Abstract
In mammals, spermatogenesis is maintained throughout life by a small subpopulation of type A spermatogonia called spermatogonial stem cells (SSCs). In rodents, SSCs, or Asingle spermatogonia, form the self-renewing population. SSCs can also divide into Apaired (Apr) spermatogonia that are predestined to differentiate. Apaired spermatogonia produce chains of Aaligned (Aal) spermatogonia that divide to form A1 to A4, then type B spermatogonia. Type B spermatogonia will divide into primary spermatocytes that undergo meiosis. In human, there are only two different types of A spermatogonia, the Adark and Apale spermatogonia. The Adark spermatogonia are considered reserve stem cells, whereas the Apale spermatogonia are the self-renewing stem cells. There is only one generation of type B spermatogonia before differentiation into spermatocytes, which makes human spermatogenesis less efficient than in rodents. Although the biology of human SSCs is not well known, a panel of phenotypic markers has recently emerged that is remarkably similar to the list of markers expressed in mice. One such marker, the orphan receptor GPR125, is a plasma membrane protein that can be used to isolate human SSCs. Human SSCs proliferate in culture in response to growth factors such as GDNF, which is essential for SSC self-renewal in mice and triggers the same signaling pathways in both species. Therefore, despite differences in the spermatogonial differentiation scheme, both species use the same genes and proteins to maintain the pool of self-renewing SSCs within their niche. Spermatocytic seminomas are mainly found in the testes of older men, and they rarely metastasize. It is believed that these tumors originate from a postnatal germ cell. Because these lesions can express markers specific for meiotic prophase, they might originate form a primary spermatocyte. However, morphological appearance and overall immunohistochemical profile of these tumors indicate that the cell of origin could also be a spermatogonial stem cell.
Keywords: spermatogonial stem cells, human, mouse, spermatocytic seminoma
Introduction
Adult spermatogenesis is a complex and tightly regulated process in which a small pool of germ-line stem cells develops to ultimately form spermatozoa (de Rooij and Russell, 2000). These stem cells, called spermatogonial stem cells (SSCs) are found in the basal compartment of the seminiferous epithelium, where they adhere to the basement membrane. SSC self-renewal ensures the maintenance of the stem cell pool, while their differentiation will generate a large number of germ cells. Therefore, a balance between SSC self-renewal and differentiation in the adult testis is essential to maintain normal spermatogenesis and fertility throughout life. The fate of SSCs is controlled by their microenvironment, or niche (Spradling et al., 2001). Essential components of the niche are the nursing Sertoli cells that physically support the SSCs and provide them with growth factors (Hofmann, 2008). In addition, recent work from several research groups revealed the importance of factors produced by cells in the interstitium between the seminiferous tubules, and also by peritubular myoid cells (Kokkinaki et al., 2009; Oatley et al., 2009). An important characteristic of SSCs is their ability to acquire pluripotency and to differentiate into tissues belonging to the three embryonic germ layers when transferred onto different niche microenvironments (Kanatsu-Shinohara et al., 2004; Guan et al., 2006; Seandel et al., 2007; Conrad et al., 2008; Kossack et al., 2008; Golestaneh et al., 2009). Alteration of signals from the niche can produce drastic changes in the behavior and morphology of spermatogonial stem cells, allowing them to develop in many different tissues without prior genetic modifications (Simon et al., 2009).
According to the WHO classification of testicular tumors (Mostofi and Sesterhenn, 1985), there are two types of human testicular seminomas. The first type is the classical seminoma (SE), which is more common in young adults between 20 and 45 years (Misener and Fuller, 1995) (Bergstrom et al., 1996). It is now established that SE originates from carcinoma in situ cells (CIS) (Skakkebaek et al., 1987; Rorth et al., 2000), which represent the pre-cancerous counterpart of primordial germ cells (PGC) and gonocytes. The neoplastic transformation of normal PGC and gonocytes might in part result from hormonal or environmental imbalance (Skakkebaek et al., 1998; Dieckmann and Skakkebaek, 1999). The second type is called spermatocytic seminoma (SS), a benign tumor that may become a life threatening disease if it progresses to sarcoma (Mikuz, 2002). A series of studies investigating the cellular origin of spermatocytic seminoma revealed that these tumors do not arise from embryonic/fetal germ cells since they do not express embryonic markers. Rather spermatocytic seminoma seems to originate from more differentiated, postnatal germ cells (Stoop et al., 2001; Satie et al., 2002; Rajpert-De Meyts et al., 2003; Verdorfer et al., 2004; Looijenga et al., 2006; De Jong et al., 2007; Gillis et al., 2007). Expression profiling of spermatocytic seminoma samples have indicated that this type of tumor might originate from primary spermatocytes (Looijenga et al., 2006). However, recent advances in the characterization of markers for human type A spermatogonia also suggest a postnatal gonocyte, or a spermatogonial stem cell origin.
Spermatogonial self-renewal and differentiation in the mouse testis
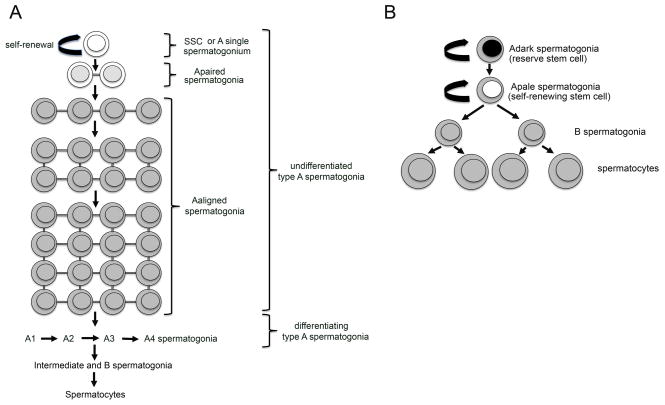
After birth, gonocytes (prespermatogonia) that were quiescent in the center of the seminiferous tubules resume proliferation and migrate to the basement membrane where they will become SSCs (de Rooij and Grootegoed, 1998). This pool of SSCs established during the first 10 days after birth will maintain spermatogenesis throughout life. SSCs (Asingle or As) are a subpopulation of type A spermatogonia. Asingle spermatogonia are isolated cells able to self renew, or to divide and differentiate into Apaired (Apr) spermatogonia. Apaired spermatogonia are cell doublets that remain linked by a cytoplasmic bridge. They divide synchronously into chains of Aaligned (Aal) spermatogonia. Because morphologically they show few characteristics of nuclear or cytoplasmic differentiation, Asingle, Apaired and Aaligned cells have been collectively called undifferentiated spermatogonia (de Rooij and Russell, 2000). Recently, Nakagawa and colleagues elegantly demonstrated that some Aaligned spermatogonia may possess the potential for self-renewal (potential stem cells) upon loss of the actual stem cells in cases of injury or natural depletion during lifelong spermatogenesis (Nakagawa et al, 2007). Aaligned spermatogonia further differentiate into A1 spermatogonia that undergo 6 divisions (A1 to A4, or differentiating spermatognia), to become intermediate and B spermatogonia. B spermatogonia give rise to spermatocytes that enter meiosis to produce haploid spermatids (de Rooij and Russell, 2000) (Figure 1A). Spermatids become spermatozoa through the process of spermiogenesis. In the adult male mouse, the population of SSCs is about 0.03% of the total content of germ cells (Tegelenbosch and de Rooij, 1993).
Figure 1. Schematic representation of the first steps of spermatogenesis in rodents and human.
A: In rodents, the SSCs (or Asingle spermatogonia) are the only self-renewing spermatogonia. They can divide to produce 2 Apaired spermatogonia linked by a cytoplasmic bridge (incomplete cytokinesis). Germ cell number increases by clonal expansion of Aaligned spermatogonia that produce expanding chains of cells. Aaligned spermatogonia further divide into A1 to A4 differentiating spermatogonia. In turn, intermediate and B spermatogonia are produced that will differentiate into spermatocytes.
B: In the human, it is generally believed that 2 types of stem cells are present, the Adark spermatogonia (reserve stem cell) and the Apale spermatogonia (self-renewing stem cell). Apale divide into type B spermatogonia that divide into spermatocytes.
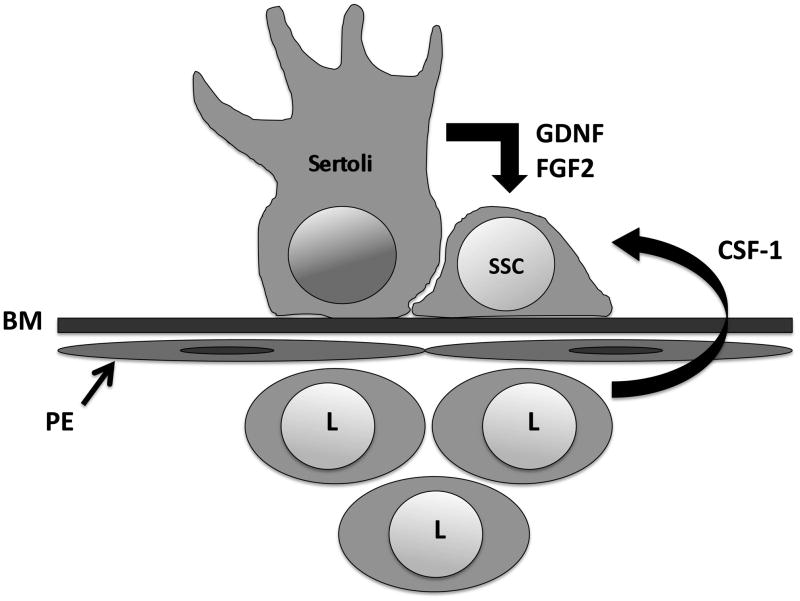
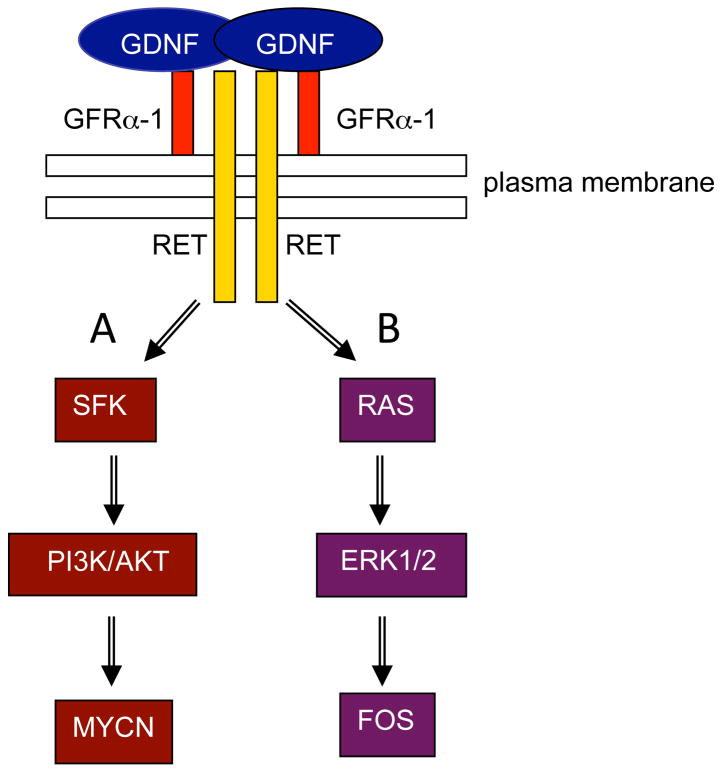
As mentioned above, the spermatogonial stem cell niche controls the balance between proliferation and differentiation of SSCs. Sertoli cells are a crucial component of this niche and produce glial cell line-derived neurotrophic factor (GDNF), a distant member of the TGFβ family, which controls SSC self-renewal (Meng et al., 2000) (Figure 2). GDNF is critical for the maintenance of permanent spermatogenesis (Naughton et al., 2006). For instance, transgenic animals over-expressing the Gdnf gene develop a phenotype with seminiferous tubules invaded by undifferentiated spermatogonia (Meng et al., 2001). Conversely, a Gdnf knockout model develops a Sertoli cell-only phenotype (Naughton et al., 2006). Furthermore, several groups reported an increase of proliferation of SSCs from diverse species when GDNF was added to freshly isolated germ cells in culture (Kanatsu-Shinohara et al., 2003; Hofmann et al., 2005; Aponte et al., 2008; Wu et al., 2009). GDNF binding to the GFRA1/RET receptor complex (GDNF Receptor Alpha-1/REarranged during Transfection) induces the activation of two signaling pathways involved in the survival and self-renewal of SSCs (Figure 3). The transcription factors MYCN (Braydich-Stolle et al., 2007) and FOS (He et al., 2008) are known GDNF signaling pathways targets. In one pathway, phosphorylation of RET and SRC-kinase family proteins (SFKs) followed by phosphatidylinositol 3-kinase (PI3K) phosphorylation, activates the AKT pathway and finally Mycn gene expression (Braydich-Stolle et al., 2007; Lee et al, 2007)(Figure 3A). Binding of GDNF to GFRA1 and RET also triggers the activation of the canonical RAS-ERK1/2 pathway and regulates SSCs proliferation through the activation of another transcription factor, FOS (Figure 3B). In the SSCs, FOS controls the expression of the gene Ccna2 (Cyclin A2) (He et al., 2008). Another gene that GDNF up-regulates is Fgfr2, which encodes the receptor for FGF2, itself produced by Sertoli cells (Hofmann et al., 2005)(Figure 2). Addition of both GDNF and FGF2 in SSC cultures indicated cooperation between these pathways, leading to improved SSC self-renewal (Kubota et al., 2004). Other factors within the niche influence the fate of SSCs, such as colony stimulating factor 1 (CSF1) which is produced by Leydig cells and some peritubular myoid cells (Oatley et al., 2009) and also plays a role in self-renewal (Figure 2). Another member of the fibroblast growth factor family, FGF9, has been recently shown to maintain expression of pluripotency-related genes and acts as an inhibitor of meiosis (Barrios et al, 2010; Bowles et al, 2010). Finally, KIT ligand (KITLG, stem cell factor, SCF), which is also produced by Sertoli cells, induces the differentiation of Aaligned spermatogonia into A1 cells (Pellegrini et al, 2008). Interestingly, Kanetsky and colleagues recently demonstrated that in humans, common genetic variations in KITLG are often associated with testicular germ cell tumors (Kanetsky et al, 2009).
Figure 2. A simplified view of the spermatogonial stem cell niche showing the main extrinsic factors driving SSC maintenance and self-renewal.
Sertoli cells and spermatogonial stem cells (SSCs) are both attached to the basement membrane (BM). Sertoli cells provide for structural support and produce glial cell line-derived neurotrophic factor (GDNF) and basic fibroblast growth factor (bFGF) which are crucial for SSC self-renewal in vitro and in vivo. Leydig cells (L) and peritubular cells (PE) produce colony-stimulating factor-1 (CSF-1), also essential for self-renewal.
Figure 3. Signaling pathways triggered by GDNF in spermatogonial stem cells.
GDNF dimerizes and binds to the GFRα-1/RET receptor complex. A: Binding of GDNF activates RET, which triggers SRC kinase phosphorylation and the downstream activation of PI3K/AKT. Ultimately the transcription factor MYCN is up-regulated. B: Binding of GDNF also can activate the RAS-mediated signaling pathway, which triggers ERK1/2 phosphorylation and up-regulation of the transcription factor FOS.
Meng and colleagues also reported that transgenic mice overexpressing GDNF exhibited an increase in proliferation of clusters of undifferentiated type A spermatogonia (Meng et al., 2000; Meng et al., 2001). These cell clusters were stained by EE2, a monoclonal antibody recognizing a glycoprotein at the surface of spermatogonia (Koshimizu et al, 1995). They also expressed GFRA1 and RET, which are markers for undifferentiated spermatogonia. The clusters did not express c-KIT, a marker for differentiating spermatogonia. Testicular tumors develop regularly in older GDNF-overexpressing mice. In those mice, the clusters invariably start to spread into the testicular interstitium and form non-metastatic tumors (Meng et al., 2001). Although these experimentally induced tumors mimic human classical seminomas in several aspects (Viglietto et al., 2000), some differences are evident such as the appearance of tumors at old age in mouse versus at young age in man, tumor development in the context of severely distorted spermatogenesis in mouse vs. normal spermatogenesis in man, high frequency of bilateral tumors in mouse (unilateral in man), and absence of large lymphocyte infiltrates in mouse while these are present in most classical seminomas (Mostofi, 1980; Skakkebaek et al., 1987). In addition, c-KIT expression is negative in the mouse model vs positive in human seminoma (Skakkebaek et al., 1987; Looijenga and Oosterhuis, 1999). Interestingly, GDNF-induced tumors showed aneuploidy associated to a distinct triploid peak observed with flow cytometry, which is a common finding in classical seminoma (Dekker et al., 1992; Looijenga et al., 1994). It is highly possible that GDNF-induced tumors are heterogeneous in the context of their cell of origin: expression of spermatogonial markers such as EE2 (Koshimizu et al, 1995) and TRA98 (Tanaka et al, 1997), and positivity for alkaline phosphatase suggest that both SSCs and gonocytes might have contributed to their neoplastic content (Meng et al, 2001).
Spermatogonial self-renewal and differentiation in the human testis
The earlier studies of Clermont (Clermont, 1963; Clermont, 1966b) on human spermatogenesis revealed two types of spermatogonia according to the staining pattern of their nucleus, the Adark and Apale spermatogonia. Both cell types are generally considered stem cells (Clermont, 1966a; Clermont, 1966b). Adark spermatogonia function as reserve stem cells that divide rarely but can be triggered to self-renew in case of injury or disease, while the Apale spermatogonia are the renewing stem cells (Clermont, 1963; Clermont, 1966a; Clermont, 1966b; Clermont, 1972)(Figure 1B). Apale will divide into B spermatogonia, which further divide into spermatocytes (Clermont, 1966b) (Figure 1B). Therefore, in humans, like in non-human primates, fewer mitotic steps are required to obtain spermatocytes and the efficiency of clonal expansion is very low in comparison to rodents (Bustos-Obregon et al., 1975; Johnson, 1994; Johnson et al., 1999; Johnson et al., 2001). Recently, Ehmke and colleagues proposed that in primates, to generate the same number of differentiated germ cells, a higher mitotic turnover is required from spermatogonia (Ehmcke et al., 2006). This higher mitotic turnover would increase the risk for germline mutations and vulnerability to cytotoxic events. To minimize this risk, Apale spermatogonia take the role of progenitors, whose proliferation increases the total number of germ cells. The role of stem cells is therefore limited to Adark spermatogonia, which will replenish the progenitor compartment in case of cytotoxic or natural depletion (Ehmcke et al., 2006).
Markers for human SSCs and their expression in spermatocytic seminoma
Over the past 10 years, a panel of markers has been assessed to characterize and identify human spermatogonial stem cells (Dym et al., 2009). In a recent study, He and colleagues isolated, characterized and cultured putative human spermatogonial stem cells based on their expression of the cell surface marker GPR125 (G protein-coupled receptor 125) (He et al., 2010). GPR125 positive cells are very rare (only one or two per tubule) possibly being Adark spermatogonia or a sub-population of the self-renewing Apale spermatogonia. Human SSCs were also positive for some markers identified in mouse SSCs and other undifferentiated spermatogonia, such as GFRA1, UCHL1 (also known as PGP 9.5), ZBTB16 (also known as PLZF), and THY1 (CD90) (Dym et al., 2009; He et al., 2010). MAGEA4, a member of the cancer-testis antigens family involved in DNA repair and cell differentiation, was also expressed by all human spermatogonia (Adark, Apale and B spermatogonia) (Dym et al., 2009; He et al., 2010). In another study, global gene expression analysis of human spermatogonia derived from adult testicular biopsies, and in which spermatogonia were the only germ cell type, revealed high expression of genes specifically involved in spermatogonial function in mice (von Kopylow et al., 2010). Of these genes, fibroblast growth factor receptor 3 (FGFR3), desmoglein 2 (DSG2), cancer testis antigen NY-ESO-1 (CTAG1A/B), and undifferentiated embryonic cell transcription factor 1 (UTF1) showed the most highly significant expression. Expression of these markers was confirmed at the protein level using immunohistochemistry of normal testis samples.
Remarkably, most of the aforementioned spermatogonial markers are expressed in human spermatocytic seminoma (Table 1). MAGEA4 is highly expressed in these tumors at the protein level (Rajpert-De Meyts et al., 2003). The MAGE family includes 12 related genes, which are activated in tumors of various histological types including bladder carcinoma (Patard et al., 1995), gastric carcinoma (Li et al., 1996), and breast carcinoma (Russo et al., 1995). Classical seminomas also express MAGEA4 uniformly and specifically, and a subpopulation of CIS cells was also positive for this protein (Aubry et al., 2001). Human gonocytes and spermatogonia, strongly express MAGEA4, which is sharply down-regulated at the onset of meiosis (Aubry et al., 2001; Jungbluth et al, 2000; Yakirevich et al, 2003; Gaskell et al, 2004; Pauls et al, 2006; Hudolin et al, 2009; He et al, 2010). Rajpert-De Meyts and colleagues demonstrated the expression of additional premeiotic markers in spermatocytic seminoma using immunohistochemistry (Rajpert-De Meyts et al., 2003). CHK2 was expressed in 93.7% of the tested samples. CHK2 positive staining is also found in normal gonocytes, spermatogonia and also in classical seminoma (Bartkova et al., 2001). Neurone-specific enolase (NSE) is expressed in normal spermatogonia and in germ cell tumors of young adults, including CIS (Kang et al., 1996), and was also found in 88% of spermatocytic seminomas (Rajpert-De Meyts et al., 2003). The meiotic germ cell marker p19-INK4d was negative in the tumor samples, which support the notion of a postnatal gonocyte/SSC origin of spermatocytic seminomas (Bartkova et al, 2000; Rajpert-De Meyts et al., 2003).
Table 1.
Normal human germ cell markers and their expression in spermatocytic seminoma.
| MARKER | SPERMATOCYTIC SEMINOMA | GONOCYTES (PRE- SPERMATOGONIA) | SSCS/UNDIF SPERMATO- GONIA | SPERMATOCYTES |
|---|---|---|---|---|
| MAGEA4 (P) | + a | + b, c, d, e | + f, g | +/−b,f, h) |
| SSX (P) | + i | + i | + i | + i |
| DAZ family (Tr, P) | + j | + k | + k | + k |
| CHK2 (P) | + a | + a | + a | − a |
| KIT (P) | − i, l | +/− l, m | − l | − l |
| PLAP (P) | − l | +/− l, m | − l | − l |
| POU5F1 (P) | − n | +/− c, n, l, m | − n, l | − n, l |
| Neurone-specific enolase (P) | + a | +/− a | + a, o | − a |
| p19INK4d (P) | − a, p | − a, p | − a, p | + a, p |
| UTF1 (Tr, P) | +/− q, r | +/− q | + q, r, s | − q |
| DMRT1 (Tr, P) | + j | − j | + j | + j |
| NY-ESO-1 (P) | + t | + t | + s, t | + t |
| FGFR3 (Tr, P) | + u | + v | + s, u, v | − u, v |
| RAS (Tr, P) | + u | + m,w | + g, u | + u |
| pERK1/2 (P) | + u | + m | + g | N/D |
| REX-1 (P) | +/− q | +/− q | +/− q | + q |
| SYCP1 (Tr, P) | + i, j | − i | − i | + i |
| LDHc (Tr, P) | + j | − x | − x | + x |
| CLGN (Tr, P) | + j | − y | − y | + y |
| TCFL5 (Tr, P) | + j | − z | − z | + z |
Tr: transcript analysis (RT-PCR, microarray)
P: protein analysis (immunohisto-chemistry, protein array)
ND: not done
+/−: weak or variable staining
Pauls et al, 2006
Fibroblast growth factor receptor 3 (FGFR3) is expressed in human fetal germ cells and is confined to spermatogonia in the adult testis (Juul et al, 2007; von Kopylow et al., 2010). In a recent study by Goriely et al, 20% (5/24) of spermatocytic seminoma showed increased immunoreactivity for FGFR3 (Goriely et al., 2009). The authors in this study demonstrated that paternal age-effect mutations activate a pathway that supports abnormal spermatogonial proliferation in the testis. A mutation in the FGFR3 gene was evident in a number of spermatocytic seminomas under investigation.
As mentioned above, spermatogonial stem cell self-renewal is triggered by GDNF. In the mouse, binding of GDNF to its receptor complex GFRA1/RET activates a number of SRC family kinases (SFKs) that become phosphorylated. This will further activate the PI3K/AKT pathway, which ultimately leads to an increase in Mycn gene expression (Braydich-Stolle et al., 2007; Lee et al., 2007)(Figure 3A). Alternatively, GDNF can activate the canonical RAS/ERK1/2 pathway, which results in phosphorylation and activation of transcription factors such as CREB-1, ATF-1, CREM-1 and c-FOS (He et al., 2008) (Figure 3B). He and colleagues also demonstrated that isolated human SSCs up-regulate ERK 1/2 (MAPK 1/3) when cultured for 2 weeks in media containing GDNF. Using Western blot analysis, these authors showed an increase in phosphorylated ERK1/2 in cultured cells compared to freshly isolated cells (He et al., 2010). Interestingly, positive staining for phosphorylated ERK1/2 was shown in 56% of spermatocytic seminomas studied by Goriely and colleagues (Goriely et al., 2009). In addition, 57% (15/26) of these tumors had an elevated expression of HRAS, and about 19% (5/26) carried a mutation in the HRAS gene (Goriely et al., 2009). These data indicate that pathological activation of RAS signaling and ERK1/2 phosphorylation in SSCs could be at the origin of >50% of spermatocytic seminoma.
Undifferentiated embryonic cell transcription factor 1 (UTF1) is a transcription factor involved in pluripotency and self-renewal of human and mouse embryonic stem cells (Okuda et al., 1998; Fukushima et al., 1998; Richards et al., 2004). UTF-1 is a target gene of the pluripotency marker POU5F1 (OCT3/4), which, together with SOX2, regulates its transcription in embryonic stem cells, inducing their proliferation (Nishimoto et al., 2005). In the normal human testis, expression of UTF1 was shown mainly postnatally in infantile and adult spermatogonia (Kristensen et al., 2008; Wang et al., 2010), although these cells do not express POU5F1 and SOX2 (Looijenga et al., 2003; Perrett et al., 2008; de Jong et al., 2008). UTF1 expression was variably detected in CIS and CIS-derived tumors, but was consistently observed in spermatocytic seminoma, with some samples staining intensely (Kristensen et al., 2008). Therefore, UTF-1 expression pattern in normal human testis and testicular tumors indicated that this protein might play a role in spermatogonial stem cell self-renewal, and that spermatocytic seminoma can derive from these cells. In the same study, another pluripotency marker, REX-1 was detected in gonocytes, CIS, seminomas and some non-seminoma tumors. In normal adult testicular tissue, REX-1 was revealed mainly in meiotic germ cells with weak expression in spermatogonia. Compared to UTF1, the expression of REX-1 in spermatocytic seminoma was weak and some samples were negative, confirming that this tumor type can originate from post-natal spermatogonial stem cells.
Cancer testis antigen NY-ESO-1 is another spermatogonial marker that is expressed in spermatocytic seminoma (Satie et al., 2002). In the latter study, NY-ESO-1 was detected in human gonocytes from week 18 of gestation, while in mature testis it was detected in spermatogonia and pachytene spermatocytes but not in post-meiotic germ cells. A similar pattern of expression was described for SSX (synovial sarcoma on X chromosome) (Stoop et al., 2001), another member of the cancer testis antigen family (see ref (Kalejs and Erenpreisa, 2005) for review) that is expressed in spermatocytic seminoma. In fact, SSX gene and its variants (SSX2-SSX4) were amongst the top discriminating biomarkers that distinguish spermatocytic seminoma from seminoma and dysgerminoma in a gene/protein profiling study of germ cell tumors (Looijenga et al., 2006).
DAZ (deleted in azoospermia) family proteins, including the closely related autosomal gene DAZL (DAZ-like), are expressed in human fetal gonocytes and spermatogonia (Xu et al, 2001). Noticeably, unlike DAZ, DAZL protein expression persists in post-meiotic germ cells. It is believed that DAZ family proteins have a dual pivotal role in male germ cell development, acting both during meiosis and earlier during establishment of the SSCs population (Reijo-Pera et al, 2000). DAZL protein was detected in CIS and seminomas (Lifschitz-Mercer et al., 2002), while DAZ protein was detected in spermatocytic seminoma (Looijenga et al., 2006). According to the latter study, genes belonging to the DAZ family such as DAZ and DAZ4 were drastically up-regulated (~100 fold) in spermatocytic seminoma in comparison to seminoma and dysgerminoma.
DMRT1 is a novel candidate protein, which might be involved in the development of spermatocytic seminoma. DMRT1 orthologues have highly conserved roles in sexual differentiation from flies and worms to humans (Raymond et al., 2000). DMRT stands for Doublesex and mab-related transcription factor, and is essential for postnatal testis differentiation in vertebrates (Pask et al., 2003). Looijenga and colleagues demonstrated that DMRT genes (DMRT1, DMRT2, and DMRT3) are up-regulated in spermatocytic seminomas as a result of gain of chromosome 9 where these genes map (Looijenga et al., 2006). Only DMRT1 was overexpressed in tumors where specific amplification of the subchromosomal region (p21.3-pter) of chromosome 9 was detected. Using immunohistochemistry, DMRT1 was detected in normal human spermatogonia and pre-meiotic spermatocytes (Looijenga et al., 2006). Studies using conditional gene targeting in mice recently demonstrated the importance of DMRT1 as a protein essential for the development of differentiating spermatogonia, since it promotes mitosis associated to differentiation, rather than meiosis (Matson et al., 2010). Therefore these studies show that in addition to self-renewing stem cells and spermatocytes, more mature spermatogonia (type B?) might be at the origin of spermatocytic seminoma. Finally, Looijenga and colleagues demonstrated by microarray analysis and immunohistrochemistry of testis samples that genes/proteins exclusively expressed in primary spermatocytes such as SYCP1 (synaptonemal complex protein 1)(Kondoh et al, 1997), LDHc (lactate dehydrogenase, testicular isoform)(Goldberg, 1990), CLGN (calmegin) (Tanaka et al, 1997), and TCFL5 (transcription factor-like 5)(Maruyama et al, 2008) are strongly expressed in spermatocytic seminomas (Stoop et al, 2001; Looijenga et al, 2006). Thus, although spermatocytic seminomas share many phenotypical features with postnatal gonocytes and SSCs, there is no doubt that they also can originate from primary spermatocytes.
Conclusion
Throughout the years much has been learned about the incidence and histology of spermatocytic seminoma. Presently, based on immunohistochemistry data, the postnatal germ cell origin of these tumors is unequivocal since no embryonic germ cell marker such as c-KIT, POU5F1 and PLAP is expressed (Raijpert-de Meyts, 2003b). In Table 1 we have provided a list of markers that have been investigated in spermatocytic seminoma, and compare their expression to what is known for postnatal gonocytes/prespermatogonia, SSCs and spermatocytes. While this list is not exhaustive, and while data obtained with immunohistochemistry may vary depending on the affinity of the antibody used, we believe that spermatocytic seminoma cells share many phenotypic markers with SSCs. A level of expression identical to that of postnatal gonocytes is seen for 11/20 markers (55%), to that of SSCs for 15/20 markers (75%), and to that of spermatocytes for 13/20 markers (65%). Therefore, the cellular origin of these tumors is a postnatal germ cell that can be arrested at any stage of maturation between gonocyte and primary spermatocyte, with slight predominance of the spermatogonial stem cell.
Acknowledgments
Work funded by NIH-HD054607
References
- Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–557. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- Aubry F, Satie AP, Rioux-Leclercq N, Rajpert-De Meyts E, Spagnoli GC, Chomez P, De Backer O, Jegou B, Samson M. MAGE-A4, a germ cell specific marker, is expressed differentially in testicular tumors. Cancer. 2001;92:2778–2785. doi: 10.1002/1097-0142(20011201)92:11<2778::aid-cncr10125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Thullberg M, Rajpert-De Meyts E, Skakkebaek NE, Bartek J. Lack of p19INK4d in human testicular germ-cell tumours contrasts with high expression during normal spermatogenesis. Oncogene. 2000;19:4146–4150. doi: 10.1038/sj.onc.1203769. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Falck J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J. Chk2 tumour suppressor protein in human spermatogenesis and testicular germ-cell tumours. Oncogene. 2001;20:5897–5902. doi: 10.1038/sj.onc.1204746. [DOI] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19:440–449. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Bergstrom R, Adami HO, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst. 1996;88:727–733. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos-Obregon E, Courot M, Flechon JE, Hochereau-de-Reviers MT, Holstein AF. Morphological appraisal of gametogenesis. Spermatogenetic process in mammals with particular reference to man. Andrologia. 1975;7:141–163. doi: 10.1111/j.1439-0272.1975.tb01247.x. [DOI] [PubMed] [Google Scholar]
- Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Renewal of spermatogonia in man. Am J Anat. 1966a;118:509–524. doi: 10.1002/aja.1001180211. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril. 1966b;17:705–721. [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- de Jong J, Stoop H, Gillis AJ, van Gurp RJ, van de Geijn GJ, Boer M, Hersmus R, Saunders PT, Anderson RA, Oosterhuis JW, Looijenga LH. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol. 2008;215:21–30. doi: 10.1002/path.2332. [DOI] [PubMed] [Google Scholar]
- De Jong J, Weeda S, Gillis AJ, Oosterhuis JW, Looijenga LH. Differential methylation of the OCT3/4 upstream region in primary human testicular germ cell tumors. Oncol Rep. 2007;18:127–132. [PubMed] [Google Scholar]
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Dekker I, Rozeboom T, Delemarre J, Dam A, Oosterhuis JW. Placental-like alkaline phosphatase and DNA flow cytometry in spermatocytic seminoma. Cancer. 1992;69:993–996. doi: 10.1002/1097-0142(19920215)69:4<993::aid-cncr2820690427>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Dieckmann KP, Skakkebaek NE. Carcinoma in situ of the testis: review of biological and clinical features. Int J Cancer. 1999;83:815–822. doi: 10.1002/(sici)1097-0215(19991210)83:6<815::aid-ijc21>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Okuda A, Nishimoto M, Seki N, Hori TA, Muramatsu M. Characterization of functional domains of an embryonic stem cell coactivator UTF1 which are conserved and essential for potentiation of ATF-2 activity. J Biol Chem. 1998;273:25840–25849. doi: 10.1074/jbc.273.40.25840. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–2021. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- Gillis AJ, Stoop HJ, Hersmus R, Oosterhuis JW, Sun Y, Chen C, Guenther S, Sherlock J, Veltman I, Baeten J, van der Spek PJ, de Alarcon P, Looijenga LH. High-throughput microRNAome analysis in human germ cell tumours. J Pathol. 2007;213:319–328. doi: 10.1002/path.2230. [DOI] [PubMed] [Google Scholar]
- Goddard NC, McIntyre A, Summersgill B, Gilbert D, Kitazawa S, Shipley J. KIT and RAS signalling pathways in testicular germ cell tumours: new data and a review of the literature. Int J Androl. 2007;30:337–348. doi: 10.1111/j.1365-2605.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Developmental expression of lactate dehydrogenase isozymes during spermatogenesis. Prog Clin Biol Res. 1990;344:49–52. [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, Destefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent Stem Cells Derived from Adult Human Testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A, Hansen RM, Taylor IB, Olesen IA, Jacobsen GK, McGowan SJ, Pfeifer SP, McVean GA, Meyts ER, Wilkie AO. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet. 2009;41:1247–1252. doi: 10.1038/ng.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. GDNF Up-regulates c-fos Transcription via the Ras/ERK1/2 Pathway to Promote Mouse Spermatogonial Stem Cell Proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudolin T, Kastelan Z, Derezić D, Basić-Jukić N, Cesare Spagnoli G, Juretić A, Jezek D. Expression of MAGE-A1, MAGE-A3/4 and NY-ESO-1 cancer-testis antigens in fetal testis. Acta Dermatovenerol Croat. 2009;17:103–107. [PubMed] [Google Scholar]
- Johnson L. A new approach to study the architectural arrangement of spermatogenic stages revealed little evidence of a partial wave along the length of human seminiferous tubules. J Androl. 1994;15:435–441. [PubMed] [Google Scholar]
- Johnson L, Neaves WB, Barnard JJ, Keillor GE, Brown SW, Yanagimachi R. A comparative morphological study of human germ cells in vitro or in situ within seminiferous tubules. Biol Reprod. 1999;61:927–934. doi: 10.1095/biolreprod61.4.927. [DOI] [PubMed] [Google Scholar]
- Johnson L, Staub C, Neaves WB, Yanagimachi R. Live human germ cells in the context of their spermatogenic stages. Hum Reprod. 2001;16:1575–1582. doi: 10.1093/humrep/16.8.1575. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Busam KJ, Kolb D, Iversen K, Coplan K, Chen YT, Spagnoli GC, Old LJ. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer. 2000;85:460–465. [PubMed] [Google Scholar]
- Juul A, Aksglaede L, Lund AM, Duno M, Skakkebaek NE, Rajpert-De Meyts E. Preserved fertility in a non-mosaic Klinefelter patient with a mutation in the fibroblast growth factor receptor 3 gene: case report. Hum Reprod. 2007;22:1907–1911. doi: 10.1093/humrep/dem126. [DOI] [PubMed] [Google Scholar]
- Kalejs M, Erenpreisa J. Cancer/testis antigens and gametogenesis: a review and “brainstorming” session. Cancer Cell Int. 2005;5:4. doi: 10.1186/1475-2867-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Li M, Vaughn DJ, Letrero R, Ciosek SL, Doody DR, Smith LM, Weaver J, Albano A, Chen C, Starr JR, Rader DJ, Godwin AK, Reilly MP, Hakonarson H, Schwartz SM, Nathanson KL. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet. 2009;41:811–815. doi: 10.1038/ng.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JL, Meyts ER, Skakkebaek NE. Immunoreactive neuron-specific enolase (NSE) is expressed in testicular carcinoma-in-situ. J Pathol. 1996;178:161–165. doi: 10.1002/(SICI)1096-9896(199602)178:2<161::AID-PATH452>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh N, Nishina Y, Tsuchida J, Koga M, Tanaka H, Uchida K, Inazawa J, Taketo M, Nozaki M, Nojima H, Matsumiya K, Namiki M, Okuyama A, Nishimune Y. Assignment of synaptonemal complex protein 1 (SCP1) to human chromosome 1p13 by fluorescence in situ hybridization and its expression in the testis. Cytogenet Cell Genet. 1997;78:103–104. doi: 10.1159/000134637. [DOI] [PubMed] [Google Scholar]
- Koshimizu U, Nishioka H, Watanabe D, Dohmae K, Nishimune Y. Characterization of a novel spermatogenic cell antigen specific for early stages of germ cells in mouse testis. Mol Reprod Dev. 1995;40:221–227. doi: 10.1002/mrd.1080400211. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and Characterization of Pluripotent Human Spermatogonial Stem Cell-Derived Cells. Stem Cells. 2008;27:138–49. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Skakkebaek NE, Graem N, Jacobsen GK, Rajpert-De Meyts E, Leffers H. Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008;23:775–782. doi: 10.1093/humrep/den010. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Li J, Yang Y, Fujie T, Baba K, Ueo H, Mori M, Akiyoshi T. Expression of BAGE, GAGE, and MAGE genes in human gastric carcinoma. Clin Cancer Res. 1996;2:1619–1625. [PubMed] [Google Scholar]
- Lifschitz-Mercer B, Elliott DJ, Issakov J, Leider-Trejo L, Schreiber L, Misonzhnik F, Eisenthal A, Maymon BB. Localization of a specific germ cell marker, DAZL1, in testicular germ cell neoplasias. Virchows Arch. 2002;440:387–391. doi: 10.1007/s004280100528. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Hersmus R, Gillis AJ, Pfundt R, Stoop HJ, van Gurp RJ, Veltman J, Beverloo HB, van Drunen E, van Kessel AG, Pera RR, Schneider DT, Summersgill B, Shipley J, McIntyre A, van der Spek P, Schoenmakers E, Oosterhuis JW. Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 2006;66:290–302. doi: 10.1158/0008-5472.CAN-05-2936. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Olie RA, van der Gaag I, van Sluijs FJ, Matoska J, Ploem-Zaaijer J, Knepfle C, Oosterhuis JW. Seminomas of the canine testis. Counterpart of spermatocytic seminoma of men? Lab Invest. 1994;71:490–496. [PubMed] [Google Scholar]
- Looijenga LH, Oosterhuis JW. Pathogenesis of testicular germ cell tumours. Rev Reprod. 1999;4:90–100. doi: 10.1530/ror.0.0040090. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- Maruyama O, Nishimori H, Katagiri T, Miki Y, Ueno A, Nakamura Y. Cloning of TCFL5 encoding a novel human basic helix-loop-helix motif protein that is specifically expressed in primary spermatocytes at the pachytene stage. Cytogenet Cell Genet. 1998;82:41– 45. doi: 10.1159/000015061. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A, Gilbert D, Goddard N, Looijenga L, Shipley J. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromosomes Cancer. 2008;47:547–557. doi: 10.1002/gcc.20562. [DOI] [PubMed] [Google Scholar]
- Meng X, de Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Mikuz G. WHO classification of testicular tumors. Verh Dtsch Ges Pathol. 2002;86:67–75. [PubMed] [Google Scholar]
- Misener TR, Fuller SG. Testicular versus breast and colorectal cancer screening: early detection practices of primary care physicians. Cancer Pract. 1995;3:310–316. [PubMed] [Google Scholar]
- Mostofi FK. Pathology of germ cell tumors of testis: a progress report. Cancer. 1980;45:1735–1754. [PubMed] [Google Scholar]
- Mostofi FK, Sesterhenn IA. Pathology of germ cell tumors of testes. Prog Clin Biol Res. 1985;203:1–34. [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial Cell-Line Derived Neurotrophic Factor (GDNF)-Mediated RET Signaling Regulates Spermatogonial Stem Cell Fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Nishimoto M, Miyagi S, Yamagishi T, Sakaguchi T, Niwa H, Muramatsu M, Okuda A. Oct-3/4 maintains the proliferative embryonic stem cell state via specific binding to a variant octamer sequence in the regulatory region of the UTF1 locus. Mol Cell Biol. 2005;25:5084–5094. doi: 10.1128/MCB.25.12.5084-5094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda A, Fukushima A, Nishimoto M, Orimo A, Yamagishi T, Nabeshima Y, Kuro-o M, Nabeshima Y, Boon K, Keaveney M, Stunnenberg HG, Muramatsu M. UTF1, a novel transcriptional coactivator expressed in pluripotent embryonic stem cells and extra-embryonic cells. EMBO J. 1998;17:2019–2032. doi: 10.1093/emboj/17.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask AJ, Behringer RR, Renfree MB. Expression of DMRT1 in the mammalian ovary and testis--from marsupials to mice. Cytogenet Genome Res. 2003;101:229–236. doi: 10.1159/000074342. [DOI] [PubMed] [Google Scholar]
- Patard JJ, Brasseur F, Gil-Diez S, Radvanyi F, Marchand M, Francois P, Abi-Aad A, Van Cangh P, Abbou CC, Chopin D, et al. Expression of MAGE genes in transitional-cell carcinomas of the urinary bladder. Int J Cancer. 1995;64:60–64. doi: 10.1002/ijc.2910640112. [DOI] [PubMed] [Google Scholar]
- Pauls K, Schorle H, Jeske W, Brehm R, Steger K, Wernert N, Büttner R, Zhou H. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Filipponi D, Gori M, Barrios F, Lolicato F, Grimaldi P, Rossi P, Jannini EA, Geremia R, Dolci S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle. 2008;7:3878–3888. doi: 10.4161/cc.7.24.7262. [DOI] [PubMed] [Google Scholar]
- Perrett RM, Turnpenny L, Eckert JJ, O’Shea M, Sonne SB, Cameron IT, Wilson DI, Meyts ER, Hanley NA. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol Reprod. 2008;78:852–858. doi: 10.1095/biolreprod.107.066175. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jacobsen GK, Bartkova J, Aubry F, Samson M, Bartek J, Skakkebaek NE. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology. 2003;42:217–226. doi: 10.1046/j.1365-2559.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebaek NE. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003b;111:267–278. doi: 10.1034/j.1600-0463.2003.11101301.x. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo RA, Dorfman DM, Slee R, Renshaw AA, Loughlin KR, Cooke H, Page DC. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol Reprod. 2000;63:1490–1496. doi: 10.1095/biolreprod63.5.1490. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan SP, Tan JH, Chan WK, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- Rorth M, Rajpert-De Meyts E, Andersson L, Dieckmann KP, Fossa SD, Grigor KM, Hendry WF, Herr HW, Looijenga LH, Oosterhuis JW, Skakkebaek NE. Carcinoma in situ in the testis. Scand J Urol Nephrol Suppl. 2000:166–186. doi: 10.1080/00365590050509896. [DOI] [PubMed] [Google Scholar]
- Russo V, Traversari C, Verrecchia A, Mottolese M, Natali PG, Bordignon C. Expression of the MAGE gene family in primary and metastatic human breast cancer: implications for tumor antigen-specific immunotherapy. Int J Cancer. 1995;64:216–221. doi: 10.1002/ijc.2910640313. [DOI] [PubMed] [Google Scholar]
- Satie AP, Rajpert-De Meyts E, Spagnoli GC, Henno S, Olivo L, Jacobsen GK, Rioux-Leclercq N, Jegou B, Samson M. The cancer-testis gene, NY-ESO-1, is expressed in normal fetal and adult testes and in spermatocytic seminomas and testicular carcinoma in situ. Lab Invest. 2002;82:775–780. doi: 10.1097/01.lab.0000017169.26718.5f. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, Valenzuela DM, Hobbs RM, Pandolfi PP, Rafii S. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, Hofmann MC, Cooke PS. Direct Transdifferentiation of Stem/Progenitor Spermatogonia Into Reproductive and Nonreproductive Tissues of All Germ Layers. Stem Cells. 2009;27:1666–1675. doi: 10.1002/stem.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Berthelsen JG, Giwercman A, Muller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Jorgensen N, Carlsen E, Petersen PM, Giwercman A, Andersen AG, Jensen TK, Andersson AM, Muller J. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS. 1998;106:3–11. doi: 10.1111/j.1699-0463.1998.tb01314.x. discussion 12. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Stoop H, van Gurp R, de Krijger R, Geurts van Kessel A, Koberle B, Oosterhuis W, Looijenga L. Reactivity of germ cell maturation stage-specific markers in spermatocytic seminoma: diagnostic and etiological implications. Lab Invest. 2001;81:919–928. doi: 10.1038/labinvest.3780302. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Pereira LA, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y. A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int J Androl. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ikawa M, Tsuchida J, Nozaki M, Suzuki M, Fujiwara T, Okabe M, Nishimune Y. Cloning and characterization of the human Calmegin gene encoding putative testis-specific chaperone. Gene. 1997;204:159–163. doi: 10.1016/s0378-1119(97)00537-4. [DOI] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Verdorfer I, Rogatsch H, Tzankov A, Steiner H, Mikuz G. Molecular cytogenetic analysis of human spermatocytic seminomas. J Pathol. 2004;204:277–281. doi: 10.1002/path.1634. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Dolci S, Bruni P, Baldassarre G, Chiariotti L, Melillo RM, Salvatore G, Chiappetta G, Sferratore F, Fusco A, Santoro M. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol. 2000;16:689–694. doi: 10.3892/ijo.16.4.689. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, Steinkraus V, Spiess AN. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- Wang P, Li J, Allan RW, Guo CC, Peng Y, Cao D. Expression of UTF1 in primary and metastatic testicular germ cell tumors. Am J Clin Pathol. 2010;134:604–612. doi: 10.1309/AJCPB44HBKINJNYU. [DOI] [PubMed] [Google Scholar]
- Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biol Reprod. 2009;81:77–86. doi: 10.1095/biolreprod.108.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakirevich E, Sabo E, Dirnfeld M, Sova Y, Spagnoli GC, Resnick MB. Morphometrical quantification of spermatogonial germ cells with the 57B anti-MAGE- A4 antibody in the evaluation of testicular biopsies for azoospermia. Appl Immuno- histochem Mol Morphol. 2003;11:37–44. doi: 10.1097/00129039-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Xu EY, Moore FL, Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc Natl Acad Sci U S. 2001;98:7414–7419. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]