Abstract
Intra-tumoral hypoxia (low oxygen [O2] level) is an independent indicator of unfavorable patient diagnosis, and increasing evidence demonstrates that hypoxia contributes to a more aggressive tumor phenotype. Adaptation to hypoxia is predominantly regulated by two structurally related hypoxia inducible factors, HIF-1α and HIF-2α, which activate the expression of genes involved in proliferation, metabolism, angiogenesis, and metastasis. While highly homologous, HIF-1α and HIF-2α have been shown to have different roles in tumorigenesis dependent on specific tumor microenvironments. Here we summarize recent studies on HIF-2α and discuss the potential mechanisms whereby it contributes to tumor aggressiveness.
Introduction
Hypoxia is an important factor involved in the progression of solid tumors and has been associated with various indicators of tumor metabolism, angiogenesis and metastasis [1]. The presence of widespread hypoxia within tumors has been associated with reduced survival after radiotherapy or chemotherapy. Hypoxia has also been linked to poor outcome in a number of tumors regardless of the treatment modalities used.
Adaptation to hypoxia at the cellular or organismal level is predominantly regulated by hypoxia inducible factors, HIF-1α and HIF-2α (also called EPAS1, HLF and MOP2). HIFs are heterodimeric proteins belonging to the basic helix-loop-helix (bHLH) /Per-ARNT-Sim (PAS) domain family of transcription factors [2]. HIF-α (HIF-1α or HIF-2α) activates gene expression via formation of a dimeric complex with HIF-1β (also called aryl hydrocarbon receptor nuclear translocator, ARNT) and subsequent binding to hypoxia response elements (HREs) within target genes [1]. Among HIF transcription targets are genes involved in proliferation, metabolism, angiogenesis, differentiation, and metastasis [1]. Despite the pronounced sequence homology between HIF-1α and HIF-2α, they have unique tissue distributions and play critical but non-overlapping roles in tumor progression [1].
Regulation of HIF-2α expression
HIF-2α was discovered independently by several groups in an effort to identify potentially novel bHLH/PAS family proteins highly homologous to HIF-1α [3,4]. Unlike HIF-1α, which is globally expressed in organisms, HIF-2α exhibits an obvious tissue-restricted mRNA expression pattern [5]. HIF-2α mRNA is predominantly expressed in endothelial cells, kidney and lung epithelial cells, bone marrow macrophages, and neural crest derivatives during development [5]. The unique pattern of HIF-2α mRNA expression indicates that transcription of EPAS1 (encoding HIF-2α) is temporally and spatially controlled by specific chromatin remodeling factors. However, precisely how HIF-2α is regulated at the level of transcription remains largely unknown.
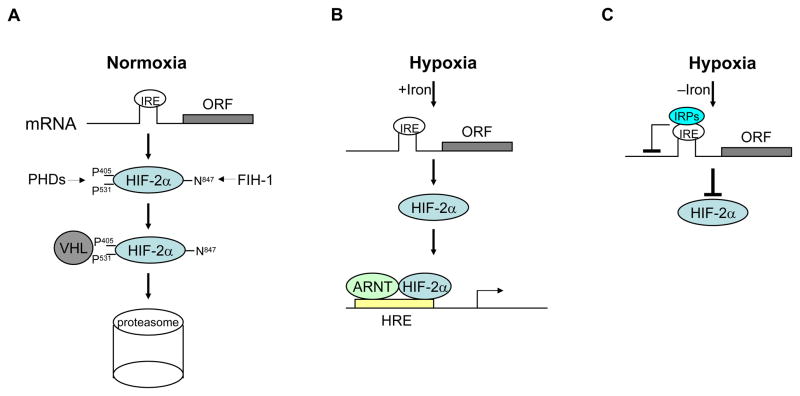
Overwhelming evidence demonstrates that HIF-2α, like HIF-1α, is predominantly regulated through hypoxia-dependent protein stabilization (Figure 1) [6,7]. The oxygen sensing mechanism controlling HIF-2α protein stability is based on posttranslational modification of its oxygen-dependent degradation domain. Under normoxia, prolyl hydroxylase domain proteins (PHD1, 2 and 3) hydroxylate two conserved proline residues (Pro 405 and 531) within HIF-2α, using oxygen, α-ketoglutarate and iron as cofactors. Hydroxylation leads to polyubiquitination of HIF-2α by the von-Hippel Lindau (pVHL) tumor suppressor protein and subsequent degradation by the 26S proteasome. In addition, hydroxylation of asparagine 847 within the C-terminal transactivation domain by factor inhibiting HIF (FIH-1) impairs HIF-2α interaction with the transcriptional coactivators CBP/p300, suppressing the activity of remaining HIF-2α proteins [8]. Lack of oxygen abrogates HIF-2α hydroxylation through PHD inhibition, resulting in increased protein stability and activity. Interestingly, expression of PHD proteins are reciprocally up-regulated by HIFα (HIF-1α and HIF-2α) under hypoxia, acting as a negative feedback to finetune HIF activities [9,10]. While previous studies demonstrated that PHD2 is a primary hydroxylase for both HIF-1α and HIF-2α, genetic studies suggest that PHD3 is mainly responsible for HIF-2α hydroxylation [9,11]. Moreover, a recent study showed that all three PHDs have an additive effect on the stability of both HIF-1α and HIF-2α proteins [12]. It seems that different PHD isoforms may be used to regulate HIF-2α (also HIF-1α) abundance depending on specific cell types, duration of hypoxic stress, and relative abundance of PHD proteins.
Figure 1. Regulation of HIF-2α expression.
A, Under normoxia, HIF-2α is hydroxylated by PHDs at proline 406 and 531. Hydroxylation leads to pVHL binding and subsequent degradation of HIF-2α by the 26S proteasome. B, Under hypoxia, when iron levels are adequate, HIF-2α is stabilized due to inhibition of PHD activities and forms complexes with ARNT to transactivate expression of hypoxia inducible genes. C, Under hypoxia, when iron is deficient, IRPs bind to the IRE within 5′UTR of HIF-2α mRNA, which in turn inhibits the translation of HIF-2α. See text for details. IRE: iron response element; IRP: IRE binding pritein; PHD: prolyl hydroxylase-domain protein; FIH: factor inhibiting HIF.
Recently, Sanchez and colleagues identified a previously unrecognized mechanism for HIF-2α regulation (Figure 1) [13]. They discovered a conserved, functional iron responsive element (IRE) in the 5′-untranslated region (UTR) of HIF-2α mRNA. Via this IRE, iron regulatory protein (IRP) binding controls EPAS1 mRNA translation in response to cellular iron availability. Increasing evidence underscores the critical roles HIF-2α plays in stimulating erythropoiesis, the major iron-utilization pathway. Short interfering RNA (siRNA) knockdown experiments in cell lines have shown that the erythropoitin (EPO) gene is selectively activated by HIF-2α, but not HIF-1α, under hypoxia [14]. Mice carrying EPAS1 hypomorphic alleles suffer from retinal degeneration associated with decreased Epo mRNA levels [15]. Notably, data from our lab demonstrate that HIF-2α plays a critical role in adult erythropoiesis, with acute deletion leading to anemia [16]. Although HIF-1α was first purified and cloned based on its affinity to the human EPO 3′ enhancer hypoxia response element (HRE) and regulates EPO expression during mouse embryogenesis, HIF-2α is the critical α-isoform regulating EPO under physiologic and stress conditions in adults. On the basis of the above reports, Sanchez’s findings uncover a regulatory link that permits feedback control between iron availability and the expression of a key transcription factor promoting iron utilization. Conceivably, iron regulation of EPAS1 modulates EPO levels, thereby adjusting the ratio of red blood cell production to iron availability.
HIF-2α in cancer
Numerous immunochemical analyses have demonstrated that both HIF-1α and HIF-2α are over-expressed in a number of primary and metastatic human cancers, and that the level of expression, either as a result of tumor hypoxia or genetic alterations, is correlated with tumor angiogenesis and patient mortality. High HIF-2α expression has been linked to poor patient outcome in several tumor types, including clear cell renal carcinoma (ccRCC), non-small cell lung cancer (NSCLC), and neuroblastoma [17–21].
(1) HIF-2α in clear cell renal carcinoma
The role of HIF-2α in tumorigenesis has been most extensively studied in ccRCC. VHL is also bi-allelically inactivated via point mutation, deletion or methylation in 70% of sporadic ccRCC [18,22]. In the absence of pVHL, HIF-α (both HIF-α and HIF-2α) is stabilized in all tumor cells independent of oxygen concentration, which in turn constitutively activates expression of genes with a hypoxia signature. While pVHL has been shown to regulate HIF-independent processes such as fibronectin assembly and microtubule stability [23], xenograft analyses indicate that its tumor suppressor function requires HIF-α degradation, and that HIF-2α, but not HIF-1α, is a primary mediator promoting tumor progression [18,19]. Consistent with this notion, HIF-2α has been linked to ccRCC development in patients with VHL disease, in which HIF-1α expression gradually decreases in more advanced lesions as HIF-2α expression increases [24]. Although HIF-2α plays undisputedly critical roles in ccRCC tumor progression based on data obtained from cell lines and animal models, few studies have employed clinical patient ccRCC samples. Therefore, our lab has categorized ccRCC into three groups based on both VHL and HIF-α status: 1) VHL wild-type/HIF-α negative; 2) VHL deficient/HIF-α positive; 3) VHL deficient/HIF-2α only [25]. Sub-classification of ccRCC based on HIF-α expression reflects underlying differences of HIF-α activities in regulation of oncogenic signaling pathway and should help to stratify patients for targeted therapy.
(2) HIF-2α in non-small cell lung cancer
About 85% to 90% of all lung cancers are of the non-small cell type. Increased tumor cell expression of HIF-α protein is associated with a worse prognosis in patients with NSCLC undergoing tumor resection [20,26]. Both HIF-1α and HIF-2α exhibit a mixed cytoplasmic/nuclear pattern of expression in cancer cells, tumor vessels, and tumor-infiltrating macrophages [20]. Interestingly, analysis of overall survival showed that HIF-2α expression was related to poor outcome, while HIF-1α expression was marginally associated with poor prognosis [20]. Thus, HIF-2α could be a useful marker in assessing risk of malignancy and a potential target for cancer prevention in NSCLC patients. However, how HIF-2α selectively promotes poor survival remains enigmatic, given that high expression of both HIF-1α and HIF-2α is related to up-regulation of multiple angiogenic factors and receptors by NSCLC cancer cells and endothelium [20]. In addition, more than 50% of tumors obtained from NSCLC patients exhibit over-expression of epidermal growth factor receptor (EGFR), a cell surface receptor involved in regulation of tumor cell proliferation, angiogenesis, and apoptosis. Immunohistochemical studies on a series of resected NSCLC tumors demonstrate a close association between expression of EGFR and HIF-1α, suggesting that EGFR might enhance the cellular response to hypoxia by increasing HIF-1α expression [26]. In support of this notion, targeting HIF-1α has been proposed as one of the emerging treatment strategies for NSCLC [26]. Further extensive studies are therefore required to identify the unique role of both HIF-α proteins, and that of HIF-2α in particular, whose expression has been linked to poor diagnosis in patients with NSCLC.
(3) HIF-2α in neuroblastoma
Human neuroblastoma is a common embryonic malignancy with a wide range of clinical outcomes, ranging from spontaneous regression to rapid tumor progression and metastasis [27]. Rapidly growing neuroblastomas invariably contain hypoxic regions and metastasize to hypoxic areas like bone and bone marrow. Accumulation of HIF-1α and/or HIF-2α proteins has been observed in vitro in various advanced-stage, metastatic human neuroblastoma cell lines exposed to hypoxia [28]. Hypoxia has a profound impact on aggressive neuroblastoma behavior: increased production of the vascular endothelial growth factor (VEGF), inhibitor of differentiation (ID), and Notch family proteins was observed in hypoxic neuroblastoma cells both in vitro and in vivo [29,30]. Interestingly, high levels of HIF-2α expression have been proposed to promote an aggressive neuroblastoma phenotype [21]. However, mechanisms whereby HIF-2α contributes to this aggressive phenotype remain largely unknown, although it has been shown that HIF-2α can be preferentially stabilized under conditions of both chronic hypoxia and physiological levels of O2 (5% O2). It seems that selective stabilization of HIF-2α in neuroblastoma is not a global phenomenon in other contexts, as obvious stabilization of HIF-1α has been shown in both macrophages and neural progenitor cells cultured under 5% O2 [31,32]. In addition, Ginouves and colleagues demonstrated in mice and in a number of cell lines from different tumor types that chronic hypoxia destabilizes both HIF-1α and HIF-2α with similar kinetics [12]. Thus, to clearly dissect the functional significance of individual HIF-α subunits in neuroblastoma tumorigenesis, it is necessary to conditionally knockout either HIF-α gene in an appropriate neuroblastoma mouse model, e.g the MYCN transgenic mouse model. Moreover, investigation of how selective stabilization of HIF-2α is achieved will shed a new light on neuroblastoma treatment, given that high HIF-2α protein levels correlate with poor prognosis in this tumor.
Mechanisms whereby HIF-2α promotes an aggressive tumor phenotype
Acquisition of aggressive tumor phenotypes is not simply the result of genetic mutations, but instead is achieved through a step-wise selection process driven in part by hypoxia [33]. While multiple mechanisms contribute to hypoxia-mediated tumor progression, activation of HIF-α signaling pathways is still among the most important for tumor cell evolution and adaptation. Here, we will focus on discussing how HIF-2α contributes to an aggressive tumor phenotype (Figure 2).
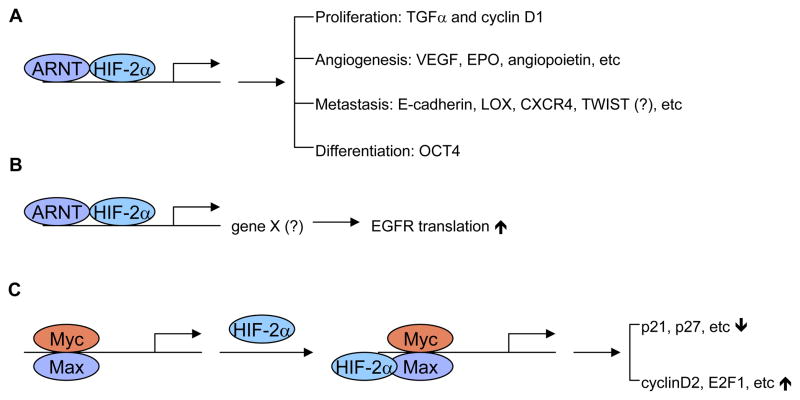
Figure 2. Mechanisms whereby HIF-2α contributes to an aggressive tumor phenotype.
A, HIF-2α activates expression of genes involved in proliferation, angiogenesis, metastasis, and differentiation. Regulation of TWIST by HIF-2α seems to be controversal. B, HIF-2α selectively activates expression of unknown gene X, which may enhance binding of polysomes to EGFR mRNA and subsequently its translation efficiency. C, HIF-2α enhances c-Myc activities by stabilizing c-Myc-Max complexes, increasing expression of c-Myc targets involved in cell cycle progression. See text for details.
(1) Activation of hypoxia inducible genes involved in tumor cell proliferation, angiogenesis, metastasis and differentiation
Proliferation
HIF-2α plays a pivotal role in promoting tumor cell growth. It is both necessary and sufficient to maintain tumor growth in VHL deficient RCC cells [18,19]. HIF-2α specifically activates the expression of TGFα and cyclin D1 in ccRCC cells [17,34]. Both TGFα and cyclin D1 are well-characterized, cell-growth regulatory proteins whose expression is frequently up-regulated in a number of tumors. Whether HIF-2α can activate TGFα and cyclin D1 in other tumor types and whether both are direct HIF-2α targets remained to be determined. Another mechanism by which HIF-2α regulates cell proliferation is through modulation of c-Myc activity [35]. This will be discussed later.
Angiogenesis
Angiogenesis is critical for tumor progression as tumor cell growth frequently outstrips the supply of O2 and nutrients. HIF-2α can directly activate the expression of genes encoding a number of pro-angiogenic factors, including VEGF, EPO, angiopoietin and TIE-2 receptors [36]. VEGF may represent one of the most important targets preferentially regulated by HIF-2α. In support of this, our lab showed that teratomas derived from ES cells with HIF-2α knocked into the HIF-1α locus were larger and exhibited increased vascularity and VEGF expression [37]. However, HIF-1α could also be a major HIF-α subunit controlling VEGF expression in other contexts [38,39]. Thus, individual contributions of HIF-α to angiogenesis are tumor-type dependent.
Metastasis
Metastasis is a complex multi-step process involving a series of tumor/host interactions. HIF activation correlates with metastasis in numerous tumors. HIF-2α promotes metastasis through regulation of critical factors controlling tumor cell metastatic potential, including E-cadherin, LOX, CXCR4, and TWIST [40–45]. E-cadherin is involved in epithelial-mesenchymal transition and HIF-2α is known to repress its expression under hypoxia [41]. CXCR4 is the most common chemokine expressed in tumors, while its ligand, SDF1, is highly expressed at sites of metastasis [40]. Expression of CXCR4 and SDF1 are significantly increased under hypoxia in a number of cell types [40]. LOX is a direct HIF target in hypoxic tumor cells, and inhibition of LOX function is sufficient to prevent hypoxia-mediated metastasis both in vitro and in vivo [42]. TWIST, a bHLH transcription factor involved in metastasis, appears to be a unique HIF-2α target [44]. However, a recent study showed direct regulation of TWIST by HIF-1α promotes metastasis [45]. In support of this notion, co-expression of HIF-1α and TWIST in primary tumors obtained from patients with head and neck cancers correlated with metastasis and the worst prognosis [45]. Examination of co-expression of HIF-2α and TWIST in human primary tumors is therefore essential to define the roles HIF-2α plays in TWIST-mediated tumor progression.
Differentiation
The cancer stem cell hypothesis proposes that tumors are originated from a small population of cells with extensive potential for self-renewal and differentiation [46]. Hypoxia and HIF-α have been shown to promote the dedifferentiation of numerous cell types including cancer cells [47,48]. However, few studies aimed at defining the mechanisms whereby HIF-α promotes an undifferentiated state have been performed. Studies from our lab have determined that HIF-2α activates the expression of Oct4, one of the most important transcription factors regulating stem cell maintenance [37]. Interestingly, Oct4 is a direct and selective target of HIF-2α. Tumors derived from ES cells with HIF-2α over-expression exhibit an abundance of undifferentiated tissue, indicating that Oct4 expression contributes to the maintenance of stem cell identity. Conceivably, hypoxia-mediated HIF-2α activation could promote the formation and/or maintenance of cancer stem cells.
(2) Promotes translation efficiency of EGFR
Over-expression of EGFR, a recurrent theme in human cancers, has been thought to cause aggressive phenotypes and resistance to standard therapy. However, genetic alterations of EGFR rarely occur in most types of cancer, suggesting the existence of a more general physiological trigger for aberrant EGFR expression. Franovic et al. showed that, surprisingly, over-expression of wild-type EGFR is selectively induced at the translational level by activation of HIF-2α in the core of solid tumors [49]. Their data suggest that HIF-2α activation may represent a common mechanism for EGFR over-expression by increasing EGFR mRNA translation. This allows for the accumulation of EGFR, increasing its availability for the autocrine signaling required for autonomous tumor cell growth. However, the precise mechanisms by which HIF-2α selectively promotes EGFR translation remain to be delineated. Presumably, HIF-2α activation leads to expression of a hypoxia-inducible activator of EGFR synthesis, or, alternatively, inhibits the expression of negative regulator-controlling receptor translation.
(3) Crosstalk between c-Myc and HIF-2α
MYC is a potent oncogene that drives unrestrained cell growth and proliferation. Over-expression of MYC occurs in 70% of human tumors, and suppression of its expression can lead to tumor regression. It is well documented that, under hypoxia, HIF-1α inhibits cell cycle progression by counteracting c-Myc transcriptional activity [50]. Interestingly, our lab showed that HIF-2α, in contrast to HIF-1α, promotes cell proliferation through enhancing c-Myc activities [51]. HIF-2α promotes cell cycle progression by further inhibiting the expression of genes encoding p21 and p27, at the same time augmenting the expression of those encoding cyclinD2 and E2F1. One mechanism whereby HIF-2α preferentially promotes c-Myc activity is through enhanced binding of c-Myc to other transcription cofactors, including Sp1, Miz1, and Max [51]. As C-MYC deregulation occurs in most types of human cancers, promotion of cell proliferation through cooperation between HIF-2α and c-Myc may represent a common mechanism for HIF-2α-mediated aggressive tumor phenotypes.
Conclusions
Over-expression of HIF-1α and HIF-2α is a common event in human cancer, and is frequently associated with poor prognosis. The HIF pathway may therefore be a useful biomarker for assessing disease states as well as a target for cancer prevention. Pharmacologic inhibition of VEGF, a well-known HIF target, has proven efficacy as a potent cancer therapeutic [52]. Hypoxia enhances resistance to radiation therapy, at least partially due to dramatic increases in HIF-α protein levels. Both HIF-1α and HIF-2α have been associated with radiation resistance [53,54]. Thus, inhibition of HIF-α expression may further enhance the benefits of cancer treatment. In fact, the mTOR inhibitor CCI-79, which inhibits the translation of HIF-1α, has been used for treatment of RCC patients [55]. However, HIF-2α is the primary HIF-α subunit accounting for RCC aggressiveness. It is likely that mTOR inhibitors may function through HIF-αindependent mechanisms in ccRCC patients as they are unable to decrease HIF-2α protein abundance [18,19,54]. Identification of other small molecules selectively inhibiting HIF-2α expression may provide opportunities for effective treatment of tumors exclusively expressing HIF-2α.
References
- 1.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheuermann TH, Zhang L, Gardner KH, Bruick RK. Hypoxia-inducible factors Per/ARNT/Sim domains: structure and function. Methods Enzymol. 2007;435:3–24. doi: 10.1016/S0076-6879(07)35001-5. [DOI] [PubMed] [Google Scholar]
- 3.Ema M, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 5.Gruber M, Simon MC. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol. 2006;13:169–174. doi: 10.1097/01.moh.0000219663.88409.35. [DOI] [PubMed] [Google Scholar]
- 6•.Jaakkola P, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. In a series of biochemical studies, these authors showed a novel mechanism dependent on O2 concentration to regulate HIF-α stability. [DOI] [PubMed] [Google Scholar]
- 7•.Maxwell PH, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. In a series of biochemical studies, these authors showed a novel mechanism dependent on O2 concentration to regulate HIF-α stability. [DOI] [PubMed] [Google Scholar]
- 8.Mahon PC, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berra E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aprelikova OCG, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- 11.Bishop T, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/−mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginouvès A, Macías N, Pouysségur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci U S A 2008. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Sanchez M, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. This study identified a novel mechanism selectively controlling HIF-2α translation via iron levels, which links HIF-2α regulation to EPO production. [DOI] [PubMed] [Google Scholar]
- 14.Warnecke C, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 15.Ding K, Seaman R, Birch DG, Garcia JA. Retinal disease in mice lacking hypoxia-inducible transcription factor-2alpha. Invest Ophthalmol Vis Sci. 2005;46:1010–1016. doi: 10.1167/iovs.04-0788. [DOI] [PubMed] [Google Scholar]
- 16•.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. These authors clearly demonstrated that HIF-2α selectively regulates Epo production in a conditional knockout mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raval RR, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5678–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo K, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 19.Kondo K, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. Plos Biol. 2003;1:439–444. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giatromanolaki A, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmquist-Mengelbier L, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Påhlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2007;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Lonser RR, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;14:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 23.Hergovich A, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 24.Mandriota SJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 25•.Gordan JD, Lal P, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Simon MC, Nathanson KL. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. manuscript in revivion. By employing clinical patient ccRCC samples, Gordan and colleagues, for the first time, categorized ccRCC into three groups based on the VHL and HIF-α status. [Google Scholar]
- 26.Swinson DE, O’Byrne KJ. Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer. 2006;7:250–256. doi: 10.3816/CLC.2006.n.002. [DOI] [PubMed] [Google Scholar]
- 27.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 28.Jögi A, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Påhlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasorella A, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2002;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- 30.Puppo M, Melillo G, Pezzolo A, Varesio L, Bosco MC. Induction of apoptosis by flavopiridol in human neuroblastoma cells is enhanced under hypoxia and associated with N-myc proto-oncogene down-regulation. Clin Cancer Res. 2004;10:8704–8719. doi: 10.1158/1078-0432.CCR-03-0422. [DOI] [PubMed] [Google Scholar]
- 31.Rius J, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao T, Liu ZH, Wu LY, Huang X, Wu HT, Xiong L, Wang X, Wang XM, Zhu LL, Fan M. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells--role of hypoxia-inducible transcription factor-1alpha. FEBS J. 2008;275:1824–1834. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]
- 33.Graeber TG, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 34.Baba M, Yamada-Okabe H, Hamada K, Tabuchi H, Kobayashi K, Kondo K, Yoshida M, Yamashita A, Kishida T, Nakaigawa N, Nagashima Y, Kubota Y, Yao M, Ohno S. Loss of von Hippel-Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene. 2003;22:2728–2738. doi: 10.1038/sj.onc.1206373. [DOI] [PubMed] [Google Scholar]
- 35.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruber M, Simon MC. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol. 2006;13:169–174. doi: 10.1097/01.moh.0000219663.88409.35. [DOI] [PubMed] [Google Scholar]
- 37••.Covello KL, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. This study, for the first time, demonstrated a unique and novel role of HIF-2α in embryonic development and adult stem cell biology via regulating Oct4 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan HE, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmeliet P, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 40.Ceradini DJ, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 41.Krishnamachary B, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 42.Erler JT, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 43.Staller P, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 44•.Gort EH, Verlaan I, Groot AJ, Plasterk RH, Shvarts A, Suijkerbuijk KP, van Laar T, van der Wall E, Raman V, van Diest PJ, Tijsterman M, Vooijs M. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. In a study utilizing both tumor cell lines and primary patient samples, these authors showed that HIF-α directly regulates expression of TWIST, a critical mediator of metastasis. [DOI] [PubMed] [Google Scholar]
- 45.Yang MH, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 46.Reya T, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–110. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 47•.Gustafsson MV, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. This study showed that crosstalk between Notch and HIF-α promotes cell dedifferentiation under hypoxia. [DOI] [PubMed] [Google Scholar]
- 48.Holmquist L, Påhlman S. Effect of hypoxia on the tumor phenotype: the neuroblastoma and breast cancer models. Adv Exp Med Biol. 2006;587:179–193. doi: 10.1007/978-1-4020-5133-3_16. [DOI] [PubMed] [Google Scholar]
- 49•.Franovic A, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A. 2007;104:13092–13097. doi: 10.1073/pnas.0702387104. Franovic and colleagues demonstrated that HIF-2α selectively enhances EGFR translation, providing a non-mutational explanation for its overexpression in human cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Koshiji M, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. This study showed an important role of HIF-1α in regulating cell cycle progression by countering c-Myc function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Gordan JD, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. In contrast to HIF-1α, HIF-2α was shown to enhance c-Myc activity and cell cycle progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gasparini G, Toi M, Ferrara N. Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat Clin Pract Oncol. 2005;2:562–577. doi: 10.1038/ncponc0342. [DOI] [PubMed] [Google Scholar]
- 53.Moeller BJ, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 54•.Bhatt RS, Zimmer M, Torregrossa J, Chen S, Sukhatme VP, Iliopoulos O, Balk S, Bubley GJ. Hypoxia-inducible factor-2alpha: effect on radiation sensitivity and differential regulation by an mTOR inhibitor. BTU Int. 2008;102:358–363. doi: 10.1111/j.1464-410X.2008.07558.x. Here Bhatt and colleagues showed that mTOR inhibitors are unable to decrease HIF-2α protein abundance, indicating that mTOR inhibitors function in ccRCC through HIF-independent mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atkins MB, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]