Abstract
Objectives/Hypothesis:
Hepatocyte Growth Factor (HGF) demonstrates beneficial properties in the treatment of aged vocal folds. However, the optimal concentration of HGF remains unknown. The purpose of the present study was to investigate the effects of HGF concentration on treatment of the aged rat vocal fold.
Study Design:
Prospective animal study.
Methods:
Seventy-five rats were studied. The rats were divided into five groups and received serial injections of HGF in 10μl of phosphate-buffered saline (PBS) at the following concentrations: 10 ng/10 μl, 50 ng/10 μl, 100 ng/10 μl, 200 ng/10 μl, or control (PBS only). Alcian blue staining was performed to investigate hyaluronan (HA), and immunohistochemistry (IHC) was performed to investigate collagen type I and III. Gene expression of hyaluronic acid synthase (HAS) −1, −2 matrix metalloproteinases (MMP) −2, −9, and procollagen I, and III were also investigated using reverse transcriptase polymerase chain reaction (RT-PCR).
Results:
Histological analyses revealed increased HA and decreased collagen type I in rats receiving injections of HGF at 100 ng/10 μl. Results were supported by RT-PCR revealing upregulated expression of HAS-2, decreased expression of procollagen I, and a significant increase of MMP-9 mRNA in rats receiving HGF at 100 ng/10 μl.
Conclusions:
We report the first in vivo concentration study of HGF for treatment of the aged vocal fold. Results revealed desirable biochemical effects of HGF at 100 ng/10 μl. These data will be used to provide immediate direction to programmatic efforts aimed at examining future applications of HGF for treatment of the aged vocal fold.
Keywords: aged vocal fold, hepatocyte growth factor, concentration study, extracellular matrix
INTRODUCTION
Age-related voice disorders have a significant impact on quality of life. Hoarseness, low pitch, weak voice, and vocal strain are commonly observed in age-related dysphonia and can severely impair communication.1,2 Endoscopic examination reveals glottic insufficiency caused by atrophy and bowing of the vocal folds, along with a reduction in mucosal wave and the amplitude of vibration.3,4 Age-related dysphonia has been attributed to laryngeal muscle atrophy, decreased motor-neuron function, reduced pulmonary function and decreased vocal fold viscoelasticity. During early stages of the dysphonia, voice therapy is effective in restoring the balance between airflow and resonance, and improving overall voice quality. However, in more advanced stages, surgical medialization and/or injection laryngoplasty are often needed to augment the severely atrophic vocal fold.5 These treatments are effective in reducing glottic insufficiency, however their effect is inherently limited as severely atrophic vocals folds have poor viscoelastic properties. Impaired viscoelasticity is the result of alterations in the extracellular matrix (ECM) of the vocal fold lamina propria.6,7 The aged vocal fold lamina propria is characterized by excessive and irregular collagen bundles, 8,9,10 decreased elastic fibers,11 and decreased hyaluronan (HA).12,13 These alterations disturb the tissues native viscoelastic properties resulting in a debilitating dysphonia.
In the search for treatments that improve age-related dysphonia, our laboratory has undertaken efforts directed towards reversing these detrimental changes to the vocal fold ECM through growth factor therapy. Prior work conducted in our laboratory has provided initial support for the regenerative effects of hepatocyte growth factor (HGF) for treatment of the aged rat vocal fold.14 HGF has high antifibrotic potential. However, the optimal dose of HGF for restoration of the biochemical changes associated with vocal fold aging has yet to be determined. Therefore, in the current study, we sought to examine the effect of HGF concentration on restoration of the changes associated with aging of the vocal fold in a rat model, previously described by our laboratory.14
MATERIALS AND METHODS
Animals
Seventy-five 18 month-old (±2 weeks) male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were used in this study. The rats were randomly divided into five groups and received serial bilateral injections of HGF (human recombinant HGF, PeproTech EC, Ltd., London, UK) to the vocal folds at varying concentrations: 10 ng of HGF in 10 μl of PBS (10 ng/10 μl group), 50 ng of HGF in 10 μl of PBS (50 ng/10 μl group), 100 ng of HGF in 10 μl of PBS (100 ng/10 μl group), 200 ng of HGF in 10 μl of PBS (200 ng/10 μl group), and 10 μl PBS without HGF (control group). The concentrations of HGF investigated in this study were selected a priori, and based on initial work conducted in our laboratory showing acute changes in ECM gene expression and histologic changes in the deposition of collagen and HA from aged rat vocal folds treated with HGF at a concentration of 100 ng/10 μl.14 Therefore, the concentrations investigated in the present study were selected to represent HGF at concentrations 1/10 (10 ng/10 μl) and 1/2 (50 ng/10 μl) lower than therapeutic concentration (100 ng/10 μl), and a concentration 2X higher (200 ng/10 μl) than therapeutic concentration (100 ng/10 μl).
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
Surgical Procedure
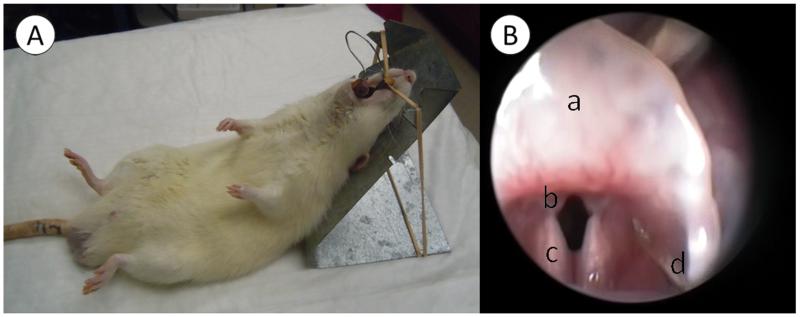
Animals received intramuscular injections of ketamine (60 mg/kg) and xylazine (6 mg/kg) for general anesthesia. Rats were positioned on a custom-made operation platform and larynges were exposed using a custom-made spring-like device as reported in detail elsewhere.15 Following adequate visualization of the larynx using a 1.9 mm 30° telescope (Karl Storz Endoscopy-America, Inc. Culver City, CA), a 30-gauge needle and a microliter syringe (Hamilton Co., Reno, NV) were used to inject 10μl of HGF solution into both vocal folds (Fig 1). To minimize leakage, the needle was inserted and advanced from the lateral aspect of the vocal fold and the solution was infiltrated into the deep layer of lamina propria. To maximize the effect of HGF, a 2-week course and 6 total injections were performed in series, as reported previously.14
Fig 1.
(A) Rats were positioned on a custom-made suspension platform and the larynx was exposed using a custom-made laryngoscope. (B) Endoscopic view of a: epiglottis, b: vocal folds, c: arytenoids, and d: injection needle.
Histological Analysis
Five rats from each group were euthanized four weeks after the first injection. Whole larynges were harvested following an overdose intracardiac injection of pentobarbital sodium (150 mg/kg). The specimens were dehydrated in 15% sucrose solution for 3 hours and in 30% sucrose solution for 18 hours at 4°C. Larynges were then transferred into embedding medium (OCT compound, Tissue-Tek, Torrance, CA), and snap frozen using a combination of acetone and dry ice, and stored at −80°C for later analysis. In preparation for histology, 10-μm-thick frozen sections were created using a cryostat (Leica, Bannockburn, IL). Sections at the middle portion of the membranous portion were used for histologic and immunohistochemical staining.
Deposition of HA was detected using Alcian blue staining. The hyaluronidase (HAase) digestion technique was used to determine HA content from other glycosaminoglycans. For the HAase digestion procedure, 50mg of bovine testes HAase (Sigma) was diluted in 100ml PBS solution, and slides were incubated in this solution for 1 hour at 37°C. Slides were then stained with Alcian blue (pH 2.5). HA deposition was detected by comparing the slides with HAase treatment to those without HAase treatment.
Deposition of collagens type I and type III were detected by IHC. Antigen retrieval procedure was performed by incubating the slides in a microwave oven for 2 minutes with 0.01M citric acid solution. After blocking for 1 hour at room temperature, sections were incubated in primary antibody solution 18 hours at 4°C. The primary antibodies used in this study were rabbit anti-collagen type I (Abcam, Cambridge, MA) at 1:100 and rabbit anti-collagen type III (Abcam) at 1:100. After washing, sections were incubated in secondary antibodies for 1 hour at room temperature. The secondary antibodies used in this study were FITC-conjugated goat anti-rabbit (Abcam) at 1:150 and Texas-Red conjugated goat anti-rabbit (Abcam) at 1:150. Nuclear staining was performed using DAPI (20ng/μl). After washing, the slides were coverslipped with Vectashield (Vector Laboratories, Burlingame, CA).
Images were captured with a NIKON Eclipse 90i microscope (NIKON, Tokyo, Japan). The following exposure times were used to capture each image: 150 ms for Alcian blue staining, 150 ms for DAPI, and 1s for FITC and Texas Red. Image analysis was performed using NIS-Elements software (NIKON, Tokyo, Japan) by calculating total stained area in each section and normalized by the total area of thyroid cartilage for each animal in order to account for individual differences in the size of the larynx.
Gene Expression Analysis
The vocal folds of ten rats from each group were used for gene expression assays. Both vocal folds were dissected from the larynx and immediately stabilized in RNAlater (Ambion, Austin, TX). An ultrasonic homogenizer (Fisher Scientific, Pittsburgh, PA) was used to homogenize the vocal fold tissues. Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA) and treated with deoxyribonuclease I (Qiagen) to minimize contamination from genomic DNA. The quantity of total RNA was determined by using the A260/A280 ratio of optical density, and electrophoresis was used to evaluate the quantity based on the appearance of the 18S and 28S ribosomal RNA bands. Reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) using the manufacturer’s recommended reaction protocol. Reactions were performed with a Veriti Thermal Cycler (Applied Biosystems) at the following parameters: 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes. Rat-specific primers for HAS-1 and −2, procollagen I and III, MMP-2 and −9, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were synthesized by Sigma-Aldrich Corp. (St. Louis, MO). Sequences of the primers are shown in Table I. Real-time Polymerase chain reaction was performed in a final volume of 20 μl reaction mixture composed of 2 μl of template complementary DNA, 0.9 μl of forward primer (20pmol/μl), 0.9 μl of reverse primer (20 pmol/μl), 10ml of Power SYBR Green PCR Master Mix (Applied Biosystems), and 6.2 ml of ribonuclease-free water. The following protocol was used for real-time PCR: 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Fluorescence was detected using the StepOnePlus Real-time PCR System (Applied Biosystems). Standard curve methods were used to determine the relative ratio of gene expression. Target gene ratios from each sample were normalized using expression ratios of the internal control gene (GAPDH). These normalized gene expression ratios were averaged for both vocal folds, and then comparisons between each group were made.
Table.
| Primer Sequences (5′-3′) | ||
|---|---|---|
| HAS-1 | Forward | TAG GTG CTG TTG GAG GAG ATG TGA |
| Reverse | AAG CTC GCT CCA CAT TGA AGG CTA | |
| HAS-2 | Forward | ACT GGG CAG AAG CGT GGA TTA TGT |
| Reverse | AAC ACC TCC AAC CAT CGG GTCTTC TT | |
| Procollagen I | Forward | AGG CAT AAA GGG TCA TCG TGG CTT |
| Reverse | AGT CCA TCT TTG CCA GGA GAA CCA | |
| Procollagen III | Forward | ATG AGC TTT GTG CAA TGT GGG ACC |
| Reverse | ACT GAC CAA GGT AGT TGC ATC CCA | |
| MMP-2 | Forward | GTC ACT CCG CTG CGC TTT TCT CG |
| Reverse | GAC ACA TGG GGC ACC TTC TGA | |
| MMP-9 | Forward | CGG AGC ACG GGG ACG GGT ATC |
| Reverse | AAG ACG AAG GGG AAG ACG GGT ATC | |
| GAPDH | Forward | GAG TCA ACG ATT TGG TCG T |
| Reverse | GAC AAG CTT CCC GTT CTC AG | |
Statistical Analysis
Histology
Separate, one-way analysis of variance (ANOVA) tests were used to investigate differences in gene expression ratios across groups. An adjusted alpha level of 0.0167 (0.05/3) was used to control for type I error. If the overall F test revealed a significant main effect, the following four planed comparisons were made: 100 ng/10 μl and control, 100 ng/10 μl and 10 ng/10 μl, 100 ng/10 μl and 50 ng/10 μl, and 100 ng/10 μl and 200 ng/10 μl using a modified Bonferroni adjusted t-test, [adjusted alpha level of 0.013 (0.05/4)] to account for multiple pairwise comparisons.
Gene expression
Separate, one-way analysis of variance (ANOVA) tests were used to investigate differences in gene expression ratios across groups. An adjusted alpha level of 0.0083 (0.05/6) was used to control for type I error. If the overall F test revealed a significant main effect, the following four planed comparisons were made: 100 ng/10 μl and control, 100 ng/10 μl and 10 ng/10 μl, 100 ng/10 μl and 50 ng/10 μl, and 100 ng/10 μl and 200 ng/10 μl using a modified Bonferroni adjusted t-test, [adjusted alpha level of 0.013 (0.05/4)] to account for multiple pairwise comparisons.
Results
Histological Analysis
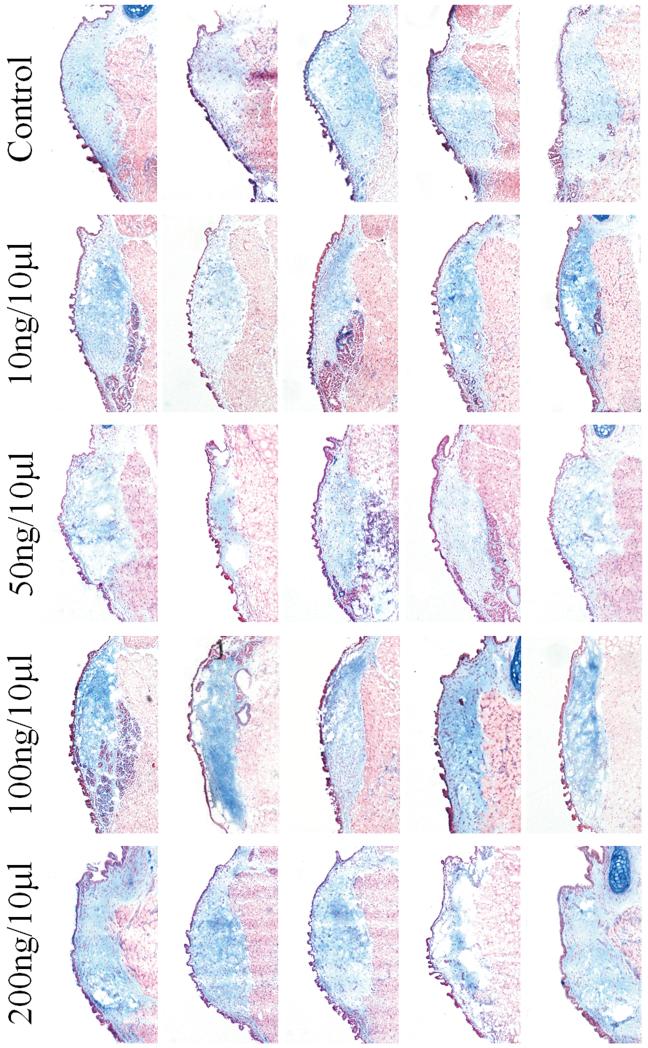
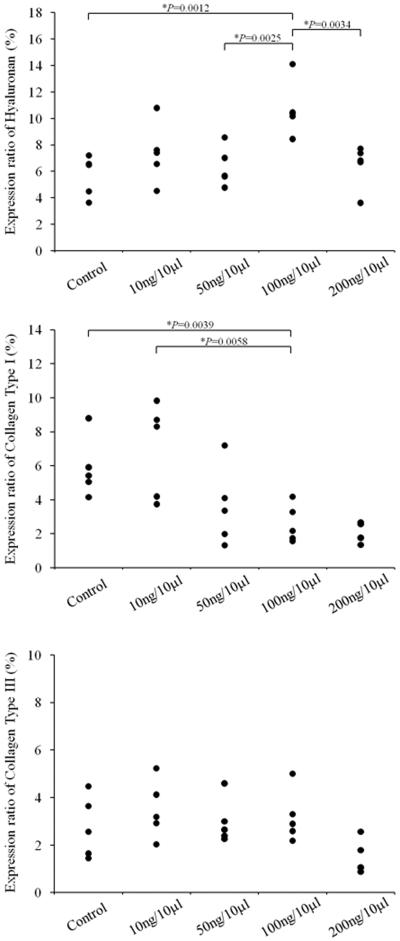
The results of ANOVA revealed a significant main effect for HA (p=0.0024) between groups. Pairwise comparisons revealed significantly increased deposition of HA in the 100 ng/10 μl concentration compared to the control group (p=0.0012), in the 100 ng/10 μl concentration compared to the 50 ng/10 μl concentration (p=0.0025), and in the 100 ng/10 μl concentration compared to the 200 ng/10 μl concentration (p=0.0034). No significant differences were observed between the 100 ng/10 μl and 10 ng/10 μl concentration (p= 0.0208). Figure 2 shows the results of Alcian Blue staining for all animals.
Fig 2.
Alcian blue staining of control and HGF-treated coronal vocal fold sections (n=5) for HA, showing HA stained blue and muscle stained red.
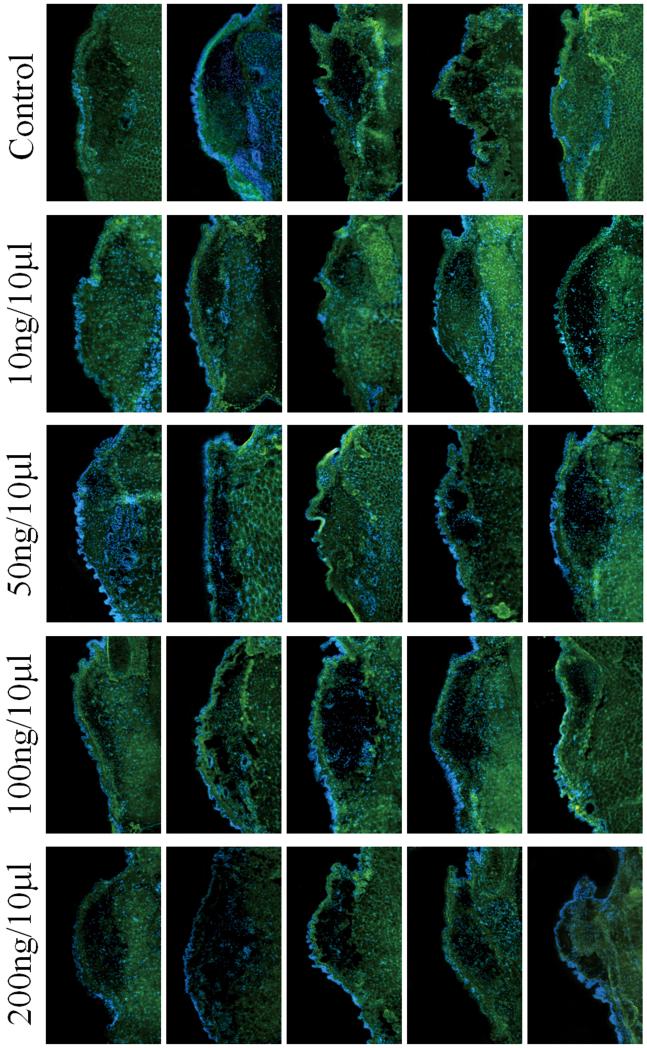
The results of ANOVA revealed a significant main effect for collagen type I (p=0.0040) between groups. Pairwise comparisons revealed significantly decreased deposition of collagen type I in the 100 ng/10 μl concentration compared to the control group (p=0.0039), and in the 100 ng/10 μl concentration compared to the 10 ng/10 μl concentration (p=0.0058). No significant differences were observed between the 100 ng/10 μl and the 50 ng/10 μl concentration (p=0.2023), or the 100 ng/10 μl and the 200 ng/10 μl concentration (p= 0.1688). Figure 3 shows the results of IHC for collagen type I for all animals.
Fig 3.
Immunohistochemistry of control and HGF-treated coronal vocal fold sections (n=5) for collagen type I, showing staining of Collagen type I fibers (indicated by green fluorescence, FITC) in lamina propria and muscle
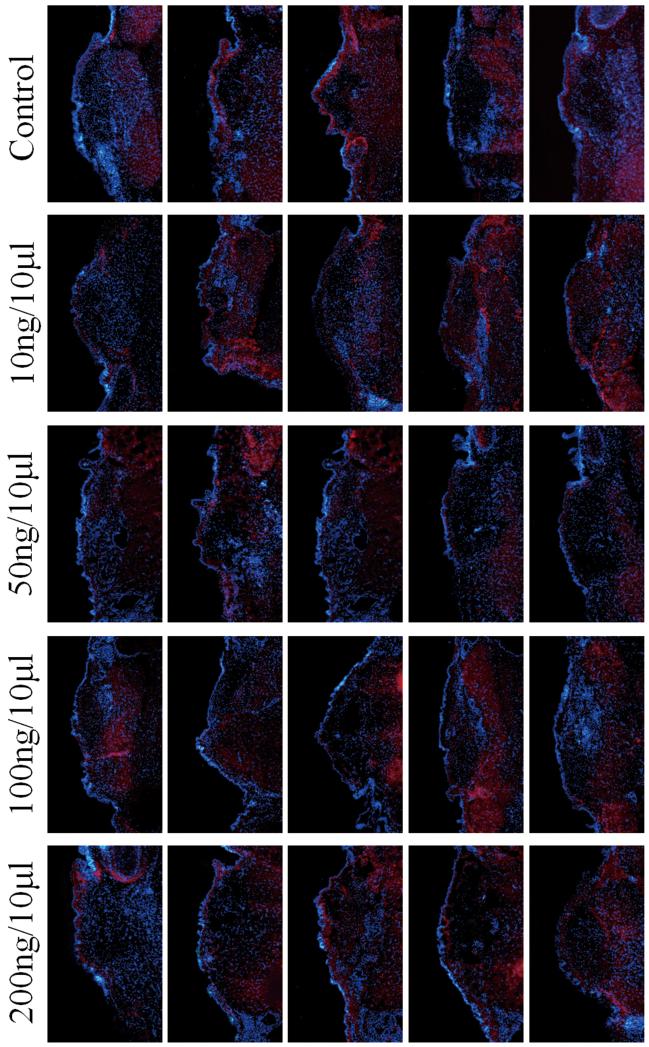
The results of ANOVA revealed no significant differences between groups for collagen type III (p=0.0628). Figure 4 shows the results of IHC for collagen type III for all animals. The results of image analysis for HA, collagen type I, and collagen type III are displayed in Figure 5.
Fig 4.
Immunohistochemistry of control and HGF-treated coronal vocal fold sections (n=5) for collagen type III, showing staining of Collagen type III fibers (indicated by red fluorescence, Texas red) in lamina propria and muscle.
Fig 5.
Results of image analysis for hyaluronan (HA), collagen type I, and collagen type III for animals in the control group, and animals receiving HGF at 10 ng/10 ⎧l, 50 ng/10 ⎧l, 100 ng/10 ⎧l, and 200 ng/10 ⎧l concentrations. Results of image analysis for HA revealed significantly increased HA in the lamina propria of rats receiving HGF at 100 ng/10 ⎧l, compared to control (p=0.0012), 50 ng/10 ⎧l (p=0.0025), and 200 ng/10 ⎧l (p=0.0034). Results of image analysis for collagen type I revealed significantly less dense staining of collagen type I in the lamina propria of rats receiving HGF at 100 ng/10 ⎧l, compared to control (p= 0.0039) and 10 ng/10 ⎧l (p=0.0058). No differences were observed for collagen type III between groups.
Gene expression Analyses
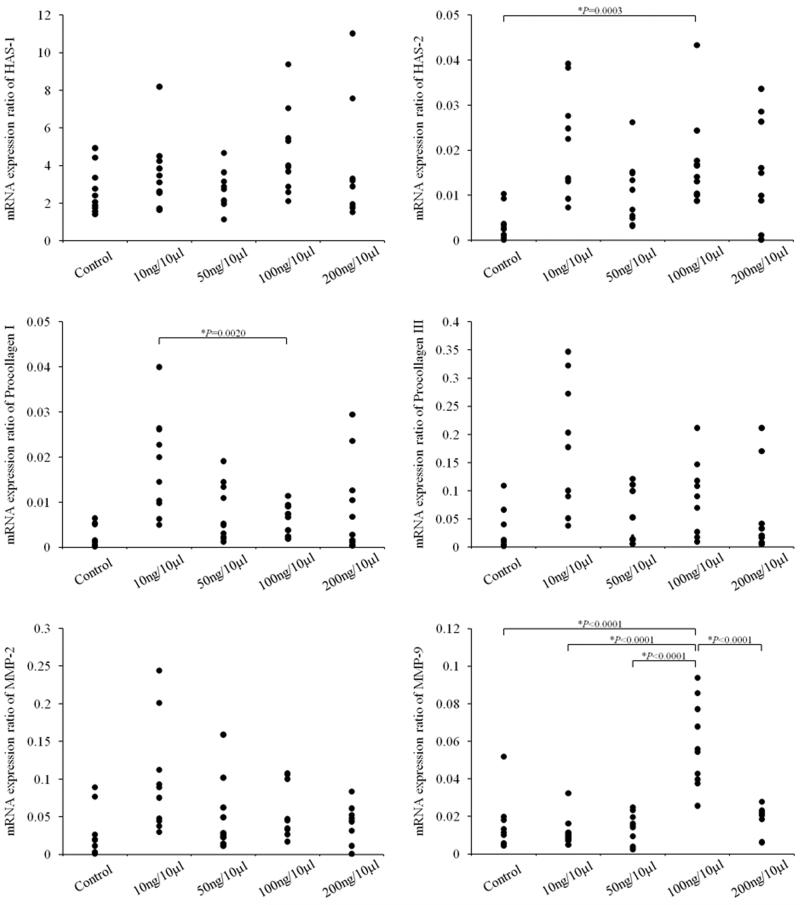
The results of ANOVA revealed a significant main effect for HAS-2 (p=0.0019) between groups. Pairwise comparisons revealed significantly increased HAS-2 gene expression in the 100 ng/10 μl concentration compared to the control group (p=0.0003) (Figure 6). No significant differences were observed between the 100 ng/10 μl concentration and the 10 ng/10 μl (p=0.2431), 50 ng/10 μl (p=0.0453), or 200 ng/10 μl (p=0.2455) concentrations. The results of ANOVA revealed no significant differences between groups for HAS-1 (p=0.1595)
Fig 6.
Results of RT-PCR for HAS-1 and −2, procollagen I and III, and MMP-2 and −9 for animals in the control group, and animals receiving HGF at 10 ng/10 μl, 50 ng/10 μl, 100 ng/10 μl, and 200 ng/10 μl concentrations. Statistical analysis for HAS −2 revealed significantly increased HAS-2 in the lamina propria of rats receiving 100 ng/10 μl compared to control (p=0.0003). Statistical analysis for procollagen I revealed significantly decreased procollagen I in the lamina propria of rats receiving 100 ng/10 μl compared to 10 ng/10 μl (p=0.0020). Statistical analysis for MMP-9 revealed significantly increased MMP-9 in the lamina propria of rats receiving 100 ng/10 μl compared to control (p<0.0001), 10 ng/10 μl (p<0.0001), 50 ng/10 μl (p<0.0001), and 200 ng/10 μl (p<0.0001).
The results of ANOVA revealed a significant main effect for procollagen I (p=0.0005) between groups. Pairwise comparisons revealed significantly downregulated expression of procollagen I in the 100 ng/10 μl concentration compared to the 10 ng/10 μl concentration (p=0.0020) (Figure 6). No significant differences were observed between the 100 ng/10 μl concentration and the control group (p= 0.9957), the 100 ng/10 μl concentration and the 50 ng/10 μl concentration (p= 0.2546), or the 100 ng/10 μl concentration and the 200 ng/10 μl concentration (p=0.2134).
The results of ANOVA revealed a significant main effect for procollagen III (p=0.0008) between groups. However, no significant differences were observed among the planned pairwise comparisons of 100 ng/10 μl and control (p=0.0020), 100 ng/10 μl and 10 ng/10 μl(p=0.0247), 100 ng/10 μl and 50 ng/10 μl(p=0.1206), or 100 ng/10 μl and 200 ng/10 μl(p=0.2222).
The results of ANOVA revealed a significant main effect for MMP-9 (p<0.0001) between groups. Pairwise comparisons revealed significantly increased MMP-9 gene expression in the 100 ng/10 μl concentration compared to the control group (p<0.0001), 10 ng/10 μl (p<0.0001), 50 ng/10 μl (p<0.0001), and 200 ng/10 μl (p<0.0001) concentrations. The results of ANOVA revealed no significant differences between groups for MMP-2 (p=0.0118)
Discussion
Age-related voice disorders have a significant impact on communication.1,2 Laryngeal videostroboscopy shows glottic insufficiency marked by atrophy and bowing of the vocal folds, with reduced mucosal wave and amplitude of vibration.3,4 Surgical treatments for correcting glottic insufficiency are available.5 However, interventions that restore the viscoelastic properties of aged vocal folds to their native state are needed.
Growth factors, especially hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF), have important roles in tissue regeneration and display high potential for restoration of the vocal fold ECM. Hirano et al. investigated the therapeutic potential of growth factors for aged vocal folds in vitro and found that fibroblasts harvested from older rats that were cultured with either HGF or bFGF increased the production of HA and decreased the production of collagen in cell culture supernatants.16 The effects were robust in the bFGF-treated cells, and dose dependent in the HGF treated cells. The effects of HGF are believed to be mediated by regulation of the matrix metalloproteinase (MMP) family. MMPs are enzymes involved in turnover of the ECM. Our group has previously demonstrated that local administration of HGF into the vocal folds of aged rats significantly increases the expression of MMP-2 and MMP-9 mRNA levels. 14 These changes in MMP mRNA are accompanied by significantly decreased expression of procollagen I (a precursor to collagen type I), and a non-significant increase in HAS-2 mRNA. Histologically, aged vocal folds treated with HGF showed decreased collagen deposition and increased deposition of HA compared to sham-treated vocal folds. Though these results are noteworthy, the optimal dose of HGF for restoration of the biochemical changes associated with vocal fold aging has yet to be established. Therefore, to address this, in the current study we examined the effects of HGF at concentrations 1/10 (10 ng/10 μl) and 1/2 (50 ng/10 μl) lower than therapeutic concentration (100 ng/10 μl), and a concentration 2X higher (200 ng/10 μl) than therapeutic concentration (100 ng/10 μl), to determine the effects of HGF concentration on gene expression and deposition of the ECM in an aged rat vocal fold model described previously by our laboratory.13
Results of RT-PCR revealed significantly upregulated expression of HAS-2, an enzyme responsible for HA synthesis, in rats receiving the 100 ng/10 μl concentration of HGF solution. These results were supported by Alcian blue staining, which showed significantly increased deposition of HA in rats receiving vocal fold injections of HGF at 100 ng/10 μl. Additionally, results of RT-PCR revealed downregulated expression of procollagen I mRNA (a precursor to collagen type I) and a significant upregulation of MMP-9 mRNA in rats receiving the 100 ng/10 μl concentration of HGF solution. MMPs are the major catalysts of collagen degradation. These transcript level findings were supported by IHC, which revealed significantly downregulated collagen type I deposition in rats receiving vocal fold injections of HGF at 100 ng/10 μl. There were no significant differences in collagen type III gene transcripts or protein levels.
Results of the concentration study provided support for the effects of HGF at 100 ng/10 μl in the treatment of ECM-related changes associated with aging of the rat vocal fold. These data should be useful to other investigators interested in the application of HGF for treatment of age-related vocal fold changes in basic animal investigations. However, investigators should use prudence when attempting to generalize these findings to humans, as it may be necessary to adjust the concentration used in future human trials to take into account differences in the volume of human and rat vocal fold lamina propria. Additionally, HGF treatment for age-related dysphonia in humans will require further investigation into issues such as tolerability, safety, and efficacy of treatment.
Conclusions
We report a concentration study on the effects of HGF on restoration of the ECM changes associating with aging in a rat model. Results supported the use of HGF at 100 ng/10 μl. These data should provide immediate direction to inform future basic and translational systematic investigations into the use of HGF for treatment of age-related vocal fold changes.
Acknowledgements
The authors would like to acknowledge Shan Huang, M.D. and Laurence James for technical assistance during surgical procedures.
Financial disclosure: Research supported by National Institutes of Health grant R21 DC 009873 from the National Institute of Deafness and Other Communication Disorders.
Footnotes
Conflict of Interest: None
References
- 1.Ramig LO, Gray S, Baker K, Corbin-Lewis K, Buder E, Luschei E, Coon H, Smith M. The aging voice: a review, treatment data and familial and genetic perspectives. Folia Phoniatr Logop. 2001;53:252–65. doi: 10.1159/000052680. Review. [DOI] [PubMed] [Google Scholar]
- 2.Ford C. Voice restoration in presbyphonia. Arch Otolaryngol Head Neck Surg. 2004;130(9):1117. doi: 10.1001/archotol.130.9.1117. [DOI] [PubMed] [Google Scholar]
- 3.Bloch I, Behrman A. Quantitative analysis of videostroboscopic images in presbylarynges. Laryngoscope. 2001 Nov;111(11 Pt 1):2022–7. doi: 10.1097/00005537-200111000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Woo P, Casper J, Colton R, Brewer D. Dysphonia in the aging: physiology versus disease. Laryngoscope. 1992 Feb;102(2):139–44. doi: 10.1288/00005537-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Damrose EJ, Berke GS. Advances in the management of glottic insufficiency. Curr Opin Otolaryngol Head Neck Surg. 2003 Dec;11(6):480–4. doi: 10.1097/00020840-200312000-00013. Review. [DOI] [PubMed] [Google Scholar]
- 6.Hirano M, Kurita S, Sakaguchi S. Ageing of the vibratory tissue of human vocal folds. Acta Otolaryngol. 1989 May-Jun;107(5-6):428–33. doi: 10.3109/00016488909127535. [DOI] [PubMed] [Google Scholar]
- 7.Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000;109:913–20. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Hirano M, Nakashima T. Age-related changes of collagenous fibers in the human vocal fold mucosa. Ann Otol Rhinol Laryngol. 2002;111:15–20. doi: 10.1177/000348940211100103. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Hirano M. Age-related changes of the macula flava of the human vocal fold. Ann Otol Rhinol Laryngol. 1995 Nov;104(11):839–44. doi: 10.1177/000348949510401102. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol. 1997 Jan;106(1):44–8. doi: 10.1177/000348949710600109. [DOI] [PubMed] [Google Scholar]
- 12.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;111(5):907–11. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Ohno T, Hirano S, Rousseau B. Age-associated changes in the expression and deposition of vocal fold collagen and hyaluronan. Ann Otol Rhinol Laryngol. 2009 Oct;118(10):735–41. doi: 10.1177/000348940911801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno T, Yoo MJ, Swanson ER, Hirano S, Ossoff RH, Rousseau B. Regeneration of aged rat vocal folds using hepatocyte growth factor therapy. Laryngoscope. 2009 Jul;119(7):1424–30. doi: 10.1002/lary.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–91. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 16.Hirano S, Bless DM, del rio AM, Connor NP, Ford CN. Therapeutic potential of growth factors for aging voice. Laryngoscope. 2004;114:2161–7. doi: 10.1097/01.mlg.0000149450.37640.db. [DOI] [PubMed] [Google Scholar]