10 µg/mL) (I-R MDP). Control hearts are subjected to 65 min of aerobic perfusion. Representative immunoblots are shown with quantitative analyses of repeat experiments (lower panel) expressed as the ratio of phosphorylated to total protein. *P < 0.05 control vs. I/R; $P < 0.05 I/R vs. I/R + MDP.
Figure 4.
Effects of the TAK1 inhibitor (5Z-7-oxozeanol, 1 µmol/L) on the activation of pTAK1 and pMKK7 during the reperfusion phase after global ischaemia of the isolated mouse heart. Hearts from wild-type mice were subjected to 30 min of aerobic perfusion, 30 min of global ischaemia, and 5 min reperfusion with muramyl dipeptide (MDP) alone (10 µg/mL) (I-R MDP) (n = 3) or MDP + 5Z-7-oxozeanol (ischaemia + MDP + 5-oxo) (n = 3). Representative immunoblots are shown (lower panel) with quantitative analyses of repeat experiments expressed as the ratio of phosphorylated to total protein. *P < 0.05.
The role of glycogen synthase kinase (GSK)3β in postconditioning is a matter of debate and has been implicated by some groups20,21 but not by others22 nor by our own lab.23 Nonetheless, we decided to check whether the GSK3 pathway could be linked to MDP-mediated protection. We looked at GSK3β phosphorylation and its upstream partner Akt. Both are phosphorylated during reperfusion; however, no difference was observed in the presence of MDP, indicating that GSK3β or Akt involvement is unlikely (data not shown).
3.5. MDP modulation of NFκB signalling
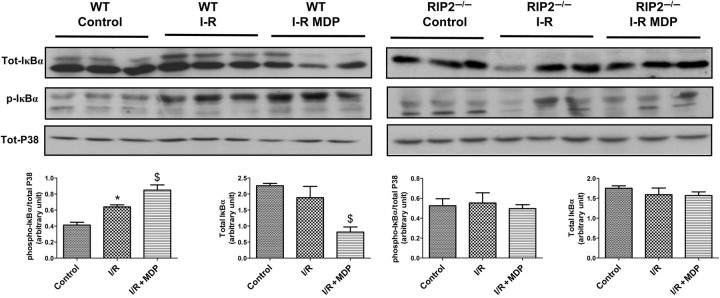
Myocardial cardioprotection conferred by modulation of cytokine production via NFκB and TAK1 has been linked to direct phosphorylation of the IkappaB kinase (IKK) complex, which in turn phosphorylates IκBα.24 IκBα binds to NFκB and sequesters it in the cytosol, preventing its role as a transcription factor. After phosphorylation by IKK complex, IκBα is rapidly degraded, and released NFκB can translocate to the nucleus where it drives the expression of many genes.25 We wanted to elucidate whether MDP-TAK1 cardioprotection signals through NFκB. In Figure 5, we studied IκBα phosphorylation and the level of total IκBα after ischaemia–reperfusion with or without MDP. In the WT, IκBα was phosphorylated during reperfusion, an effect enhanced by MDP. The IκBα protein was degraded in the presence of MDP as reflected by a reduction in total IκBα. In the RIP2−/− mice, the IκBα phosphorylation level was similar between control and ischaemia/reperfusion with or without MDP and IκBα protein did not seem to be degraded. These results indicate that the modulation of the NFκB pathway may be involved in cardioprotection by MDP.
Figure 5.
Effects of muramyl dipeptide (MDP) at reperfusion on the activation of IκBα pathway in isolated mouse heart. Hearts from wild-type (n = 3) and RIP2-deficient (RIP2−/−, n = 3) mice were subjected to 30 min of aerobic perfusion, 30 min of global ischaemia, and 5 min reperfusion with Krebs buffer (I-R Krebs) or with MDP (10 µg/mL) (I-R MDP). Control hearts were subjected to 65 min of aerobic perfusion. Representative immunoblots are shown with quantitative analyses of repeat experiments expressed as the ratio of phosphorylated to total protein. *P < 0.05 control vs. I/R; $P < 0.05 I/R vs. I/R + MDP.
4. Discussion
The major finding in this study is that MDP at reperfusion induced cardioprotection, mediated via an RIP2/TAK1-dependent mechanism. We demonstrated that administration of a pharmacological inhibitor of TAK1 abrogated the protective postconditioning effect of MDP in myocardial I/R injury. We also showed that the cardioprotective effect of MDP administrated during reperfusion was abolished in RIP2 null hearts. Thus, RIP2 and TAK1 are involved in MDP-initiated postconditioning. Furthermore, our data suggest that the activation of several downstream kinases (such as MKK7/JNK pathway) results directly, or indirectly, in the modulation of NFκB activation. To our knowledge, this is the first report which causally links MDP-induced cardioprotection with the RIP2/TAK1 signalling pathway.
In our previous study,14 we did not find any evidence that RIP2 contributed to the development of myocardial infarction; however, RIP2 may be involved in the myocardial response to MDP. LPS and MDP are both components of bacterial cell wall that act synergistically, so it is possible that some cardioprotective pathways activated by LPS such as Akt, ERK, and IKKβ could be shared by MDP. Previous studies have shown that pre-treatment of animals with a low dose of LPS for 24 h results in protection against I/R injury through the PI3K/Akt pathway.9 The role of MDP after acute myocardial ischaemia has been studied in vivo in a different protocol and shown to facilitate infarct expansion in association with the promotion of monocyte recruitment and inappropriate collagen synthesis.26 But in our hands in a different species and model with a different protocol where monocyte recruitment was impossible, we demonstrated a cardioprotective effect of MDP. It is interesting to note that in addition to LPS, the toll-like receptor-4 recognizes heat shock proteins (Hsp-60 and Hsp-70) that are produced in response to myocardial ischaemia and confer potent cardioprotective effects in the heart. So further studies are necessary to delineate the complex cardioprotective effect of MDP and identify possible common partners with the better characterized LPS cardioprotective pathway (for review, see Chao27).
Another cardioprotective pathway GSK3β and other reperfusion injury salvage kinase (RISKs) have been implicated by others in postconditioning,20,21 but in our previous study23 and in the current study, we could not associate the cardioprotective effect of MDP with GSK3β inactivation. This controversial observation may be explained by in vivo vs. ex vivo models, different triggers of protection (isoflurane vs. MDP) or temporal variations (duration of I/R cycle) in the postconditioning protocol.
In this study, by inhibiting TAK1 activity with 5Z-7-oxozeanol, we revealed the cardioprotective role of TAK1. TAK1 has already been associated with cell survival in other compartments, i.e. osteoclast cells,28 and studies in adult mouse myocardium, where TAK1 is activated after aortic banding, and was sufficient to provoke heart failure.29 Thus, the role of TAK1 in heart pathophysiology is likely complex with an early cardioprotective role during short-term activation over minutes during ischaemia–reperfusion but a possible deleterious role with more prolonged activation over days. Superficially, at least, it seems surprising that TAK1 activation can be beneficial. However, the suggested paradigm of brief activation having a beneficial consequence is very similar to that seen in the related TNF signalling cascade. Here, it is clear that prolonged TNF exposure leads to cardiac dilatation, heart failure, and premature death,30 in a manner analogous to chronic TAK1 activation,29 while brief exposure is cardioprotective.31
Since postconditioning could be useful for clinicians, the identification of a new mechanism of postconditioning is valuable. Obviously, the direct use of MDP in patients is unlikely but the modulation of the signalling pathway identified in this study could be the target for new cardioprotective drugs. Further studies are required to determine the safety of modulation of RIP2/TAK1 pathway in an in vivo model and whether other survival kinases activated at the time of myocardial reperfusion contribute to the protection observed in the postconditioned heart.
Conflict of interest: none declared.
Funding
This work was supported by projects grants from the British Heart Foundation (07/073/23432) and Guy’s and St Thomas Charity (R060701). The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London.
References
- 1.Lim SY, Davidson SM, Paramanathan AJ, Smith CC, Yellon DM, Hausenloy DJ. The novel adipocytokine visfatin exerts direct cardioprotective effects. J Cell Mol Med. 2008;12:1395–1403. doi: 10.1111/j.1582-4934.2008.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 5.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, et al. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA. 1989;86:2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Z, Auchampach JA, Pieper GM, Gross GJ. Cardioprotective effects of monophosphoryl lipid A, a novel endotoxin analogue, in the dog. Cardiovasc Res. 1993;27:832–838. doi: 10.1093/cvr/27.5.832. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Ao L, Brown JM, Meldrum DR, Sheridan BC, Cain BS, et al. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-alpha. Am J Physiol. 1997;273:H1894–H1902. doi: 10.1152/ajpheart.1997.273.4.H1894. [DOI] [PubMed] [Google Scholar]
- 9.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, et al. Modulating Toll-like receptor mediated signaling by (1–>3)-beta-d-glucan rapidly induces cardioprotection. Cardiovasc Res. 2004;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2006;290:H709–H715. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet S, Nishino Y, Kumphune S, Sicard P, Clark JE, Kobayashi KS, et al. The role of RIP2 in p38 MAPK activation in the stressed heart. J Biol Chem. 2008;283:11964–11971. doi: 10.1074/jbc.M707750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwanaga Y, Davey MP, Martin TM, Planck SR, DePriest ML, Baugh MM, et al. Cloning, sequencing and expression analysis of the mouse NOD2/CARD15 gene. Inflamm Res. 2003;52:272–276. doi: 10.1007/s00011-003-1170-z. [DOI] [PubMed] [Google Scholar]
- 16.Murch O, Abdelrahman M, Kapoor A, Thiemermann C. Muramyl dipeptide enhances the response to endotoxin to cause multiple organ injury in the anesthetized rat. Shock. 2008;29:388–394. doi: 10.1097/shk.0b013e3181453e59. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 18.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, et al. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93:254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- 19.Bellahcene M, Jacquet S, Cao XB, Tanno M, Haworth RS, Layland J, et al. Activation of p38 mitogen-activated protein kinase contributes to the early cardiodepressant action of tumor necrosis factor. J Am Coll Cardiol. 2006;48:545–555. doi: 10.1016/j.jacc.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 22.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 23.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, et al. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem. 2008;283:24497–24505. doi: 10.1074/jbc.M802825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 26.Maekawa Y, Anzai T, Yoshikawa T, Sugano Y, Mahara K, Kohno T, et al. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–1520. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 27.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ. TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp Cell Res. 2008;314:2725–2738. doi: 10.1016/j.yexcr.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 30.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 31.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]