Abstract
Objectives/Hypothesis
1. To determine the phonation threshold pressure (PTP) and phonation threshold flow (PTF) in excised human larynges. 2. To determine the effects of posterior glottal width, glottal area, and gender on PTP and PTF. 3. To test the hypothesis that hysteresis is present in excised human laryngeal phonation. 4. To compare these results to those from canine experiments and human subject measurements.
Study Design
Induced phonation of excised human larynges in the laboratory.
Methods
Nine human larynges were harvested within 24 hours post-mortem. PTP and PTF at phonation onset and offset were measured on a bench apparatus. The effects of posterior glottal width, glottal area, and gender were examined.
Results
Large inter-subject variability was observed in PTP and PTF. PTP was comparable to those measured in vivo, whereas PTF was substantially higher. One-way ANOVA showed no significant dependence of PTP and PTF on posterior glottal width. Hysteresis was observed, with offset PTP and PTF lower than onset values. Offset measurements had significantly less variability than onset measurements (P = 0.012 for PTP, P = 0.0001 for PTF).
Conclusions
This study is one of the first to report onset and offset PTP and PTF in fresh excised human larynges. The high PTF observed likely reflects a large DC flow component due to vocal fold bowing. Offset PTP and PTF values may be intrinsically more reliably measured than onset values. The large inter-subject variability in PTP and PTF may have implication for the clinical application of these aerodynamic parameters of phonation.
Level of Evidence
N/A (Laboratory study).
Keywords: phonation threshold pressure, phonation threshold flow, excised larynx, hysteresis, vocal fold
INTRODUCTION
Aerodynamic parameters of phonation reflect vocal fold geometry, biomechanical properties of the vocal fold, and vocal tract acoustics. Phonation threshold pressure (PTP), the minimum subglottal pressure required to initiate or sustain phonation, is a fundamental indicator of vocal function because it directly relates to the viscous shear properties of the vocal fold cover, the mucosal wave velocity, and the prephonatory glottal geometry.1,2 PTP is increased by vocal fold pathology such as dehydration.3 Since direct measurement of PTP in a live subject requires invasive placement of the pressure transducer in the subglottis, PTP is most commonly estimated from intraoral pressure measurements using airflow interruption techniques. Phonation threshold flow (PTF), the minimum glottal airflow required to initiate or sustain phonation, has been proposed as a more practical aerodynamic parameter for clinical use. Like PTP, PTF is predicted to be a function of vocal fold tissue properties, glottal geometry, and vocal tract loading.4 Unlike PTP, PTF can be measured directly noninvasively and thus has the potential to be utilized for routine clinical assessment. PTF has also been shown to be more sensitive to glottal width changes than PTP and therefore may have more utility in conditions such as vocal fold paralysis or paresis.5
Much of the work on characterizing the aerodynamics of phonation has been done in the excised larynx model using animal larynges. Unlike in live subjects, parameters affecting phonation can be precisely controlled and quantified in excised larynx experiments. PTP and PTF can be directly measured as a function of independent variables. Most of the experimental data to date derive from excised canine larynges, as the canine has long been the principal animal model for both in vivo and ex vivo laryngeal investigations.6 The canine larynx is comparable in morphology and glottal anatomy to the human larynx. Canine vocal folds also display similar vibratory properties to those of humans.6,7 However, human vocal folds possess a unique layered structure unlike those in any animals analyzed.7 Differences between canine and human glottal mechanics have also been noted.6 With respect to aerodynamic parameters, PTPs measured in excised canine larynges are generally higher than those measured in humans in vivo or ex vivo, presumably in part due to the higher tissue stiffness and viscosity of the canine vocal fold mucosa compared to that of humans.8
We report PTP and PTF at phonation onset and offset in a series of excised human larynges. Our first objective was to determine whether they were sensitive to changes in posterior glottal width as an indicator of the potential utility of these aerodynamic parameters to quantify pathologies that affect glottic closure. In excised canine larynges, PTF had been found to correlate with posterior glottal width, defined as the distance between the vocal processes.5 A second objective was to verify the presence of hysteresis in human vocal fold oscillation ex vivo. Hysteresis, a phenomenon of energy loss in nonlinear systems, has been predicted to occur in phonation9 and has been observed in excised canine larynges, where the threshold conditions observed during oscillation onset differed from those during offset. 10 We interpret the current empirical findings with attention to features specific to humans, in particular the effect of gender and of vocal fold bowing in excised larynges.
MATERIALS AND METHODS
Larynges
Excised human larynges were procured through the UT Southwestern Willed Body Program from donors with no history of smoking or any known history of laryngeal pathology. The larynges were harvested within 24 hours post-mortem and kept hydrated in a sealed beaker containing phosphate-buffered saline (PBS). Immediately prior to the phonation experiment, the extrinsic laryngeal muscles and associated tissue were dissected away, leaving only the strap muscles overlying the thyroid lamina. In the endolarynx, the ventricular folds and all soft tissue superior to them were removed to provide an unobstructed view of the true vocal folds. Limited resection of the posterior borders of the thyroid lamina was performed to provide transverse access to the muscular processes of the arytenoid cartilages. This allowed not only placement of arytenoid adduction sutures but also stabilization of the arytenoids by horizontal metal probes on the excised larynx phonation set-up. All phonation experiments took place within 24 hours post-mortem.
Excised Larynx Phonation Apparatus
A bench apparatus similar to that described in Alipour and Jaiswal11 and Chan and Titze2 was constructed. Compressed air supply was passed through a desiccating air filter (DeVilbiss DAD-500, Swanton, OH) and controlled by a pressure regulator with a range of 0–2 psi (Fairchild Model 10, Winston-Salem, NC). The air was passed through an in-line flowmeter (Gilmont, New York, NY) to monitor mean flow. AC flow was monitored by a pneumotach (Han Rudolph 4700, Shawnee, KS) connected to a differential pressure transducer (Validyne DP103, Northridge, CA). The air was then heated and humidified to about 37°C and 100% humidity (ConchaTherm III, Hudson RCI, Temecula, CA) before exiting in a short section of PVC pipe above the bench top. A pressure tap located 5 cm below the pipe opening was connected to a water manometer (Dwyer 1230-8, Michigan City, IN) to measure mean subglottal pressure, and the AC subglottal pressure was monitored by a pressure transducer (Microswitch 26PCA, Freeport, IL) mounted across the pipe from the manometer pressure tap. Acoustic signal was monitored by a condenser microphone positioned 8 cm from the glottis, at an azimuth angle of 45°. Electrode plates from an electroglottograph (EGG) device (Glottal Enterprises EG-2, Syracuse, NY) placed in contact with the strap muscles overlying either side of the thyroid lamina monitored the EGG signal. For the latter half of the experiments, a sound level meter (Quest Technologies Model 1800, Oconomowoc, WI) was also mounted 15 cm from the glottis at 60° azimuth. Analog signals of AC subglottal pressure, AC flow, acoustics, sound pressure level, and EGG were sampled at 5000 samples/sec per channel by a DATAQ DI-720 A/D converter and recorded on a computer using WINDAQ (DATAQ Instruments, Akron, OH).
Larynx Mounting and Data Acquisition
A 1 to 2-in segment of the proximal trachea was secured to the pipe with a hose clamp so that the cricoid sat atop the pipe. Anterior-posterior stabilization of the larynx was provided by a screw secured to the posterior cricoid lamina in the midline and a second screw at the thyroid notch, each connected to a micrometer. The micrometers were adjusted to maintain neutral vocal fold length and tension, i.e. in situ length and natural tension of the vocal folds with the excised larynx in a relaxed state. Vocal fold adduction was manipulated by arytenoid adduction sutures. Transverse screw heads placed in contact with the muscular processes further stabilized the arytenoids. Posterior glottal width, defined as the distance between the vocal processes, was controlled by placement of plastic shims of defined thicknesses (0.5, 1, 2, 3 mm) between the arytenoids so the shim did not protrude anteriorly beyond the vocal processes. The no-shim condition with the vocal processes in contact was considered to have a nominal posterior glottal width of 0 mm. Once the larynx was mounted on the pipe, PBS was dripped at regular intervals onto the vocal folds to maintain tissue hydration.
Five phonation trials were carried out at each posterior glottal width for each larynx. For each phonation trial, the subglottal presure was gradually increased by adjusting the pressure regulator slowly until vocal fold vibration occurred (phonation onset). The subglottal pressure was then gradually decreased until phonation ceased (offset). Threshold flow and pressure were determined based on discrete changes in the signal amplitude and periodicity in the pressure and EGG signals. The determination was facilitated by custom routines written in MATLAB 7.8.0 (The MathWorks, Natick, MA) and confirmed visually via a graphical user interface written for this purpose. To determine the mean subglottal pressure, flow, and relative amplitude of the subglottal pressure wave form immediately after phonation onset for the “flow-drop” analysis, an algorithm was used to select a 20-cycle window as follows. A 200-msec window was scanned from phonation onset forwards to cover an 800-msec search space in the pressure wave signal. At each point, the standard deviation of the pressure wave amplitude within the 200-msec window was calculated. The standard deviation was then plotted as a function of time within the 800-msec search space. A 20-cycle window at 60% on the standard deviation curve was used for analysis. Glottal areas were determined using ImageJ (NIH, Bethesda, MD) from images taken at each posterior glottal width before the first phonation trial.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to determine if significant differences existed between the aerodynamic parameters measured at different posterior glottal widths. To determine whether PTP or PTF in individual larynges correlated with posterior glottal width, Spearman rank-order correlation analysis (α = 0.05) was performed. The 0-mm posterior glottal width data were excluded for this purpose because in several larynges a large posterior glottal chink in the absence of a shim likely resulted in unusually large flow rates for the 0-mm condition. Statistical calculations were performed with SAS 9.2 (SAS Institute Inc., Cary, NC) and Excel 2007 (Microsoft, Redmond, WA).
RESULTS
Aerodynamic measurements
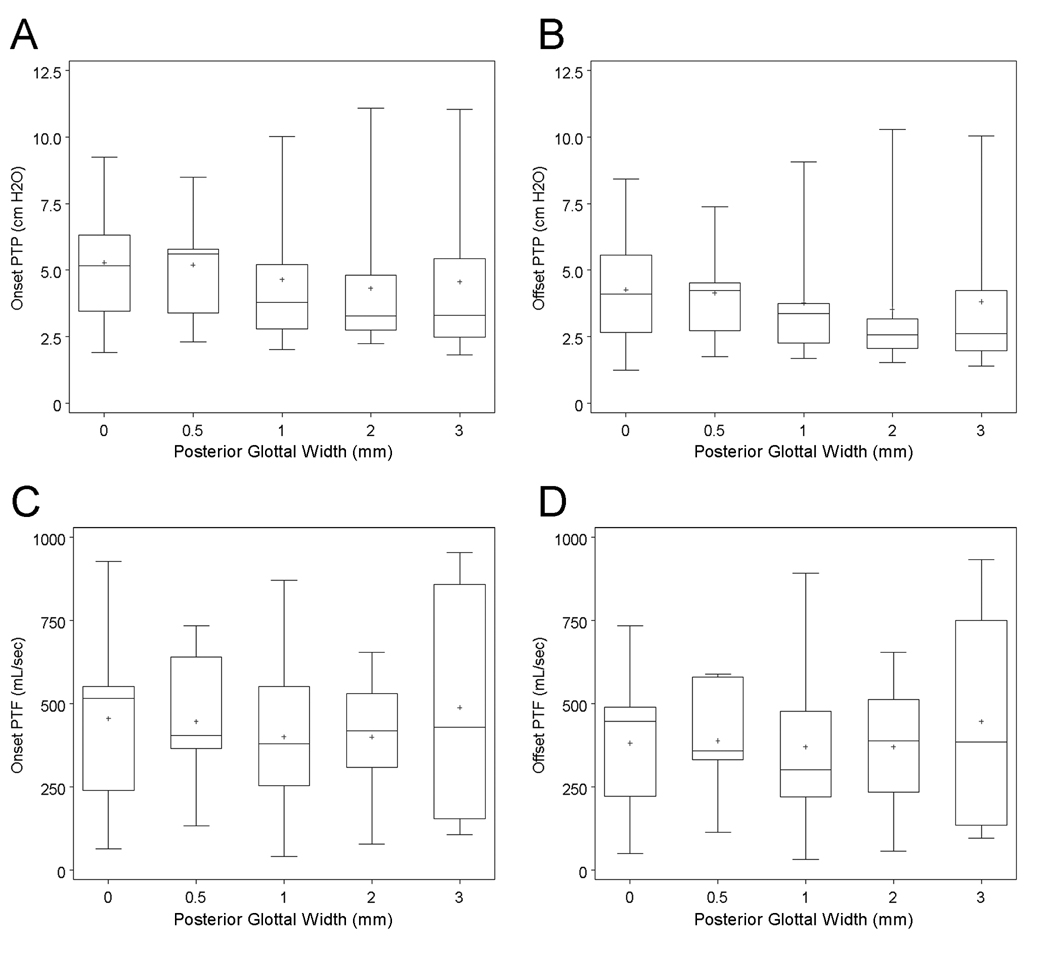
Aerodynamic data were collected from nine excised human larynges at posterior glottal widths defined by shims of varying thicknesses (Fig. 1). Table 1 summarizes the subject information. PTP and PTF were determined at both phonation onset and offset (Fig. 2).
Figure 1.
Excised human larynx mounted on the induced phonation apparatus with posterior glottal widths of (A) 0.0, (B) 0.5, and (C) 1 mm as dictated by the thickness of an inter-arytenoid shim.
Table 1.
Demographics of Laryngeal Donors.
| Larynx | Gender | Age |
|---|---|---|
| A | Male | 87 |
| B | Male | 85 |
| C | Male | 71 |
| D | Female | 86 |
| E | Male | 90 |
| F | Male | 62 |
| G | Female | 90 |
| H | Female | 81 |
| I | Female | 92 |
Figure 2.
Sample subglottal pressure, air flow, sound pressure level, acoustic, and EGG signals as a function of time at phonation onset and offset.
Offset vs. Onset PTP and PTF
To determine if the onset and offset PTP and PTF data exhibited a hysteresis phenomenon, the offset-onset ratios as defined by Regner et al.10, RPTP = PTPoffset/PTPonset and RPTF = PTFoffset/PTFonset, were calculated for each trial. A total of 197 trials were conducted, with a mean RPTP of 0.783 ± 0.093 (range 0.564–1.038). Almost all trials (98.5%) produced a RPTP less than 1.0, consistent with theoretical considerations of hysteresis.9 The mean RPTF was 0.880 ± 0.087 (range 0.705–1.114), with a majority of trials (89%) producing a RPTF less than 1.0, again consistent with theoretical considerations.10
Stability of Offset vs. Onset PTP and PTF measurements
We noted during the course of data collection that within the 5 trials for a given condition, the onset PTP and PTF measurements tended to fluctuate more from trial to trial than the offset measurements. To test the hypothesis that the offset measurements were more stable than onset measurements, their standard deviations were compared by one-tailed, paired t-tests. The results showed that the PTPoffset measurements fluctuated to a significantly lesser extent across trials than PTPonset measurements (P = 0.012). The PTFoffset measurements were also significantly more stable than PTFonset measurements (P = 0.0001).
Effect of posterior glottal width on PTP and PTF
PTP and PTF as functions of posterior glottal width are plotted in Figure 3. The means and standard deviations are listed in Table 2. When PTP and PTF data were averaged across all larynges (Fig. 3), there were no significant differences in the aerodynamic parameters as a function of posterior glottal width as determined by one-way ANOVA. The P values for PTPonset, PTPoffset, PTFonset, and PTFoffset were 0.94, 0.98, 0.95, and 0.98, respectively. The within-group variances accounted for the vast majority of the total variance. In other words, there was substantial larynx-to-larynx variability in PTP and PTF values at the same posterior glottal width. We therefore examined data for each larynx individually. The Spearman rank-order correlation analysis was applied to PTP and PTF vs. posterior glottal width. For PTPonset, 6 of 8 larynges had negative Spearman correlation coefficients (ρ), with 4 of the 6 with a magnitude close to 1 and statistically significant (Table 3). The two positive correlations did not reach statistical significance. Similarly, 5 of 8 larynges had negative Spearman correlation coefficients for PTPoffset vs. posterior glottal width that were statistically significant. Larynx B was excluded from analysis because of incomplete data.
Figure 3.
Box plots of (A) PTPonset, (B) PTPoffset, (C) PTFonset, and (D) PTFoffset data as a function of posterior glottal width. The lower and upper boundaries of each box denote the 25th and 75th percentiles. Horizontal bar within the box marks the median, and the cross marks the mean. Whiskers show the maximum and minimum values.
Table 2.
Phonation Threshold Pressure (PTP) and Phonation Threshold Flow (PTF) Data.
| Posterior Glottal Width (mm) |
PTPonset (cm H2O) |
PTPoffset (cm H2O) |
PTFonset (mL/s) |
PTFoffset (mL/s) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 0.0 | 5.29 | 2.42 | 4.27 | 2.30 | 455 | 267 | 382 | 212 |
| 0.5 | 5.20 | 2.16 | 4.14 | 1.91 | 447 | 213 | 389 | 178 |
| 1.0 | 4.65 | 2.66 | 3.77 | 2.31 | 400 | 253 | 370 | 256 |
| 2.0 | 4.32 | 2.84 | 3.54 | 2.81 | 400 | 200 | 371 | 210 |
| 3.0 | 4.56 | 3.40 | 3.82 | 3.20 | 488 | 352 | 447 | 335 |
SD = standard deviation.
Table 3.
Spearman Rank-Order Correlation Coefficients Between PTP/PTF and Posterior Glottal Width
| PTPonset | PTPoffset | PTFonset | PTFoffset | |||||
|---|---|---|---|---|---|---|---|---|
| Spearman ρ |
P | Spearman ρ |
P | Spearman ρ |
P | Spearman ρ |
P | |
| Larynx A | −0.11 | 0.69 | −0.83 | 0.0001 | −0.17 | 0.54 | −0.28 | 0.31 |
| Larynx C | 0.32 | 0.24 | 0.66 | 0.01 | 0.85 | <.0001 | 0.94 | <.0001 |
| Larynx D | −0.90 | <.0001 | −0.97 | <.0001 | 0.92 | <.0001 | 0.95 | <.0001 |
| Larynx E | −0.95 | <.0001 | −0.97 | <.0001 | −0.92 | <.0001 | −0.93 | <.0001 |
| Larynx F | 0.27 | 0.25 | 0.11 | 0.65 | 0.29 | 0.21 | 0.25 | 0.29 |
| Larynx G | −0.94 | <.0001 | −0.70 | 0.004 | 0.54 | 0.01 | 0.06 | 0.84 |
| Larynx H | −0.84 | <.0001 | −0.69 | 0.001 | 0.81 | <.0001 | 0.74 | 0.00 |
| Larynx I | −0.12 | 0.60 | −0.12 | 0.63 | 0.18 | 0.45 | 0.32 | 0.17 |
Spearman ρ = Spearman correlation coefficient. P = P value.
In contrast, a generally positive correlation was found between PTFonset and posterior glottal width. Six of the 8 larynges had positive correlation coefficients, with 4 of the 6 correlations statistically significant. Of the 2 larynges with negative correlation coefficients, Larynx A had a coefficient near zero, and Larynx E showed a strongly negative correlation between PTFonset and posterior glottal width. PTFoffset showed a similarly overall positive correlation with posterior glottal width.
Effect of prephonatory glottal area on PTP and PTF
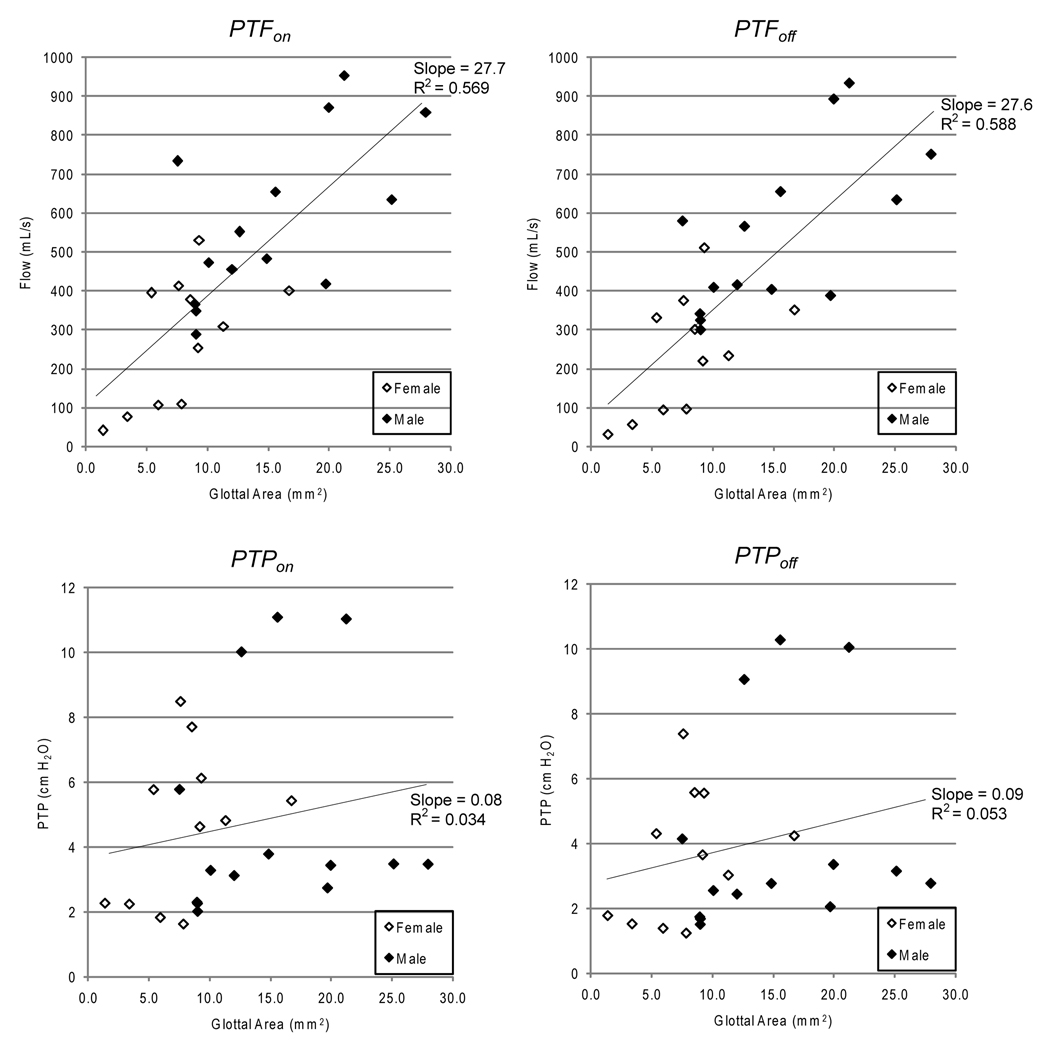
Scatter plots of PTP and PTF vs. glottal area are shown in Figure 4. Values for each larynx at each posterior glottal width are plotted. As such, the displayed data do not represent independent measurements, since each larynx accounts for multiple data points. The linear fits should therefore only be interpreted qualitatively. With this caveat, both PTFonset and PTFoffset showed a generally positive correlation with glottal area. No correlation was seen for PTPonset and PTPoffset.
Figure 4.
Scatter plots of PTP and PTF vs. glottal area. Data from female larynges are shown with open symbols, and those from male larynges are shown with solid symbols. The line in each graph represents the linear least-squares fit, and the associated slope and coefficient of determination (R2) are shown.
Effect of gender on PTP and PTF
An important determinant of glottal area is gender, as male vocal folds are 40–60% longer than female vocal folds with correspondingly larger glottal areas.12,13 As the analytical expressions for PTF were shown to increase with glottal area,4 we hypothesized that PTF was greater in males than in females. Table 4 shows results of one-tailed Student’s t-tests. Both PTFonset and PTFoffset were significantly greater in male larynges than in female larynges at 1, 2, and 3 mm posterior glottal widths (P < 0.05), with the difference at 0.5 mm approaching significance. In contrast, PTP showed no gender-related difference overall.
Table 4.
Statistical Results (P Values) of One-Tailed t-Tests for Comparisons Between Male and Female Aerodynamic Data
| P value | 0.5 mm | 1 mm | 2 mm | 3 mm |
|---|---|---|---|---|
| PTFonset | 0.068 | 0.013 | 0.034 | 0.020 |
| PTFoffset | 0.058 | 0.013 | 0.034 | 0.021 |
| PTPonset | 0.062 | 0.391 | 0.392 | 0.201 |
| PTPoffset | 0.052 | 0.319 | 0.339 | 0.193 |
Decrease in flow immediately after phonation onset
A decrease in flow immediately after phonation onset was noted in at least one condition in eight of the nine larynges. An example is shown in Figure 2. We observed that this phenomenon seemed to occur when the phonation was perceptually the loudest as the posterior glottal width was varied. To quantify this observation, we examined the increase in sound pressure level. As sound pressure level data were not available for all larynges, we also examined the relative amplitude of the subglottal pressure waveform immediately after phonation onset. As shown in Figure 5, the “flow-drop” phenomenon in each larynx tended to occur under conditions that also produced large increases in sound pressure level and large pressure amplitudes.
Figure 5.
Aerodynamic and acoustic parameters immediately following phonation onset compared to those at onset. From top to bottom: ΔFlow = Flow immediately after onset – PTFonset; ΔSPL = sound pressure level immediately after onset – sound pressure level at onset; Pressure Wave Amplitude = amplitude of subglottal pressure wave immediately after onset; ΔPressure = mean subglottal pressure immediately after onset – PTPonset. Alternating groups of solid bars and open bars distinguish data from different subjects as labeled at the bottom of the graph. ΔSPL data were only collected for the 4 larynges shown.
DISCUSSION
This study provides detailed analysis of some key aerodynamic parameters at phonation onset and offset in nine human excised larynges. While significant knowledge of aerodynamic measurements at phonation threshold has been gained from laboratory investigations of excised canine larynges, this study is among the first to provide this type of data with human larynges. As such, the findings have particular relevance to clinical applications of aerodynamic measurements. Since the study was meant to provide normative data, we applied strict subject inclusion criteria. Only fresh larynges from non-smokers were used. Adverse effects on tissue properties due to post-mortem changes and preservative methods such as freezing were minimized by harvesting the larynges and collecting data within 24 hours post-mortem.14
The range of measured PTP was consistent with those obtained by direct measurement of subglottal pressure in human subjects15 or via airflow interruption techniques.16–18 The mean PTPoffset values in this study (3.5–4.3 cm H2O) were comparable to those reported in normal subjects (2.4–3.9 cm H2O).16–18 Two factors could presumably contribute to slightly higher PTP in this study. Our sample was drawn from an aged population, which is expected to have higher PTP due to decreased vocal fold vertical thickness19 and increased tissue stiffness.20 Dehydration effects in excised larynx experiments could also lead to higher PTP compared to in vivo. The fact that our results were comparable to those obtained in vivo suggested that dehydration effects were minimal in our experiments.
The PTFoffset measured in this study (mean 0.37–0.45 L/s) were substantially higher than those reported in a series of normal human subjects (mean 0.06 L/s).21 One factor to account for this difference could be the vocal fold bowing observed in denervated, excised human larynges (Fig. 1). Bowing is associated with a large component of direct current airflow (DC flow).22 The DC flow represents a large part of the input aerodynamic energy that remains with the kinetic energy of glottal air flow and does not contribute to the energy of phonation.4 The large difference between PTF measured in this study and PTF in human subjects suggested there are likely mechanisms other than vocal fold shape that may increase the efficiency of energy conversion from input flow to tissue oscillation in vivo versus in the excised larynx. These mechanisms may include vocal fold muscle tone, active posturing (length changes and abduction/adduction), and acoustic coupling effects of the vocal tract.4
Our results were consistent with excised canine larynx data and theoretical predictions on the hysteresis phenomenon in vocal fold oscillation. Hysteresis refers to the observation that the threshold conditions during oscillation onset are different from those during offset. Applying the theory of nonlinear dynamics to Titze’s mucosal wave model of vocal fold oscillation1, Lucero9 predicted the offset/onset ratio RPTP to be in the range [0.5–1]. Regner et al.10 extended the prediction to RPTF to be in the range [0.707–1]. 98.5% of the RPTP in this study fell within the predicted range, with the rare outlier ratios exceeding 1.0 likely the result of measurement error. 89% of the RPTF ratios were in the range [0.707, 1], compared to 80% in the canine data reported in Regner et al.10 Except for a single trial with an RPTF of 0.705, all out-of-range ratios (about 10% of the data) exceeded 1, and most could not be explained by measurement error since they were consistent across trials under the same conditions. It is not clear why this small percentage of data deviated from theoretical prediction, but it could be related to the deviations from an idealized glottal geometry that was assumed for the theoretical analysis. An RPTP ratio unexpectedly greater than 1.0 was reported in one out of six normal subjects studied in Plant15, but a similar exception for RPTF has not been reported.
We noted that the PTPoffset and PTFoffset measurements had significantly smaller variability than the corresponding onset measurements obtained over repeated phonation trials under the same conditions. The difference was particularly significant for the standard deviations of PTFoffset vs. those of PTFonset (P = 0.0001). The basis for this observation was not entirely clear, but an implication was that it provided additional support for the preferential use of offset rather than onset parameters in the clinical setting. Zhuang et al.21 noted that since the onset of phonation in humans is commonly associated with a prephonatory airflow peak, PTFoffset provides a more accurate measure of flow threshold than PTFonset. Similarly, current noninvasive clinical measurements of PTP using airflow interruption are taken at phonation offset.16 Data from excised human larynges in this study suggested offset parameters may be inherently more reliable than onset parameters independent of subject training.
Previously, PTF was found to increase linearly with posterior glottal width in excised canine larynx experiments and was therefore proposed to have potential clinical utility in the assessment of conditions involving incomplete glottic closure.5 PTF was indeed found to differ between normal human subjects and those with vocal fold polyps.21 Nonetheless, in the present study, we were not able to demonstrate a positive relationship of PTF with posterior glottal width in excised human larynges. Part of the discrepancy with canine data could arise from the large inter-subject variability in aerodynamic properties due to a presumably more heterogeneous sample population in the present study. The human larynges came from donors who died from a variety of natural causes at mostly advanced ages. Their vocal fold tissue and vibratory properties reflected the end result of a lifetime of divergent genetic and environmental influences. In contrast, canine larynges derive from experimental animals that are likely to be more uniform in age and physiologic conditions. The present series of excised human larynges probably captures some of the heterogeneity present in a human population and thus may be more representative of what could be expected in the clinical setting. The large inter-subject variability in aerodynamic properties in this study was also consistent with similar variability in the viscoelastic shear properties of the vocal fold cover harvested from autopsied human subjects.20 Significant variability in aerodynamic properties has indeed been reported among live normal controls.15 We suggest that some of the discrepancy between the excised human larynx data and canine data may predict possible limitations in the usefulness of PTF in the clinical assessment of conditions where the degree of glottic closure is the primary variable.
Even when inter-subject variability is removed by analyzing individual human larynx data, we still did not find consistent variation of PTF with posterior glottal width. We suspect the absence of correlation may be attributable to vocal fold bowing. To understand the potential effect of vocal fold bowing, let us consider the notion of transglottal air flow being proportional to the horizontal cross sectional area of the glottis at the level of the vocal fold free edges. With straight vocal folds, the glottal area A can be approximated by A=xh/2=xLcos(θ/2)/2, where x is the posterior glottal width, h is the anterior-posterior length of the glottis, L is the length of the vocal fold, and θ is the abduction angle (the angle between the vocal folds at the anterior commissure). If θ is small, cos(θ/2)≈1, and A≈xL/2. In excised canine larynges, the vocal folds tend to be relatively straight (e.g. Fig. 2 in Ref. 5), so that as the posterior glottal width is increased, the glottal area increases linearly at small angles of abduction. PTF has been predicted to vary proportionally with glottal area in an analytical model.4 In the canine larynx, since the glottal area is proportional to posterior glottal width, the increase in PTF with increasing posterior glottal width is consistent with theoretical prediction. This may not be the case in excised human larynges, which tend to have bowed vocal folds, especially those from elderly subjects. Bowing removes the linear relationship between glottal area and posterior glottal width in two ways. First, bowing creates a non-zero glottal area and allows a constant DC flow when the posterior glottal width is zero (Fig. 1A). Second, the glottal area increases less than linearly with increase in posterior glottal width. For example, increasing the posterior glottal width from 1 to 2 mm in this series of human larynges only increased the glottal area by an average of 37%. PTF is therefore not expected to be as responsive to the change in posterior glottal width in excised human larynges as compared to canine larynges. Canine larynges therefore may be a better model to investigate the effect of adduction on aerodynamic parameters, whereas human larynges could more closely simulate the effect of vocal fold atrophy or tissue loss on aerodynamic parameters. On the other hand, the insensitivity to posterior glottal width could be less pronounced when PTF is measured in innervated larynges in vivo, since adductory muscle activity can eliminate or significantly reduce bowing. The potential utility of PTF in the clinical assessment of vocal fold paralysis or paresis, which may entail varying degrees of vocal fold bowing, will require further investigation in vivo to substantiate.
We found a positive correlation of glottal area with PTF and no correlation with PTP. This was consistent with data from excised canine larynx experiments that showed PTF to correlate positively with posterior glottal width but not PTP.5 PTF was also found in this study to be higher in male larynges than females for posterior glottal widths of 1–3 mm, whereas PTP did not exhibit any gender-dependence. The higher PTF in males can be attributed to the generally larger glottal areas in male larynges (Fig. 4). This gender difference was also noted in a population of normal human subjects.21 Such gender-dependence suggests that normative values of PTF for clinical use should be established for each gender separately.
Finally, we made the observation that while the air flow increased monotonically through phonation onset in many cases, under certain conditions of posterior glottal widths the flow actually decreased immediately after phonation onset and appeared to be associated with loud phonation. We found that, for most larynges, this “flow-drop” phenomenon was generally associated with larger increases in sound pressure level (SPL) and larger relative amplitudes of the subglottal pressure waveform after phonation onset. To rationalize this observation, we consider the concept of glottal resistance R = subglottal pressure/transglottal flow. In most larynges, conditions that produced a drop in flow immediate after phonation onset also had larger increases in the mean subglottal pressure (Fig. 5, Larynges A, B, D, E, G, H, I). The sudden decrease in flow and increase in pressure reflected an increase in glottal resistance. The data therefore seemed to suggest that for some larynges certain pre-phonatory glottal geometries could produce higher glottal resistance associated with higher acoustic power. Further studies will be required to investigate this possible relationship. From the standpoint of hysteresis, our observation of “flow-drop” after phonation onset was consistent with the prediction that the minimum airflow needed to sustain phonation could be lower than the PTFonset.4
CONCLUSION
PTP and PTF measurements at phonation onset and offset in excised human larynges confirmed some features of these aerodynamic parameters previously reported in excised canine larynx experiments, such as the observation of hysteresis, where offset PTP and PTF were generally lower than onset values. Offset PTP and PTF values also appeared to be more reliably measured than onset values. There were some key differences that may impact the clinical application of these measures, such as the lack of a positive relationship with posterior glottal width for both PTP and PTF. The magnitude of PTP was comparable to those measured in vivo, but PTF was substantially higher, possibly reflecting a large DC flow component due to vocal fold bowing in the excised human larynx. Canine larynges may be a better model to investigate the effect of adduction on PTP and PTF, whereas human larynges could more closely simulate the effect of vocal fold atrophy or tissue loss. Finally, the large inter-subject variability in PTP and PTF compared to those obtained in excised canine larynx experiments suggested that the interpretation of these aerodynamic parameters in the clinical setting may be less straightforward than previously thought and may need to be controlled for patient-specific factors.
ACKNOWLEDGMENTS
We thank Kipton Sheek, Chet C. Xu, Erin Henslee, and Harry Tibbals for assistance in constructing the excised larynx phonation apparatus and in data collection.
Financial disclosure: Laboratory equipment was funded by the Department of Otolaryngology-Head and Neck Surgery, UT Southwestern Medical Center. The participation of JM and KTW was supported by the UT Southwestern Summer Medical Student Research Program. This work was supported by NIDCD grants R01 DC006101S1, R01 DC006101, and R01 DC005788. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness And Other Communication Disorders or the National Institutes of Health.
Footnotes
To be presented at the annual meeting of the American Laryngological Association, Chicago, Illinois, April 28, 2011.
Conflict of interest: None.
REFERENCES
- 1.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 2.Chan RW, Titze IR. Dependence of phonation threshold pressure on vocal tract acoustics and vocal fold tissue mechanics. J Acoust Soc Am. 2006;119:2351–2362. doi: 10.1121/1.2173516. [DOI] [PubMed] [Google Scholar]
- 3.Verdolini K, Min Y, Titze IR, et al. Biological mechanisms underlying voice changes due to dehydration. J Speech Lang Hear Res. 2002;45:268–281. doi: 10.1044/1092-4388(2002/021). [DOI] [PubMed] [Google Scholar]
- 4.Jiang JJ, Tao C. The minimum glottal airflow to initiate vocal fold oscillation. J Acoust Soc Am. 2007;121:2873–2881. doi: 10.1121/1.2710961. [DOI] [PubMed] [Google Scholar]
- 5.Hottinger DG, Tao C, Jiang JJ. Comparing phonation threshold flow and pressure by abducting excised larynges. Laryngoscope. 2007;117:1695–1699. doi: 10.1097/MLG.0b013e3180959e38. [DOI] [PubMed] [Google Scholar]
- 6.Berke GS, Moore DM, Hantke DR, Hanson DG, Gerratt BR, Burstein F. Laryngeal modeling: theoretical, in vitro, in vivo. Laryngoscope. 1987;97:871–881. [PubMed] [Google Scholar]
- 7.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110:814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kimura M, Mau T, Chan RW. Rheometric properties of canine vocal fold tissues: Variation with anatomic location. Auris Nasus Larynx. 2010 Oct 27; doi: 10.1016/j.anl.2010.09.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucero JC. A theoretical study of the hysteresis phenomenon at vocal fold oscillation onset-offset. J Acoust Soc Am. 1999;105:423–431. doi: 10.1121/1.424572. [DOI] [PubMed] [Google Scholar]
- 10.Regner MF, Tao C, Zhuang P, Jiang JJ. Onset and offset phonation threshold flow in excised canine larynges. Laryngoscope. 2008;118:1313–1317. doi: 10.1097/MLG.0b013e31816e2ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alipour F, Jaiswal S. Phonatory characteristics of excised pig, sheep, and cow larynges. J Acoust Soc Am. 2008;123:4572–4581. doi: 10.1121/1.2908289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mau T, Courey MS. Influence of gender and injection site on vocal fold augmentation. Otolaryngol Head Neck Surg. 2008;138:221–225. doi: 10.1016/j.otohns.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Titze IR. Principles of Voice Production. Iowa City, IA: National Center for Voice and Speech; 2000. pp. 185–189. [Google Scholar]
- 14.Chan RW, Tize IR. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482–491. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 15.Plant RL. Aerodynamics of the human larynx during vocal fold vibration. Laryngoscope. 2005;115:2087–2100. doi: 10.1097/01.mlg.0000184324.45040.17. [DOI] [PubMed] [Google Scholar]
- 16.Rieves AL, Regner MF, Jiang JJ. Phonation threshold pressure estimation using electroglottography in an airflow redirection system. Laryngoscope. 2009;119:2378–2383. doi: 10.1002/lary.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J, O'Mara T, Conley D, Hanson D. Phonation threshold pressure measurements during phonation by airflow interruption. Laryngoscope. 1999;109:425–432. doi: 10.1097/00005537-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. J Speech Hear Res. 1994;37:1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 19.Ximenes Filho JA, Tsuji DH, do Nascimento PH, Sennes LU. Histologic changes in human vocal folds correlated with aging: a histomorphometric study. Ann Otol Rhinol Laryngol. 2003;112:894–898. doi: 10.1177/000348940311201012. [DOI] [PubMed] [Google Scholar]
- 20.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang P, Sprecher AJ, Hoffman MR, et al. Phonation threshold flow measurements in normal and pathological phonation. Laryngoscope. 2009;119:811–815. doi: 10.1002/lary.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitajima K. Airflow study of pathologic larynges using a hot wire flowmeter. Ann Otol Rhinol Laryngol. 1985;94:195–197. doi: 10.1177/000348948509400220. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, O'Mara T, Chen HJ, Stern JI, Vlagos D, Hanson D. Aerodynamic measurements of patients with Parkinson's disease. J Voice. 1999;13:583–591. doi: 10.1016/s0892-1997(99)80012-5. [DOI] [PubMed] [Google Scholar]