Abstract
Reductive alkylation of Diels-Alder derived ring junction– α-cyanoketones provides a route to trans-fused bicyclic systems.
Keywords: Diels–Alder, Reductive Alkylation, trans-Octalone, Cyanoketone
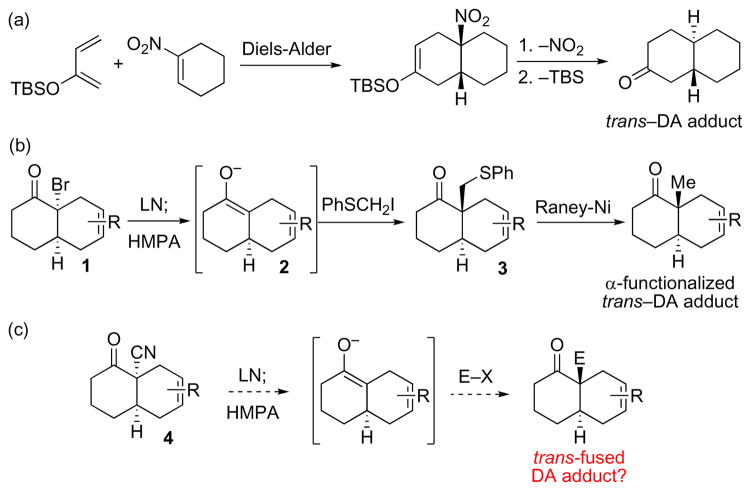
In the context of our “pattern recognition” approach as an aid in retrosynthetic analysis, we seek to discern substructural units – or motifs – within the target structures that are accessible through reasonable chemistry.1 Translating a target synthesis problem to the identification of such an accessible pattern (or patterns) may well be non-obvious but may, in the end, provide a particularly clear path for progress. Obviously, the value of this type of thought process grows as the number of accessible patterns (“safe havens”) increases. For example, recognition of a cis–fused junction in a bicyclic motif where at least one of the rings is 6-membered, typically suggests a classical Diels-Alder (DA)–based strategy. In an effort to expand the menu of useful patterns available, we recently undertook to study a possible trans–DA paradigm,2 The logic involves equipping a cyclic dienophile with a temporary activating group which enhances proclivities for formation of the bicicyclic adduct. Subsequent cleavage of this angular functionality and introduction of a new function at the same site, with overall inversion of configuration provides entry into the trans-fused series (Figure 1a).2a In pursuit of such a “trans–DA” paradigm, we have developed the capacity to rapidly construct trans-fused decalinoid motifs bearing angular methyl groups (Figure 1b).2c Thus, following DA cycloaddition, cis-fused α-bromoketones (1) are obtained. Exposure to lithium naphthalide (LN), and alkylation of the resultant enolate 2 with iodomethyl phenyl sulfide provides the angularly functionalized trans-fused adduct with high trans stereoselectivity in the very important octalin series (3). Following Raney Ni-mediated desulfurization, the angularly methylated trans-octalone system is in hand. While direct methylation also provides preferential transfused stereoisomer in the octalin series, the two-step protocol described above affords significantly higher trans-selectivities.
Figure 1.
Based on precedent from our α-bromoketone-based studies we hypothesized that it should also be possible to functionalize an analogous system bearing an angular cyano moiety (Figure 1c). It seemed likely that an α-cyanocyclenone system (cf. 4) would be a more reactive dienophile than is its corresponding α-bromo counterpart.
Indeed, Liu and co-workers had reported that angularly cyanylated systems of the type 4 could be prepared by DA cycloaddition. Interestingly, they had reported that methylation of these compounds gave cis-fused products. However, the systems employed in their studies incorporated structural features which would be expected to dispose them toward cis-alkylation.3,4 Thus, we sought to explore the feasibility of converting cis-fused α-cyanoketones (4) to trans-fused angularly alkylated products through recourse to the conditions which were successful in the α-bromoketone alkylation (see Figure 1b). In this context, we could also explore a tangential, nonetheless interesting, question, i.e. whether the distereoselectivies of enolate alkylations would be essentially the same starting with a α-bromo or α-cyano precursors.
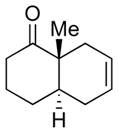
We first examined the direct methylation of α-cyanoketones. Thus, a variety of cis-fused cycloadducts (4a–4d) readily underwent angular alkylation with MeI as an electrophile (Method A). As anticipated, trans-fused adducts were predominantly formed, albeit with moderate levels of selectivity (Table 1). The isolated cis- and trans- isomers were rigorously characterized through spectral comparison with known standards prepared by alternative methods.2c
Table 1.
Angular alkylation of α-cyanoketones.a
 | |||||
|---|---|---|---|---|---|
| entry | α-cyanoketone | Method A | Method B | ||
| product | yield trans:cis | product | yield trans:cis | ||
| 1 |
 4a |
 5a |
78% 7:1 |
 6a |
72% 7:1 |
| 2 |
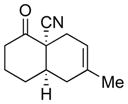 4b |
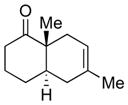 5b |
65% 2:1 |
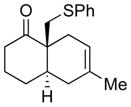 6b |
75% 8:1 |
| 3 |
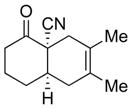 4c |
 5c |
75% 3:1 |
 6c |
67% 7:1 |
| 4 |
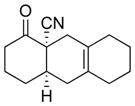 4d |
 5d |
67% 2:1 |
 6d |
62% 7:1 |
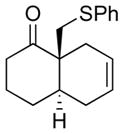
Having established the propensity of the enolate derived from the α-cyanoketone to undergo alkylation to provide trans-fused adducts, we next sought to improve the selectivity ratios of the products (Table 1). As precedented,2c when iodomethyl phenyl sulfide5 was used as the electrophile, the product stereoselectivities were markedly improved. The levels of alkylation selectivity achieved under these conditions were quite similar to those observed in our previous study using α-bromoketone substrates. However, we note that, in general, the analogous α-bromoketones appear to afford somewhat higher trans:cis ratios.5
Finally, we examined the reductive allylation of α-cyanoketones 4b and 4c, with allylbromide as the electrophile. The reactions were conducted under conditions that were previously reported to provide exclusively cis-fused products.3 Interestingly, we also obtained predominantly cis-fused adducts, though in our hands, isomeric mixtures (2:1 and 3:1, respectively) were observed (Scheme 1).
Scheme 1.
Allylation of α-cyanoketones 4a and 4b.
In summary, we describe herein a useful extension of our trans-Diels-Alder paradigm, through the development of a trans-selective reductive alkylation of cis-fused α-cyanoketone Diels-Alder adducts. We note that our stereochemical assignments are fully supported by comparisons with known standards in the readily accessible (via Diels-Alder reactions) cis series. Moreover, the selectivities reported herein are entirely consistent with those obtained in the context of our analogous α-bromoketone alkylation protocol. The method described here has the major advantage in that it exploits the cyano substituted dienophile which is much more reactive than is the previously used bromo version. Experiments building upon these findings are in progress.
Supplementary Material
Acknowledgments
This work was supported by the NIH (HL25848 to S.J.D.). F.P. thanks Eli Lilly and Company for a graduate fellowship. Special thanks to Ms. Rebecca Wilson for valuable help in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson RM, Danishefsky SJ. J Org Chem. 2007;72:4293–4305. doi: 10.1021/jo070871s. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kim WH, Lee JH, Danishefsky SJ. J Am Chem Soc. 2009;131:12576–12578. doi: 10.1021/ja9058926. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim WH, Lee JH, Aussedat B, Danishefsky SJ. Tetrahedron. 2010;66:6391–6398. doi: 10.1016/j.tet.2010.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee JH, Zhang Y, Danishefsky SJ. J Am Chem Soc. 2010;132:14330–14333. doi: 10.1021/ja1073855. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shibatomi K, Futatsugi K, Kobayashi F, Iwasa S, Yamamoto H. J Am Chem Soc. 2010;132:5625–5627. doi: 10.1021/ja1018628. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Shia K, Liu H. Chem Comm. 2000:1599–1600. [Google Scholar]
- 4.(a) Liu HJ, Yip J, Shia KS. Tetrahedron Lett. 1997;38:2253–2256. [Google Scholar]; (b) Liu HJ, Zhu JL, Shia KS. Tetrahedron Lett. 1998;39:4183–4186. In some instances, Liu and coworkers reported very high levels of cis selectivity. However, these were in cases bearing a methyl group para to the ketone. We, too, have found this trend. [Google Scholar]
- 5.(a) Trost BM, Kunz RA. J Org Chem. 1974;39:2648–2650. [Google Scholar]; (b) Trost BM, King SA. J Am Chem Soc. 1990;112:408–422. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.