Abstract
The integration of chemistry and molecular biology with imaging is providing some of the most exciting opportunities in the treatment of cancer. The field of theranostic imaging, where diagnosis is combined with therapy, is particularly suitable for a disease as complex as cancer, especially now that genomic and proteomic profiling can provide an extensive `fingerprint' of each tumor. Using this information, theranostic agents can be shaped for personalized treatment to target specific compartments, such as the tumor microenvironment (TME), whilst minimizing damage to normal tissue. These theranostic agents can also be used to target multiple pathways or networks by incorporating multiple small interfering RNAs (siRNAs) within a single agent. A decade ago genetic alterations were the primary focus in cancer research. Now it is apparent that the tumor physiological microenvironment, interactions between cancer cells and stromal cells, such as endothelial cells, fibroblasts and macrophages, the extracellular matrix (ECM), and a host of secreted factors and cytokines, influence progression to metastatic disease, aggressiveness and the response of the disease to treatment. In this review, we outline some of the characteristics of the TME, describe the theranostic agents currently available to target the TME and discuss the unique opportunities the TME provides for the design of novel theranostic agents for cancer therapy.
Keywords: tumor microenvironment, theranostic imaging, invasion, metastasis
INTRODUCTION
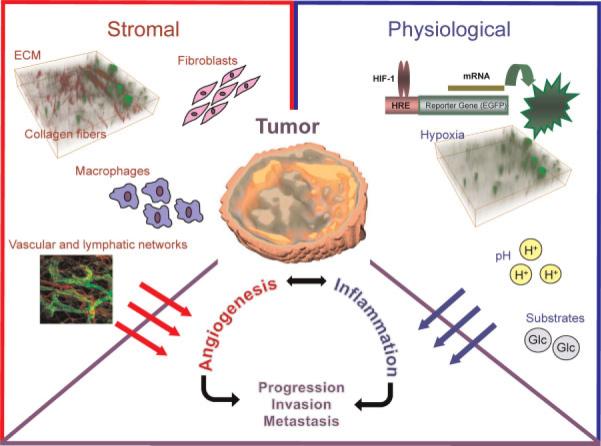
The past decade has provided irrefutable evidence that the tumor microenvironment (nurture) is as important as the genetic content (nature) in the evolution of the malignant phenotype. The three major characteristics of cancer – uncontrolled proliferation, the ability to establish vasculature and the ability to metastasize – although, singly, not life-threatening, collectively confer lethality (1). Cancer cells also have a formidable capacity to adapt and survive the hostile physiological environments found in tumors, such as hypoxia, acidic pH and substrate deprivation, in addition to having the ability to survive treatments that are ordinarily lethal to normal cells. It is probable that the resilience of the cancer cell and several of its lethal phenotypic traits arise from tumor microenvironmental interactions as well as from its genomic plasticity. The term `tumor microenvironment' (TME) is used to describe the infrastructure that surrounds and supports cancer cells, and broadly covers the extracellular matrix (ECM), cancer-associated fibroblasts (CAFs), adipocytes, pericytes, multiple immune cells, such as tumor-associated macrophages (TAMs), and vascular and lymphatic endothelial cells (Fig. 1). Given the complexity both within and outside the cancer cell, and the interactions between cancer cells and the surrounding stroma, it is not surprising that a single perturbation within a tumor can create a cascade of changes in multiple pathways and networks, some of which may have lethal repercussions, e.g. metastasis, that may only be evident several years later.
Figure 1.
Schematic diagram to show the different components of the tumor microenvironment. The image of collagen fibers overlaid with hypoxic regions was obtained from an MDA-MB-231 tumor expressing enhanced green fluorescent protein (EGFP) under the control of a hypoxia response element (HRE). Collagen fiber images were acquired with second harmonic generation (SHG) microscopy. The three-dimensional display of the tumor is a parametric image of extracellular transport of a macromolecular contrast agent. The vascular and lymphatic network image is from http://www.imm.ox.ac.uk/wimm-research/mrc-human-immunology-unit/david-jackson/Fig3.jpg/image_preview. ECM, extracellular matrix; HIF-1, hypoxia-inducible factor-1.
To effectively treat cancer, critical networks that are vital for the adaptive or compensatory mechanisms of cancer cells, but are not required by normal cells, need to be identified and targeted, as a major limiting factor in successful treatment is the collateral damage to normal tissue. Multimodality and multiparametric molecular and functional imaging provide unprecedented opportunities to image the TME and the interactions between cancer cells and stromal cells.
In theranostics (or theragnostics), noninvasive imaging-based detection of a target is combined with the delivery of a therapeutic payload to that target. The TME is primed with targets to exploit for therapy (2), which can be used in combination with imaging for `theranostic' imaging agents. In this era of personalized molecular medicine, the effective implementation of theranostic agents that target the TME may achieve cancer cures, a goal that remains elusive despite the technological advances available in the 21st century. Here, we review different aspects of the TME and provide examples of theranostic agents, where available, or describe potential agents for future strategies, for theranostic imaging of the TME.
THERANOSTIC IMAGING OF THE PHYSIOLOGICAL TME
The physiological environment of tumors is uniquely characterized by areas of poor flow, hypoxia, high lactate and low extracellular pH (pHe) (3). Unlike other diseases in which hypoxia induces severe damage, cancer cells have the remarkable ability to adapt, survive and disseminate from physiological environments characterized by hypoxia and extracellular acidosis. The hypoxic environment of solid tumors has also proven to be a major obstacle for successful radiation and chemotherapy of cancer for several decades (4,5).
Hypoxia
The existence of hypoxia in tumors and its implications for the failure of radiation therapy were predicted in 1955 by Thomlinson and Gray (6) on the basis of observations that necrosis in human lung carcinomas occurred at approximately 150 μm, the calculated diffusion distance of oxygen, from the nearest capillary. Four decades later, hypoxia and hypoxia-inducible factors (HIF-1 and HIF-2) are being associated with the transcriptional activation of an ever-increasing number of genes that regulate several phenotypic characteristics of cancer (7). Tumor hypoxia can be diffusion-limited or chronic, or can arise acutely from vascular collapse. Most treatments, especially radiation therapy, result in reoxygenation (8,9).
HIF-1 is a heterodimeric protein that consists of a constitutively expressed β-subunit and an oxygen-dependent α-subunit that is rapidly degraded through polyubiquitination and proteasomal degradation under well-oxygenated conditions. Under hypoxic conditions, HIF-1α or HIF-2α undergo heterodimerization with HIF-1β and bind to hypoxia response elements (HREs) to activate the transcription of several target genes. These target genes control angiogenesis, cell survival, resistance to chemo- and radiation therapy, metabolism, pH regulation, invasion and metastasis, increased genetic instability and dedifferentiation (7,10). Increased invasion of ECM by colon cancer cells under hypoxic conditions (11) can be partly reversed by a HIF-specific small interfering RNA (siRNA). Antisense RNA also limits the invasion and metastasis of pancreatic cancer cells (12). HIF stabilization has also been linked to the epithelial-to-mesenchymal transition observed in many cancer cells, as well as the loss of E-cadherin, an extracellular marker of differentiated epithelial cells (13). HIF-1 has also been shown to promote lysyl oxidase-associated invasion and metastasis of cancer cells (14). A detailed review on the causes, consequences and detection of hypoxia can be found in this issue.
Efforts to target hypoxia, initially to improve the outcome of radiation therapy through the use of radiation sensitizers, and by using hyperbaric oxygen, have not been successful and require further optimization (15,16). As hypoxic areas are, by default, also poorly perfused, the limited success of radiation sensitizers and hyperbaric oxygen could be a result, in part, of the limited delivery of agents and oxygen in these poorly perfused regions. More recently, low-molecular-weight HIF-1 inhibitors have achieved better success. In addition, the discovery that several chemotherapeutic agents also inhibit HIF-1 is providing new opportunities for the targeting of HIF-1 (17).
The development of a theranostic agent for HIF-1 requires the expression of an imaging reporter in the tumor under hypoxic conditions, and the delivery of a therapeutic payload only where hypoxia is present. HRE has been exploited in several studies to target hypoxia using either prodrug enzymes or suicide genes (18,19), although, to date, this has not been combined with imaging. As mentioned earlier, the minimization of damage to normal tissues is one of the most sought-after goals in cancer treatment. One strategy is to deliver a drug-activating enzyme to the tumor, followed by the administration of a nontoxic prodrug administered systemically. Several prodrug–enzyme systems exist that can be easily incorporated for the theranostic imaging of hypoxia (20,21). HRE-driven expression of the prodrug enzyme cytosine deaminase (CD) has already been demonstrated (19). CD converts a nontoxic prodrug 5-fluorocytosine (5-FC) to cytotoxic 5-fluorouracil (5-FU). The conversion of 5-FC to 5-FU can be detected in vivo by 19F MRS, and such studies should be possible in the future, with a potential for clinical translation, using either viral vector, liposomal or nanoparticle delivery of cDNA encoding for HRE-driven CD expression. Image-guided delivery of carriers that deliver siRNA to directly downregulate HIF-1 would be another option to reduce the damaging effects of HIF-1 expression in tumors.
Another example of hypoxia-based theranostic imaging is to use hypoxia imaging to prescribe radiation dose delivery, employing selective subvolume boosting and dose painting for theranostic radiation therapy (22). Flynn et al. (22) have recently described the use of 61Cu(II)-diacetyl- bis(N4-methylthio-semicarbazone) (61Cu-ASTM) positron emission tomography (PET) imaging to boost the radiation dose using intensity-modulated X-ray or intensity-modulated proton therapy in a patient with head and neck squamous cell carcinoma (Fig. 2). In the future, the optimization of dose prescription with the radiotracer activity concentration will be required. Dose prescriptions may also be combined with other theranostic agents or prodrug–enzyme treatments.
Figure 2.
Coronal images of a patient with a head and neck squamous cell carcinoma with an overlay of a pretreatment 61Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) (61Cu-ATSM) positron emission tomography (PET) image. 61Cu-ATSM retention was highest in the planning target volume (PTV) boost, within which the regional node (A) and the regional node and the primary tumor (B) are shown. The regional node contained a more complex distribution of 61Cu-ATSM retention than the primary tumor. The plane in (A) is inferior to the plane in image (B). (C) For intensity-modulated proton therapy-spot scanning (IMPT-SS), the entire region that is prescribed a nonuniform, nonzero dose (a) is covered with proton beam spots (an example beam spot is shown) shown as yellow dots (b). For intensity-modulated proton therapy-distal gradient tracking (IMPT-DGT), the dose prescription is thresholded to two uniform levels (c), and spots are then placed only at the locations of dose gradients (d). Reprinted with permission from ref. (22).
pH
Cells regulate pHe and intracellular pH (pHi) using an array of transporters, including Na+/H+ pumps, monocarboxylate transporters (MCTs) 1–4 and carbonic anhydrases (CAs) I–XIII (23). Cells require these pumps to balance the excess of H+ produced by cellular functions, including glucose metabolism.
pHe of tumors is usually acidic, whereas pHi is neutral to alkaline (24). Increased glycolysis, poor blood flow and hypoxia contribute to the acidic pHe observed in tumors. Cancer cells exhibit high glycolytic activity, even in the presence of oxygen, a phenomenon called the `Warburg effect' after observations made by Otto Warburg in 1930. The molecular mechanisms underlying this aerobic glycolysis are mediated, in part, through the stabilization of HIF-1α even under oxygenated conditions in cancer cells (25). HIF-1α expression mediates the switch in glucose metabolism through the induction of lactate dehydrogenase, which converts pyruvate to lactate, and the inactivation of pyruvate dehydrogenase, the enzyme responsible for the conversion of pyruvate to acetyl-coenzyme. Poor blood flow in tumors and the resulting hypoxia also contribute towards increased anaerobic glycolysis (25).
Excessive glycolysis in cancer cells results in large amounts of lactate production and secretion. Increased inflammation and hypoxia have been linked to enhanced transport of intracellular H+ through the actions of MCT-2 and CAIX and CAXII, respectively (26,27). A detailed review on the causes, consequences and detection of tumor pH can be found in this issue.
Unlike hypoxia, where HRE can be exploited to drive the expression of imaging reporters and therapeutic agents, in the absence of a clearly identifiable pH response element, theranostic imaging of tumor pH will mostly rely on the delivery of a therapeutic payload that is only activated at low pHe. Innovative advances have been made in the design of polymers that are pH responsive (28–30). Koo et al. (28) have combined the delivery of photosensitizers using pH-responsive polymeric micelles with photodynamic therapy to target acidic environments. These pH-responsive micelles show pH-dependent demicellization at pH values below 6.5. The photosensitizer released as a result of demicellization produces fluorescence and singlet oxygen following laser excitation, which can be used for diagnosis and therapy (28). Although optical imaging has limitations for the detection of signal from tissue at depths greater than a few millimeters, this advance would be useful for surface tumors or intra-operative treatments. Recently, Kato and Artemov (31) have published a novel dual-contrast MRI technique to detect the release of agents from nanocarriers using simultaneous encapsulation of superparamagnetic iron oxide (SPIO) nanoparticles and a gadolinium-based paramagnetic contrast agent, gadolinium diethylenetriaminepentaacetic acid bismethylamide (GdDTPA-BMA). This technique may have wide-ranging applications as an MRI-based theranostic agent. As a result of their widely different molecular sizes, SPIO and GdDTPA-BMA have very different diffusion properties. When the nanocarrier is intact, SPIO and GdDTPA-BMA are in close proximity, and a strong negative signal enhancement caused by the T2 effects of SPIO dominates the positive T1 contrast generated by GdDTPA-BMA. Once the nanocarrier has released its cargo, a positive T1 contrast is observed once the distance between GdDTPA-BMA and SPIO molecules is beyond the T2 enhancement range. A combination of this dual-contrast MR technique with pH-responsive polymeric micelles or liposomes could extend this pH-based theranostic approach to deep-seated tissues, and for clinical translation.
Recently, Li et al. (32) have developed a novel pH-activated near-infrared (NIR) fluorescence nanoprobe, in which a high payload of the NIR fluorophore IR783 is conjugated with biodegradable dextran via pH-labile hydrazone bonds. Self-quenching between spatially neighboring IR783 fluorophores results in a low background signal in normal tissue. However, cleavage of the fluorophores from the nanoprobe in the acidic TME results in significant fluorescence enhancement. This prototype nanoprobe can be used to deliver a therapeutic payload in acidic TMEs and to detect pH.
Neutralization of the acidic TME has been shown to reduce metastasis (33). It may be possible to design pH-targeting theranostic agents to deliver chemicals at acidic pH that would absorb protons. Strategies to exploit the existing pH gradient in tumors can also be used to improve the uptake of weakly basic chemotherapeutic agents (34,35).
Inflammation
More than a decade ago, Dvorak (36) postulated that tumors are `wounds that do not heal'. The similarities between wounds and solid tumors, such as hypoxia, high lactate and secretion of proinflammatory molecules, are remarkable. A characteristic response of living vascularized tissue to injury is inflammation, which induces the formation of eicosanoids. Three well-known classes of phospholipase, phospholipase A2 (PLA2), phospholipase C and phospholipase D, participate in the formation of free arachidonic acid (AA) from membrane phospholipids in response to mechanical, chemical and physical stimuli (37). As AA is derived from membrane phospholipids, its production and utilization in the formation of eicosanoids is closely coupled to membrane choline phospholipid metabolism (38). AA is converted to various eicosanoids by the action of lipoxygenases and cyclooxygenases (COXs) (37,39). These eicosanoids impact on cell motility, invasion, vascular characteristics and metastatic dissemination (40–42). Several tumors exhibit inflammatory properties, characterized by increased levels of prostaglandins and other proinflammatory molecules that are secreted by tumor cells, stromal cells and specialized immune cells during inflammation (43).
COX-1 and COX-2 are cytoplasmic enzymes that convert PLA2-mobilized AA into the lipid signal transduction molecules prostaglandins and thromboxanes (44). One major product of the COX-2-catalyzed reaction is prostaglandin E2 (PGE2), an inflammatory mediator participating in several biological processes, including development, pain, immunity, angiogenesis and cancer (45–47). COX-2 function has been the target of pharmaceutical intervention in several different cancers, such as gastric, lung, breast and colon cancer (42,48–52). Our studies have shown that silencing of COX-2 in highly metastatic breast cancer cells delays orthotopic tumor growth and inhibits extrapulmonary colonization by these cells (53). PGE2 has also been shown to promote the expression of genes associated with increased angiogenesis, such as vascular endothelial growth factor (VEGF), in the mammary fat pad of mice (54). COX-2 (and PGE2) also result in HIF-1 stabilization (55,56), suggesting that COX-2 function may be mediated, in part, through the HIF-1 axis, which includes drug resistance, increased invasion, increase in choline kinase (57) and the emergence of an aggressive phenotype, even under well-oxygenated conditions.
The development of COX-2 imaging agents is of major interest for several diseases including cancer. Although PET and single photon emission computed tomography (SPECT) imaging agents derived from COX-2 inhibitors have been described previously (58,59), the selective binding of these agents has not been clearly validated in vivo. A recent study demonstrated the feasibility of using indomethacin-derived fluorescent COX-2 imaging agents in vivo (60). In these studies, the retention of the probe in the tumor was found to be dependent on COX-2 activity.
Downregulation of COX-2 can be achieved by the delivery of siRNA loaded in cationic multifunctional liposomes that are decorated with imaging reporters for MRI-guided delivery of COX-2 siRNA in tumors (61). COX-2 siRNA can be loaded directly into cationic liposomes without changing the functionality of siRNA. The incorporation of MR contrast agents within liposomes creates the possibility of imaging siRNA delivery in vivo with MRI with a clear path of clinical translatability.
Ideally, a theranostic agent for COX-2 would report on its expression and activity, and an agent for PGE2 would report on its levels in the microenvironment or its binding to specific receptors (EP1–4). These agents would contain a payload, the delivery of which would be activated by COX-2 and PGE2 expression or function. Although such an agent is currently not available, a molecular theranostic agent, driven by a promoter strongly activated by PGE2, may be a candidate.
Interactions between cancer cells and the TME, which often result in inflammatory signaling, invasion and metastasis, are mediated by soluble messengers, known as chemokines, that bind to specialized chemokine receptors (62). Chemokines are small (8–17-kDa) proteins, which, like their G-protein-coupled receptors, can be categorized into four subfamilies, depending on the number and spacing of cysteine residues. One widely researched chemokine interaction in the TME involves the binding of CXCL12 (cysteine-X-cysteine ligand 12; also known as SDF-1) to CXCR4 (cysteine-X-cysteine receptor 4). CXCR4 receptor expression is regulated by hypoxia (63) and by COX-2 (27), and its expression is increased at the sites of metastasis. CXCR4 inhibitors have also been shown to reduce the incidence of metastasis (64). CXCR4 receptor imaging in preclinical studies has been performed with 125I-labeled antibodies using SPECT imaging. More recently, Nimmagadda et al. (65) have developed a PET imaging agent using [64Cu]AMD3100, a positron-emitting analog of the stem cell mobilizing agent plerixafor. The CXCR4 receptor may be a good candidate for the development of a theranostic agent for metastatic cancer. However, the payload must target pathways specific to cancer, as CXCR4 is also expressed on immune cells and CD34+-expressing hematopoietic progenitor cells, as it mediates leukocyte homing and bone marrow homeostasis (66).
THERANOSTIC IMAGING OF THE STROMA
During tumor growth and invasion, cancer cells become surrounded by and embedded within a complex mesh of ECM, small vessels, infiltrating leukocytes and varying amounts of fibroblasts, collectively referred to as the `tumor stroma'. This stroma is thought to represent a host defense reaction that impedes further tumor spread as both cell–cell and cell matrix interactions can regulate tumor dissemination. The invading cancer cell thus has to breach barriers, including the basement membrane, stromal matrix and cell–cell junctions, all of which oppose its movement. Cancer cells invade surrounding tissues to grow and disseminate by secreting proteolytic enzymes, such as aspartic, cysteine and serine proteases and matrix-degrading metalloproteinases.
The cancer ECM
The ECM comprises collagens, glycoproteins and proteoglycans, several of which are altered in tumors (67). These changes are a result of tumor- and TME-derived signaling. Tumors actively secrete a host of proteolytic enzymes that continuously remodel ECM (68). Lysosomal cathepsins participate in the cleavage of fibrillar collagen (69), laminin (70) and fibronectin (71), intracellularly, and also following secretion. Changes in hyaluronan synthesis and degradation by hyaluronan synthase 2 and hyaluronidase 2, respectively, affect the quantity and quality of hyaluronan, resulting in shorter hyaluronan fragments thought to provide hydrated pathways for metastasizing cells (72). Hyaluronidase activity has been detected using a contrast agent that combines hyaluronan with GdDTPA and nontoxic agarose beads. MRI contrast was generated from hyaluronidase activity by its action on hyaluronan, which altered the relaxation rate of water molecules in contact with the contrast agent (73).
Intrinsic second harmonic generation (SHG) microscopy is valuable for understanding the remodeling of the collagen matrix in intact tissue (74). SHG is a nonlinear optical process that requires a molecule without a center of symmetry to produce a signal. This contrast mechanism can be used to image endogenous structural proteins, such as collagen, with the advantage that differences in fiber structure and volume can be determined in three dimensions with micrometer resolution. A major component of ECM in tumors is fibrillar collagen I, which also governs the delivery of therapeutic molecules in tumors (75,76). We applied SHG microscopy to detect differences in collagen fibers in hypoxic and normoxic tumor regions using human tumor xenografts stably expressing enhanced green fluorescent protein (EGFP) under the control of the HRE of VEGF (77). Hypoxic tumor regions were found to contain fewer collagen fibers than normoxic tumor regions (78). MRI of the macromolecular contrast agent albumin-GdDTPA can be used to characterize the extravascular transport of macromolecules through the ECM of solid tumors in vivo (79,80). Macromolecular transport can be characterized by draining or pooling regions of the agent. With this method, it was possible to detect increased drainage in more invasive tumors that had a greater capacity to degrade ECM (80). We are currently relating macromolecular transport through ECM observed with MRI to collagen fiber volume and distribution in tumors to characterize the functional impact of fiber patterns in normoxic and hypoxic tumor regions.
Although imaging techniques are available to directly probe the ECM structure and protease activity (81), theranostic agents directly targeting ECM require strategies in which specific enzymes overexpressed in the tumor ECM act on a substrate, the cleavage of which should provide a reporter and a therapeutic payload.
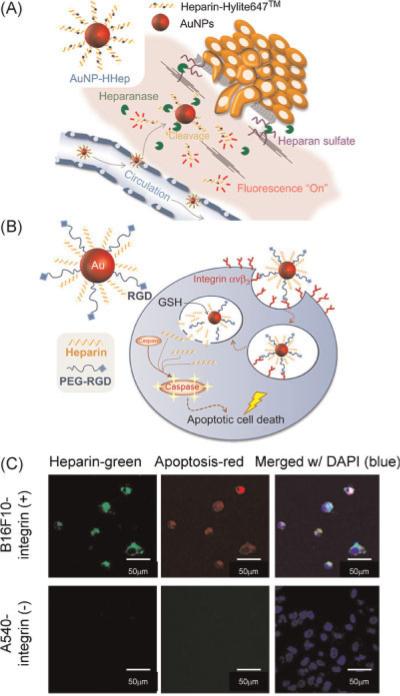
An example of such a strategy using heparin has been described recently by Lee et al. (82). Heparin, a major component of ECM, plays a role in anti-coagulation, anti-inflammation, anti-angiogenesis, and anti-tumor cell proliferation (83). Metastatic cancer cells are characterized by the overexpression of heparinase and heparanase, which facilitate cell migration through heparin-degraded ECM (82). Heparin also possesses an apoptosis-inducing activity in cells by its interaction with transcription factors. Lee et al. (82) have developed heparin-immobilized gold nanoparticles (AuNP-HHep) as a theranostic agent for metastatic cancer cells. These AuNP-HHep nanoparticles showed fluorescence quenching, but, once the heparin was cleaved, fluorescence signals were detected from dyes in the heparin chains using fluorescence resonance energy transfer microscopy.
Targeted cellular delivery of these nanoparticles was achieved by attaching an arginine–glycine–aspartic acid (RGD) peptide that resulted in receptor-mediated endocytosis through the αvβ3-integrin receptor in cells overexpressing this receptor. Quenching of fluorescein-labeled heparin, when bound to AuNP, was removed once heparin was released by the action of glutathione on the gold–thiol linkage. The released heparin induced apoptosis in the cells (Fig. 3).
Figure 3.
(A) Schematic illustration of heparin-immobilized gold nanoparticles (AuNP-HHep) for metastatic cancer cell detection. (B) Schematic illustration of targeted apoptotic cancer cell death for αvβ3-integrin-positive cells on treatment with heparin and polyethylene glycol-arginine–glycine–aspartic acid (PEG-RGD)-immobilized gold nano-particles (AuNP-Hep/PEG-RGD). (C) Confocal microscopic images of B16F10 cells (αvβ3-integrin positive) and A549 cells (αvβ3-integrin negative) following incubation with AuNP Hep/PEG-RGD. Heparin was fluorescently labeled with fluorescein (green), and apoptosis-related caspases 3 and 7 were detected by magic red assay (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Reprinted with permission from ref. (82).
Alternatively nanocarriers that can be cleaved open by tumor ECM enzymes, and that contain either siRNA or chemotherapeutic agents, as well as reporters such as the dual-contrast MR reporters (31), may provide another strategy for theranostic imaging of ECM.
Vascular and lymphatic endothelial cells
The tumor vasculature is richly primed with targets and mechanisms that can be exploited for theranostic imaging. As the delivery network that provides substrates and nutrients, as well as chemotherapeutic agents, to cancer cells, but allows cancer cells to disseminate, it is one of the most important components of the TME. Several receptors on vascular endothelial cells can serve as targets for tumor vasculature-based theranostic imaging (84), as these present easily accessible cellular targets for labeling, imaging and delivery of a therapeutic agent.
One receptor usually used for targeting vascular endothelial cells is αvβ3-integrin, as it is elevated in these cells during angiogenesis. Imaging agents targeting αvβ3 have been developed for MRI (85–88), PET (89) and fluorescence imaging (90–93). The expression of neural cell adhesion molecules (NCAM) is also elevated in tumor endothelial cells. MRI of NCAM was performed using a biotinylated peptide in combination with streptavidin-labeled gadolinium-loaded apoferritin (94).
Theranostic nanoparticles that are cross-reactive with α5β1 and αvβ3 have been used to characterize tumor angiogenesis in a breast cancer xenograft model, and to deliver and assess anti-angiogenic treatment with three-dimensional MRI (95). In this study, nanoparticles were loaded with gadolinium for MRI and rhodamine for optical imaging, and fumagillin was added as an anti-angiogenic agent. The nanoparticles were used to detect the tumor vasculature, to deliver therapy and to detect the tumor response (95) (Fig. 4).
Figure 4.
(A) Tumor neovascular morphology revealed by three-dimensional reconstructions of MR signal enhancement. The tumor volume is outlined in gray, and voxels meeting the enhancement threshold at 2 h post-injection of contrast agent are shown in blue. Left: two rotated views of an α5β1(arginine–glycine–aspartic acid, RGD)-targeted tumor. The cross-sections on the right demonstrate the paucity of angiogenesis in the core. Right: minimal enhancement associated with irrelevant arginine–glycine–serine (RGS)-targeted contrast agent. (B) Assessment of the anti-angiogenic response to integrin-targeted fumagillin nanoparticles (NPs) with α5β1(αvβ3)-targeted MR contrast agent. Top: the extent of neovascularity was quantified by calculating the amount of signal enhancement in the tumor periphery. α5β1(αvβ3)-targeted fumagillin NPs reduced peripheral tumor neovascularity relative to control (p < 0.05, n ¼ 5). αvβ3-targeted fumagillin NPs had no significant effect on angiogenesis, compared with control. Bottom: the effect of α5β1(αvβ3)-targeted fumagillin NPs on tumor neovascular morphology is clearly apparent on three-dimensional reconstructions of MR signal enhancement. Tumor volume is outlined in gray; contrast-enhanced pixels are in blue. Reprinted with permission from ref. (95).
A novel theranostic nanoparticle, termed the `nanoworm' (NW), because of its elongated shape, has been described recently by Agemy et al. (96). NW serves as an imaging agent that contains an iron oxide particle for MRI and a fluorochrome for optical imaging. The tumor-homing pentapeptide cysteine–arginine–glutamic acid–lysine–alanine (CREKA) specifically binds to fibrin and fibrin-associated clotted plasma proteins in tumor vessels and inhibits tumor growth by obstructing tumor circulation through blood clotting (96). CREKA-NW amplifies the clotting effect by accumulating in tumor vessels and inducing additional clotting, providing new binding sites for amplification. CREKA-NW was combined with NW loaded with another tumor-homing peptide to improve treatment efficacy. Extensive clotting in tumor vessels was observed following treatment of mice bearing orthotopic prostate tumors, but no clotting was observed in normal tissues. Multiple doses of NW induced tumor necrosis and reduced tumor growth (96).
The presence of cancer cells in lymph nodes is a high risk factor in the development of distant metastasis. The impact of both induction and inhibition of lymphangiogenesis is therefore of significant clinical interest, as the ability to target tumor-associated lymphatic vessels may create new opportunities for the prevention of lymph node metastasis. Although targets within blood vessels are easily accessible, the mechanisms that control passive and active lymphatic uptake of macromolecules are largely unknown, even for normal lymphatic vessels, let alone the aberrant peritumoral lymphatics channels (97,98). Theranostic agents for lymphatics can theoretically be designed by targeting specific cell surface receptors on lymphatic endothelial cells. Candidate markers for imaging include the `classical' histological markers of lymphatic vessels, such as the hyaluronan receptor LYVE-1, and podoplanin and prox1 (97,98).
CAFs
The structural components of ECM are largely synthesized and organized by fibroblasts. Fibroblasts are stromal cells that synthesize ECM, particularly collagens I, III and V, fibronectin and elastin (99). As a result, fibroblasts are particularly important during wound healing (100). CAFs are known to secrete chemoattractants or express chemoattractant receptors that may be useful for theranostic purposes. It has been proposed recently that breast CAFs secrete CXCL12, thereby stimulating the mobilization of endothelial progenitor cells from the bone marrow and promoting growth by binding to CXCR4 expressed on the surface of breast carcinoma cells (101). CAFs have been defined by the expression of α-smooth-muscle actin, neuron-glial antigen-2, platelet-derived growth factor receptor-β or fibro-blast-specific protein-1 (102).
MRI and NIR imaging studies detecting the recruitment of fibroblasts by tumors have been performed using fibroblasts labeled with biotin–bovine serum albumin (BSA)–GdDTPA. The uptake of biotin–BSA–GdDTPA is mediated by caveolae or SPIOs through endocytosis. Active recruitment of fibroblasts that infiltrate the desmoplastic tumor and populate the vascular areas surrounding tumor nodules was demonstrated (103). The recruitment of fibroblasts by tumors may be exploited for the development of fibroblast-based theranostic agents.
Immune cells
Several immune cells, normally responsible for foreign pathogen elimination, are recruited to tumors. Of these, macrophages are capable of orchestrating the pro-tumorigenic inflammatory response and modulating the effectiveness of cytotoxic T lymphocytes (CTLs). TAMs and other immune cells have also been linked to tumorigenesis, tumor promotion and metastasis (104–106). One critical determinant of the effectiveness of an immune response is the secretion of interleukin (IL)-12 or IL-10 by macrophages. Tumor or macrophage-secreted IL-10 commits macrophages to an M2-polarized activation which, together with transforming growth factor-β and PGE2, suppresses differentiation into dendritic cells, inhibits nitric oxide production, increases tumor vascularization and shows poor presentation of antigen to CTLs (107). Recently, Tie2-expressing monocytes (TEMs) have been shown to interact with Ang-2 expressed on tumor blood vessels (108). Because the expression of Ang-2 is driven by hypoxia, TEMs have been shown to accumulate in hypoxic areas of the tumor (109,110). As a result of their inherent capability to phagocytose large particles, macrophages and monocytes present intriguing target cells for theranostic agents. Like CAFs, TAMs and TEMs can easily be labeled either ex vivo or in situ through their endogenous endocytic pathways (111–113) which, together with their ability to home in on tumors, can be exploited for the development of theranostic agents. In a recent clinical study of iron-oxide-labeled autologous dendritic cell therapy of melanoma patients, MRI was used to monitor the delivery and migration of cells (114,115). Loss of label as a result of cell division which dilutes the exogenous labeling of cells, and a loss of specificity from phagocytosis of the label by other cells, may be solved using a molecular approach of expressing reporter genes.
New developments for theranostic imaging of cell surface receptors
Although the primary focus of this review is the theranostic imaging of the TME, we have included some new developments in the theranostic imaging field in this section. HER2 is overexpressed in a subset of ovarian and breast cancers. A multifunctional gold nanoshell-based theranostic complex has been developed recently to specifically target HER2, and image and induce photothermal tumor ablation with NIR illumination (116). The nanocomplexes consist of a gold nanoshell encapsulated in a silica shell, with SPIO to generate MR contrast agent, and a NIR fluorophore. In cell studies, the nanoshells converted light energy to heat, raising the local temperature and inducing the thermal ablation of cancer cells (116).
Iron oxide nanoparticles have also been coated with a polymeric matrix of polyacrylic acid containing hydrophobic pockets in which NIR dye and anticancer drugs, such as taxol, can be encapsulated (117). To specifically target cancer cells, particles are tagged with folate to bind to folate receptor-expressing cancer cells. A cell study using lung carcinoma cells demonstrated an 80% reduction in viability. These functional nanoparticles are a promising vehicle for in vivo delivery (117).
Cytochrome c (Cyt c), a small mitochondrial protein that plays a key role in cellular respiration, has been identified as an important mediator of apoptosis. Santra et al. (118) have developed a novel biocompatible and targetable polymeric nanoparticle loaded with Cyt c for cancer therapy, and fluorescent dyes for optical imaging. The particles consist of a water-soluble hyperbranched polyhydroxyl polymer tagged with folic acid to specifically target folate receptor-expressing cancer cells (118). Cell studies demonstrated the feasibility of using these particles as a theranostic agent for target-specific detection and tumor treatment.
Magnetic nanoparticles (MNPs), consisting of an iron oxide magnetic core coated with aoleic acid and stabilized with a pluronic copolymer, provided sustained delivery in an MCF-7 tumor in vivo (119). These MNPs could be developed as effective theranostic agents to target cell surface receptors and to deliver anticancer agents (119).
PET/NIR fluorescent/MRI triple iron oxide nanoparticles have also been developed, as shown in a recent study in which dopamine nanoconjugates were encapsulated in human serum albumin matrices, that are clinically utilized as drug carriers (120). These nanoparticles were labeled with 64Cu-DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) for PET imaging and Cy5.5 for optical imaging; the iron core provided MRI contrast. In vivo, these nanoparticles revealed prolonged circulation half-life, massive accumulation in U87MG tumors, a high extravasation rate and a low uptake by macrophages in the tumor region (120). It may be possible to incorporate siRNA or a prodrug enzyme into such a nanosystem for theranostic imaging. Targeted delivery of MNPs can also be combined with a high-frequency magnetic field to trigger drug release or to produce hyperthermia and ablate tissues using magnetic field hyperthermia (121).
CONCLUSION
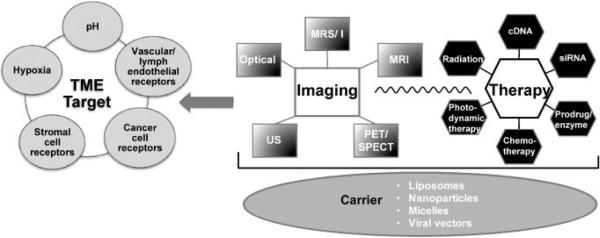
There is no dearth of innovation in the development of theranostic imaging agents for the TME. As outlined in the schematic diagram in Fig. 5, multiple imaging, therapy and TME target options exist that can be combined to design theranostic agents. Advances in molecular biology, chemistry and imaging have resulted in the molecular imaging field arriving at these new and exciting developments that hold significant promise in treating cancer, whilst minimizing damage to normal tissue. For instance, image-guided delivery of multiple siRNAs selectively to a tumor, visualization of this delivery via liposome or nanocarrier technology, and detection of a response are all well within the realm of current imaging capabilities.
Figure 5.
Schematic diagram of strategies for theranostic agents. The figure outlines the imaging modalities, therapy and potential tumor microenvironment (TME) targets that can be used for the development of such agents. PET, positron emission spectrometry; siRNA, small interfering RNA; SPECT, single photon emission computed tomography; US, ultrasound.
Exciting new areas that should develop rapidly in the future include the targeting of specific microenvironments or stromal compartments with theranostic agents. Imaging cancer stem cells, and imaging and targeting permissive or preventative microenvironmental niches for cancer stem cells, are other areas that are likely to have a significant impact on cancer research and treatment.
Although several of the approaches described are clinically compatible, hardly any have been translated clinically. This is not surprising as `theranostics' is a nascent field. A major emphasis for the future should be to rapidly translate and implement the most promising of these into the clinic, rather than allow them to languish as research agents. Although one of the examples included here (Fig. 2) is from a clinical study, clinical applications of theranostic agents are few and far between. Some of the potential barriers impeding the clinical translation of these strategies include Food and Drug Administration (FDA) clearance of the novel nanoparticles that form the major platform for these theranostic agents. A major barrier in MRI theranostics is that, unlike optical and nuclear imaging agents, higher concentrations of the agents are needed, which consequently require more stringent reviewing. Multimodality imaging systems that combine the strengths and capabilities of each modality, together with a concerted effort from industry and funding agencies to promote the clinical translation of discoveries made in this exciting new field, should facilitate this transition.
Acknowledgements
We thank Drs Dmitri Artemov and Cong Li for critical reading of the manuscript. Support from P50 CA103175, P30 CA006973, R01 CA73850, R01 CA82337, R01 CA136576, R01 CA138515, R01 CA138264, R01 CA134695 (KG), R21 CA140904 and R21 CA133600 is gratefully acknowledged.
Abbreviations used
- AA
arachidonic acid
- AuNP-Hhep
heparin-immobilized gold nanoparticles
- BSA
bovine serum albumin
- CA
carbonic anhydrase
- CAFs
cancer-associated fibroblasts
- CD
cytosine deaminase
- COX
cyclooxygenase
- CREKA-NW
cysteine–arginine–glutamic acid–lysine–alanine-nanoworm
- CTLs
cytotoxic T lymphocytes
- Cu-ASTM
61Cu(II)-diacetyl-bis(N4-methylthio-semicarbazone)
- CXCR
cysteine-X-cysteine receptor
- CXCL
cysteine-X-cysteine ligand
- Cyt c
cytochrome c
- DAPI
4′,6-diamidino-2-phenylindole
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- FC
fluorocytosine
- FU
fluorouracil
- GdDTPA-BMA
gadolinium diethylenetriaminepentaacetic acid bismethylamide
- HIF
hypoxia-inducible factor
- HRE
hypoxia response element
- IL
interleukin
- IMPT-DGT
intensity-modulated proton therapy-distant gradient tracking
- IMPT-SS
intensity-modulated proton therapy-spot scanning
- MCT
monocarboxylate transporter
- MNP
magnetic nanoparticles
- NCAM
neural cell adhesion molecule
- NIR
near-infrared
- NW
nanoworm
- PEG-RGD
polyethylene glycol-arginine–glycine–aspartic acid
- PET
positron emission tomography
- PGE2
prostaglandin E2
- pHe
extracellular pH
- pHi
intracellular pH
- PLA
phospholipase A
- RGD
arginine–glycine–aspartic acid
- RGS
arginine–glycine–serine
- SHG
second harmonic generation
- siRNA
small interfering RNA
- SPECT
single photon emission computed tomography
- SPIO
superparamagnetic iron oxide
- TAMs
tumor-associated macrophages
- TEMs
Tie2-expressing monocytes
- TME
tumor microenvironment
- US
ultrasound
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front. Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 4.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussink J, Kaanders JH, Rijken PF, Raleigh JA, Van der Kogel AJ. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat. Res. 2000;153:398–404. doi: 10.1667/0033-7587(2000)153[0398:cibpah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Milas L, Hunter NR, Mason KA, Milross CG, Saito Y, Peters LJ. Role of reoxygenation in induction of enhancement of tumor radioresponse by paclitaxel. Cancer Res. 1995;55:3564–3568. [PubMed] [Google Scholar]
- 10.Michieli P. Hypoxia, angiogenesis and cancer therapy: to breathe or not to breathe? Cell Cycle. 2009;8:3291–3296. doi: 10.4161/cc.8.20.9741. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 12.Chang Q, Qin R, Huang T, Gao J, Feng Y. Effect of antisense hypoxia-inducible factor 1alpha on progression, metastasis, and chemosensitivity of pancreatic cancer. Pancreas. 2006;32:297–305. doi: 10.1097/00006676-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 14.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 15.Gothard L, Haviland J, Bryson P, Laden G, Glover M, Harrison S, Woods M, Cook G, Peckitt C, Pearson A, Somaiah N, Stanton A, Mortimer P, Yarnold J. Randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema after radiotherapy for cancer. Radiother. Oncol. 2010;97:101–107. doi: 10.1016/j.radonc.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Spalding AC, Lawrence TS. New and emerging radiosensitizers and radioprotectors. Cancer Invest. 2006;24:444–456. doi: 10.1080/07357900600705706. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr. Pharm. Des. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- 18.Winnard PT, Jr, Botlagunta M, Kluth JB, Mukadam S, Krishnamachary B, Vesuna F, Raman V. Hypoxia-induced human endonuclease G expression suppresses tumor growth in a xenograft model. Cancer Gene Ther. 2008;15:645–654. doi: 10.1038/cgt.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marignol L, Foley R, Southgate TD, Coffey M, Hollywood D, Lawler M. Hypoxia response element-driven cytosine deaminase/5-fluorocytosine gene therapy system: a highly effective approach to overcome the dynamics of tumour hypoxia and enhance the radio-sensitivity of prostate cancer cells in vitro. J. Gene Med. 2009;11:169–179. doi: 10.1002/jgm.1281. [DOI] [PubMed] [Google Scholar]
- 20.Jamin Y, Gabellieri C, Smyth L, Reynolds S, Robinson SP, Springer CJ, Leach MO, Payne GS, Eykyn TR. Hyperpolarized (13)C magnetic resonance detection of carboxypeptidase G2 activity. Magn. Reson. Med. 2009;62:1300–1304. doi: 10.1002/mrm.22049. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Penet MF, Winnard P, Jr, Artemov D, Bhujwalla ZM. Image-guided enzyme/prodrug cancer therapy. Clin Cancer Res. 2008;14:515–522. doi: 10.1158/1078-0432.CCR-07-1837. [DOI] [PubMed] [Google Scholar]
- 22.Flynn RT, Bowen SR, Bentzen SM, Rockwell Mackie T, Jeraj R. Intensity-modulated X-ray (IMXT) versus proton (IMPT) therapy for theragnostic hypoxia-based dose painting. Phys. Med. Biol. 2008;53:4153–4167. doi: 10.1088/0031-9155/53/15/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458:981–992. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths JR. Are cancer cells acidic? Br. J. Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J. Bioenerg. Biomembr. 2007;39:251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 26.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 27.Stasinopoulos I, Mori N, Bhujwalla ZM. The malignant phenotype of breast cancer cells is reduced by COX-2 silencing. Neoplasia. 2008;10:1163–1169. doi: 10.1593/neo.08568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS, Choi Y, Kwon IC, Kim K, Jeong SY. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem. Commun. (Camb.) 2010;426:5668–5670. doi: 10.1039/c0cc01413c. [DOI] [PubMed] [Google Scholar]
- 29.Lin YH, Chang CH, Wu YS, Hsu YM, Chiou SF, Chen YJ. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials. 2009;30:3332–3342. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Chang G, Li C, Lu W, Ding J. N-Boc-histidine-capped PLGA-PEG-PLGA as a smart polymer for drug delivery sensitive to tumor extracellular pH. Macromol. Biosci. 2010;10:1248–1256. doi: 10.1002/mabi.201000117. [DOI] [PubMed] [Google Scholar]
- 31.Kato Y, Artemov D. Monitoring of release of cargo from nanocarriers by MRI/MR spectroscopy (MRS): significance of T2/T2* effect of iron particles. Magn. Reson. Med. 2009;61:1059–1065. doi: 10.1002/mrm.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Li K, Yan H, Li G, Xia J, Wei X. Dextran based pH-activated near-infrared fluorescence nanoprobe imaging of the acidic compartments in cancer cells. Chem. Commun. (Camb.) 2010;46:1326–1328. doi: 10.1039/b917368d. [DOI] [PubMed] [Google Scholar]
- 33.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem. Pharmacol. 2003;66:1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- 35.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharmacol. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 37.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu. Rev. Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser E, Chiba P, Zaky K. Phospholipases in biology and medicine. Clin. Biochem. 1990;23:349–370. doi: 10.1016/0009-9120(90)90051-u. [DOI] [PubMed] [Google Scholar]
- 39.Haeggstrom JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem. Biophys. Res. Commun. 2010;396:135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 40.Liu JF, Fong YC, Chang CS, Huang CY, Chen HT, Yang WH, Hsu CJ, Jeng LB, Chen CY, Tang CH. Cyclooxygenase-2 enhances alpha2beta1 integrin expression and cell migration via EP1 dependent signaling pathway in human chondrosarcoma cells. Mol. Cancer. 2010;9:43. doi: 10.1186/1476-4598-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin. Cancer Res. 2010;16:1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445–1461. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 43.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc. Natl. Acad. Sci. USA. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Donk WA, Tsai AL, Kulmacz RJ. The cyclooxygenase reaction mechanism. Biochemistry. 2002;41(15):451–15. 458. doi: 10.1021/bi026938h. [DOI] [PubMed] [Google Scholar]
- 45.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res. Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 47.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 48.Diaz-Cruz ES, Brueggemeier RW. Interrelationships between cyclooxygenases and aromatase: unraveling the relevance of cyclooxygenase inhibitors in breast cancer. Anticancer Agents Med. Chem. 2006;6:221–232. doi: 10.2174/187152006776930873. [DOI] [PubMed] [Google Scholar]
- 49.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 50.Krysan K, Reckamp KL, Sharma S, Dubinett SM. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med. Chem. 2006;6:209–220. doi: 10.2174/187152006776930882. [DOI] [PubMed] [Google Scholar]
- 51.Wallace JL. Recent advances in gastric ulcer therapeutics. Curr. Opin. Pharmacol. 2005;5:573–577. doi: 10.1016/j.coph.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Zhang L, Yi Y, Kang HK, Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat. Med. 2004;10:411–415. doi: 10.1038/nm1005. [DOI] [PubMed] [Google Scholar]
- 53.Stasinopoulos I, O'Brien DR, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol. Cancer Res. 2007;5:435–442. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]
- 54.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc. Natl. Acad. Sci. USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji R, Chou CL, Xu W, Chen XB, Woodward DF, Regan JW. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of a phosphoinositide-3 kinase signaling pathway. Mol. Pharmacol. 2010;77:1025–1036. doi: 10.1124/mol.110.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stasinopoulos I, O'Brien DR, Bhujwalla ZM. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol. Ther. 2009;8:31–35. doi: 10.4161/cbt.8.1.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glunde K, Shah T, Winnard PT, Jr, Raman V, Takagi T, Vesuna F, Artemov D, Bhujwalla ZM. Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model. Cancer Res. 2008;68:172–180. doi: 10.1158/0008-5472.CAN-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuge Y, Obokata N, Kimura H, Katada Y, Temma T, Sugimoto Y, Aita K, Seki K, Tamaki N, Saji H. Synthesis and evaluation of a radioiodinated lumiracoxib derivative for the imaging of cyclooxygenase-2 expression. Nucl. Med. Biol. 2009;36:869–876. doi: 10.1016/j.nucmedbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 59.de Vries EF, Doorduin J, Dierckx RA, van Waarde A. Evaluation of [(11)C]rofecoxib as PET tracer for cyclooxygenase 2 overexpression in rat models of inflammation. Nucl. Med. Biol. 2008;35:35–42. doi: 10.1016/j.nucmedbio.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, Matrisian LM, Subbaramaiah K, Dannenberg AJ, Piston DW, Marnett LJ. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010;70:3618–3127. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikhaylova M, Stasinopoulos I, Kato Y, Artemov D, Bhujwalla ZM. Imaging of cationic multifunctional liposome-mediated delivery of COX-2 siRNA. Cancer Gene Ther. 2009;16:217–226. doi: 10.1038/cgt.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose AA, Siegel PM. Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol. 2010;6:55–74. doi: 10.2217/fon.09.138. [DOI] [PubMed] [Google Scholar]
- 65.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Res. 2010;70:3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broxmeyer HE. Chemokines in hematopoiesis. Curr. Opin. Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 67.Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin. Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 68.Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J. Gastroenterol. 2005;11:1251–1266. doi: 10.3748/wjg.v11.i9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song F, Wisithphrom K, Zhou J, Windsor LJ. Matrix metalloproteinase dependent and independent collagen degradation. Front Biosci. 2006;11:3100–3120. doi: 10.2741/2036. [DOI] [PubMed] [Google Scholar]
- 70.Zheng WQ, Looi LM, Cheah PL. Correlation between laminin and cathepsin D expressions in breast carcinoma. Tumori. 2002;88:296–299. doi: 10.1177/030089160208800411. [DOI] [PubMed] [Google Scholar]
- 71.Guinec N, Dalet-Fumeron V, Pagano M. `In vitro' study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin, and release of gelatinase activities from basement membrane fibronectin. Biol. Chem. Hoppe Seyler. 1993;374:1135–1146. doi: 10.1515/bchm3.1993.374.7-12.1135. [DOI] [PubMed] [Google Scholar]
- 72.Udabage L, Brownlee GR, Waltham M, Blick T, Walker EC, Heldin P, Nilsson SK, Thompson EW, Brown TJ. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Res. 2005;65:6139–6150. doi: 10.1158/0008-5472.CAN-04-1622. [DOI] [PubMed] [Google Scholar]
- 73.Shiftan L, Israely T, Cohen M, Frydman V, Dafni H, Stern R, Neeman M. Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma. Cancer Res. 2005;65(10):316–10. 323. doi: 10.1158/0008-5472.CAN-04-3947. [DOI] [PubMed] [Google Scholar]
- 74.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 75.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 76.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 77.Raman V, Artemov D, Pathak AP, Winnard PT, Jr, McNutt S, Yudina A, Bogdanov A, Jr, Bhujwalla ZM. Characterizing vascular parameters in hypoxic regions: a combined magnetic resonance and optical imaging study of a human prostate cancer model. Cancer Res. 2006;66:9929–9936. doi: 10.1158/0008-5472.CAN-06-0886. [DOI] [PubMed] [Google Scholar]
- 78.Kakkad S, Solaiyappan M, O'Rourke B, Stasinopoulos I, Ackerstaff E, Raman V, Bhujwalla Z, Glunde K. Hypoxic tumor microenvironments reduce collagen I fiber density. Neoplasia. 2010;12:608–617. doi: 10.1593/neo.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pathak AP, Artemov D, Ward BD, Jackson DG, Neeman M, Bhujwalla ZM. Characterizing extravascular fluid transport of macromolecules in the tumor interstitium by magnetic resonance imaging. Cancer Res. 2005;65:1425–1432. doi: 10.1158/0008-5472.CAN-04-3682. [DOI] [PubMed] [Google Scholar]
- 80.Pathak AP, Artemov D, Neeman M, Bhujwalla ZM. Lymph node metastasis in breast cancer xenografts is associated with increased regions of extravascular drain, lymphatic vessel area, and invasive phenotype. Cancer Res. 2006;66:5151–5158. doi: 10.1158/0008-5472.CAN-05-1788. [DOI] [PubMed] [Google Scholar]
- 81.Bremer C, Ntziachristos V, Weitkamp B, Theilmeier G, Heindel W, Weissleder R. Optical imaging of spontaneous breast tumors using protease sensing `smart' optical probes. Invest Radiol. 2005;40:321–327. doi: 10.1097/01.rli.0000163797.23172.90. [DOI] [PubMed] [Google Scholar]
- 82.Lee K, Lee H, Bae KH, Park TG. Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomaterials. 2010;31:6530–6536. doi: 10.1016/j.biomaterials.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 83.Robert F. The potential benefits of low-molecular-weight heparins in cancer patients. J. Hematol. Oncol. 2010;3:3. doi: 10.1186/1756-8722-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neri D, Bicknell R. Tumour vascular targeting. Nat. Rev. Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 85.Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, Koning GA, Griffioen AW, Nicolay K. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19:2008–2010. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- 86.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat. Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 87.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, Lacy EK, Zhang H, Robertson JD, Wickline SA, Lanza GM. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- 88.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 89.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl. Med. Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Achilefu S, Bloch S, Markiewicz MA, Zhong T, Ye Y, Dorshow RB, Chance B, Liang K. Synergistic effects of light-emitting probes and peptides for targeting and monitoring integrin expression. Proc. Natl. Acad. Sci. USA. 2005;102:7976–7981. doi: 10.1073/pnas.0503500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, Bremner R, Bading JR, Conti PS. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia. 2005;7:271–279. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu AR, Hou LC, Veeravagu A, Greve JM, Vogel H, Tse V, Chen X. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol. Imaging Biol. 2006;8:315–323. doi: 10.1007/s11307-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 93.Wang W, Wu Q, Pasuelo M, McMurray JS, Li C. Probing for integrin alpha v beta3 binding of RGD peptides using fluorescence polarization. Bioconjug. Chem. 2005;16:729–734. doi: 10.1021/bc049763s. [DOI] [PubMed] [Google Scholar]
- 94.Geninatti Crich S, Bussolati B, Tei L, Grange C, Esposito G, Lanzardo S, Camussi G, Aime S. Magnetic resonance visualization of tumor angiogenesis by targeting neural cell adhesion molecules with the highly sensitive gadolinium-loaded apoferritin probe. Cancer Res. 2006;66:9196–9201. doi: 10.1158/0008-5472.CAN-06-1728. [DOI] [PubMed] [Google Scholar]
- 95.Schmieder AH, Caruthers SD, Zhang H, Williams TA, Robertson JD, Wickline SA, Lanza GM. Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22:4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agemy L, Sugahara KN, Kotamraju VR, Gujraty K, Girard OM, Kono Y, Mattrey RF, Park JH, Sailor MJ, Jimenez AI, Cativiela C, Zanuy D, Sayago FJ, Aleman C, Nussinov R, Ruoslahti E. Nanoparticle-induced vascular blockade in human prostate cancer. Blood. 2010;116:2847–2856. doi: 10.1182/blood-2010-03-274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 98.Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem. Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- 99.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth – bystanders turning into key players. Curr. Opin. Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 101.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 102.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 103.Granot D, Addadi Y, Kalchenko V, Harmelin A, Kunz-Schughart LA, Neeman M. In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007;67:9180–9189. doi: 10.1158/0008-5472.CAN-07-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr. Opin. Oncol. 2009;21:53–59. doi: 10.1097/CCO.0b013e32831bc38a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 107.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 108.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 109.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J. Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 110.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 111.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 112.Corot C, Petry KG, Trivedi R, Saleh A, Jonkmanns C, Le Bas JF, Blezer E, Rausch M, Brochet B, Foster-Gareau P, Baleriaux D, Gaillard S, Dousset V. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest. Radiol. 2004;39:619–625. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 113.Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, Remy C. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2007;37(Suppl 1):S47–S58. doi: 10.1016/j.neuroimage.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 114.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JW, Scheenen TW, Punt CJ, Heerschap A, Figdor CG. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 115.Verdijk P, Scheenen TW, Lesterhuis WJ, Gambarota G, Veltien AA, Walczak P, Scharenborg NM, Bulte JW, Punt CJ, Heerschap A, Figdor CG, de Vries IJ. Sensitivity of magnetic resonance imaging of dendritic cells for in vivo tracking of cellular cancer vaccines. Int. J. Cancer. 2007;120:978–984. doi: 10.1002/ijc.22385. [DOI] [PubMed] [Google Scholar]
- 116.Chen W, Bardhan R, Bartels M, Perez-Torres C, Pautler RG, Halas NJ, Joshi A. A molecularly targeted theranostic probe for ovarian cancer. Mol. Cancer Ther. 2010;9:1028–1038. doi: 10.1158/1535-7163.MCT-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santra S, Kaittanis C, Grimm J, Perez JM. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small. 2009;5:1862–1868. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santra S, Kaittanis C, Perez JM. Cytochrome c encapsulating theranostic nanoparticles: a novel bifunctional system for targeted delivery of therapeutic membrane-impermeable proteins to tumors and imaging of cancer therapy. Mol. Pharm. 2010;7:1209–1222. doi: 10.1021/mp100043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–4021. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Comes Franchini M, Baldi G, Bonacchi D, Gentili D, Giudetti G, Lascialfari A, Corti M, Marmorato P, Ponti J, Micotti E, Guerrini U, Sironi L, Gelosa P, Ravagli C, Ricci A. Bovine serum albumin-based magnetic nanocarrier for MRI diagnosis and hyperthermic therapy: a potential theranostic approach against cancer. Small. 2010;6:366–370. doi: 10.1002/smll.200901689. [DOI] [PubMed] [Google Scholar]