Abstract
Cell-based therapies are a potential therapeutic alternative for the treatment of coronary artery disease. However, transplanted cells undergo significant death in the living subject. Hypoxic preconditioning (HPC) is a potential intervention to increase transplanted cell survival. However, the biological mechanisms of this benefit remain unclear. We hypothesize that the beneficial effect of HPC on stem cell survival is in part due to preservation of oxidant status, an effect that will be monitored using state-of-the-art molecular imaging.
Methods
H9c2 rat cardiomyoblasts expressing the construct CMV-firefly luciferase (h9c2-fluc), with and without HPC, were exposed to hypoxia, and oxidative stress and cell survival were measured. Subsequently, H9c2-fluc cells, with and without HPC, were injected into the myocardium of rats and cell survival was monitored daily with Bioluminescence (BLI) using a CCD camera.
Results
Compared to controls, cells exposed to hypoxia had increased amount of reactive oxygen species (ROS, control:14.1±0.9 vs. hypoxia:19.5±2.0 RFU/μg protein, p=0.02) and decreased cell survival (control:0.29±0.005 vs. hypoxia:0.24±0.005 OD/μg protein, p<0.001). HPC treatment decreased the amount of hypoxia-induced ROS (HPC:11.5±0.7RFU/μg protein, p=0.002 vs. hypoxia and p=0.11 vs. control), associated with improved survival (HPC:0.27±0.004OD/μg protein, p=0.002 vs. hypoxia and p=0.005 vs. control). Most importantly, compared to unconditioned cells, HPC-cells had increased cell survival after transplantation to the myocardium (C:34.7±6.7% vs. HPC:83.4±17.5% at day 5 post-transplant, p=0.01).
Conclusion
The beneficial effect of HPC is in part due to preservation of oxidant status. Molecular Imaging can assess changes in cell survival in the living subject and has the potential to be applied clinically.
Keywords: Bioluminescence, molecular imaging, firefly luciferase, myoblasts, hypoxia preconditioning, oxidative stress
Introduction
Coronary artery disease (CAD) is the leading cause of death in the United States1. Despite advances in medical (e.g., beta blockers, angiotensin-converting enzyme inhibitors, and statins) and interventional therapies (e.g., stents), it continues to be a cause of significant morbidity and mortality2. Thus, novel therapies may be needed to reinstitute coronary flow and cardiac function in certain situations.
Stem cell therapy has emerged as a therapeutic potential alternative for the treatment of coronary artery disease3. However, almost invariably, transplanted cells undergo a significant rate of cell death shortly after transplantation4, 5. Thus, novel strategies to increased cell survival after transplantation are been actively investigated. Recently, hypoxic preconditioning (HPC) has been shown to preserve stem cell survival after transplantation to the myocardium6. However, the biological mechanisms underlying HPC’s beneficial effect remain unclear. Increased oxidative stress results from an imbalance between pro- and anti-oxidants, that can lead to deleterious changes in cell biology7. Furthermore, we have shown that oxidative stress blockade preserves cell survival8. However, the relationship between HPC and oxidative stress in the survival of transplanted cells remains to be determined.
The ultimate goal of regenerative medicine is the use of stem cells in the living subject. To accurately assess stem cell survival in the living subject, it is imperative that a non-invasive imaging strategy is used. Until recently, it was not possible to accurately and non-invasively assess the full extent and degree of the beneficial response of interventions (like HPC) on transplanted cells in the myocardium, in large part due to the unavailability of non-invasive modalities to accurately assess the survival of transplanted cells directly in the living subject. Novel developments in non-invasive imaging have allowed us to study transgene expression and the biology of cell therapy, using imaging modalities such as bioluminescence imaging (BLI)9-12. Our laboratory and others have previously demonstrated that the fate of transplanted cells can be monitored longitudinally in the myocardium5, 13 and peripheral muscle14 using molecular imaging modalities like optical bioluminescence (BLI)9, 12.
Thus, we tested the hypothesis that the beneficial effect of hypoxic preconditioning on transplanted cells is in part due to preservation of oxidative stress balance, and that this benefit can be assessed non-invasively using BLI in the living subject.
Material and Methods
Experimental approach
Embryonic rat H9c2 cardiomyoblasts were stably transfected with the CMV-firefly luciferase reporter gene (H9c2-fluc). Transfected cells were incubated in a hypoxic chamber (1%O2, 4%CO2, 95%N2) for 24 hours and compared to cells under normoxic conditions. Furthermore, a different group of cardiomyoblasts were treated with HPC (2 sets of 15 minutes of hypoxia-1%O2- and 30 minutes of reoxygenation-21%O2-) prior to prolonged hypoxic exposure. Comparable cells were used for both cell culture and studies in the living subject.
Development of a stable cell line expressing firefly luciferase
Embryonic rat H9c2 cardiomyoblasts (American Type Culture Collection, Manassas, VA, USA) were stably transfected with plasmids carrying the fluc gene driven by the cytomegalovirus (CMV) promoter (H9c2-fluc), and the antibiotic resistance gene G41813, 14. After antibiotic selection, high-expressor clones, assessed by firefly luciferase protein activity and detected by CCD camera, were isolated, grown, and used for the study14.
Role of hypoxia on cell viability
Cell viability was tested with the MTT assay15. Cells were incubated with the MTT reagent for 2 hours at 37C, after which a solvent (0.4N HCl isopropanol) was added, and plates placed on a shaker at room temperature for 1 hour. Lastly, absorbance was read in a spectrophotometer at a wavelength of 570nm.
Assessment of oxidative stress
Production of endogenous oxidative stress by-products was assessed using the conversion of 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA, Molecular Probes, Eugene, OR)16. After hypoxia or control conditions, cells were exposed to DCHFDA (5 μM/L) in DMEM (10% FBS) for 30 min at 37°C. To measure the catalase-inhibitable fractions of dichlorofluorescein (DCF) fluorescence, a separate group of cells were incubated with 25 units of catalase (Worthington Biochemical, Lakewood, NJ) prior to exposure to DCHFDA. For quantification of DCHFDA conversion, cells from the different groups were lysed using passive lysis buffer (Promega, Madison, WI). The cell lysate was removed and centrifuged at 4°C at 13,000 rpm for 15 minutes, and the DCHFDA fluorescence in the supernatant was read with an excitation wavelength of 488 nm and emission at 510 nm on a Spectramax Gemini EM (Molecular Devices, Sunnyvale, CA, USA) plate reader. To normalize fluorescence per cell, protein was determined by Bradford (BioRad, Hercules, CA) following manufacturer’s directions. Fluorescence was corrected for background signal and normalized for protein content, and expressed as fluorescence/μg of protein.
To further assess the involvement of oxidative stress, we measured the amount of ROS reactions by measuring the conversion of dihidroethidium (DHE) to ethidium, as previously described from our laboratory8. Cells were plated at a density of 70,000 per well in 12 well plates, 4 wells per treatment, and were treated to 24 hours of hypoxia with or without preconditioning. Cells were then treated with 10μM DHE (Molecular Probes, Eugene, OR) in serum free clear medium (to minimize the interaction of serum with DHE) for 30 min. Cells were rinsed and lysed with passive lysis buffer (Promega, Madison, WI) at 4°C, and equal volumes of the cell lysates were plated on a 96-well black fluorometer plate and fluorescence was read on a Spectramax Gemini EM plate reader with excitation at 589 nm and emission of 610 nm. Four wells of cells untreated with DHE were used to determine background fluorescence. To confirm the specificity of the DHE measurement, a separate set of cells was treated with 25 units of superoxide dismutase (SOD), prior to the DHE assay. The relative fluorescence was normalized to protein content in the lysates and corrected for background fluorescence.
To confirm the location of the signal obtained with DCF and DHE, cells were plated (7×104/well) in 2-well CC2 coated chamber slides (Nalge-Nunc) and exposed for 24 hours to hypoxia (1% O2) with or without preconditioning. Cells were treated with either DHE or DCFDA (concentrations as above) and counterstained for 5 min with DAPI and mounted with ProLong Gold (invitrogen). Slides were photographed on a Zeiss Axiovert LSM 510 inverted confocal fluorescent microscope (Carl Zeiss, Inc., Oberkochen, Germany) with excitation/emission of 480/530 nm (DCF) or 567/610nm (DHE). Representative staining was displayed for each group.
Western blotting
Western blotting was performed following standard protocols17, 18. Equal amounts of cell lysates (25 μg protein) were loaded onto 10% PAGE gels, electrophoresed for 90 min at 90V in Tris-glycine-SDS buffer, then transferred to PVDF membranes in Tris-glycine-SDS-20% methanol at 100V for one hour in an iced bath. Membranes were blocked for one hour in Tris-Buffered Saline Tween-20 (TBST) containing 5% milk (TBST:milk) followed by overnight incubation on rocker at 4°C with primary antibody diluted in TBST:milk. Antibodies used included NAD(P)H p47phox, catalase, and Mn-SOD (dilution for NAD(P)H 1:250, dilution for catalase and Mn-SOD 1:500, all from Santa Cruz Biotechnology, Santa Cruz, CA). β-actin was used as the loading control (Abcam, Cambridge, MA, 1:2000). Following incubation with primary antibody, membranes were washed once for 5 minutes, then 3 times for 10 minutes each in TBST and then incubated on rocker for one hour at room temperature with horseradish peroxidase conjugated secondary antibody diluted 1:5000 in TBST:milk. Wash steps were repeated as above, and then membranes were incubated for 5 min. with SuperSignal West Pico chemiluminescent substrate per manufacturers instructions and imaged by a 5 minute (or 15 second for β-actin) film exposure. Band densities were analyzed by ImageJ and normalized to corresponding β-actin band densities.
Studies in living subjects
Protocols were approved by the Mayo Animal Research Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). Stem cell transplantation was performed as previously described from our laboratory13. Briefly, female rats (Charles River Laboratories, Wilmington, MA, USA) weighing 140–150 g were divided into two groups: control (n=6) and HPC (n=7). Cells were platted 24 hours before transplantation and incubated with either regular medium (control) or regular medium+HPC (HPC). HPC was induced the day of the study following the protocol described above.
The day of the study, animals were anesthetized with 2% isoflurane, their anterior chest was shaved, and the animals were positioned on the surgical table. Using sterile techniques, a left thoracotomy was performed and the anterolateral wall of the LV exposed. Using a 28G needle, 1×106 cells (in 50μl of PBS) were delivered. EKG and temperature were monitored throughout the experiment. Animals were then observed and monitored until recovery for approximately 10 minutes after cell transplantation.
Before each study cardiac function was performed using a dedicated small animal High Resolution Ultrasound system (Hi-Res US, VeVo 770; Visualsonics, Inc., Toronto, ON, Canada). Global left ventricular ejection fraction was estimated using the para-sternal long axis view of the LV (frequency= 30 MHz)13.
Optical bioluminescence imaging of cardiomyoblast transplantation
Animals were imaged daily after cell transplantation until no BLI signal was detected. BLI was performed using a cooled CCD camera (Xenogen, Alameda, CA, USA)5, 13. After intravenous injection of the reporter substrate D-luciferin (50 mg/kg of body weight), rats were imaged after D-luciferin delivery using 5-min acquisition scans. Bioluminescence was quantified as maximal radiance in photons/sec/cm2/sr and to normalize for the baseline signal, data was expressed as percent change (compared to day 1).
Statistical analysis
Data are given as mean ± SEM. Comparisons were performed using unpaired Student t-test of unequal variance. Statistical significance was accepted for p<0.05.
Results
Effect of hypoxia on cell viability and survival
We examined whether hypoxic conditions (similar to what stem cells will encounter in vivo) affect cell survival. When exposed to hypoxic conditions (1% O2 for 24 hours), there was a decrease in cell survival compared to cells that were maintained under standard cell culture conditions (as assessed by MTT, Figure 1). However, preconditioning cardiomyoblasts with HPC, prior to the prolonged hypoxic challenge, resulted in preservation of cell survival (Figure 1).
Figure 1. Assessment of cell survival.
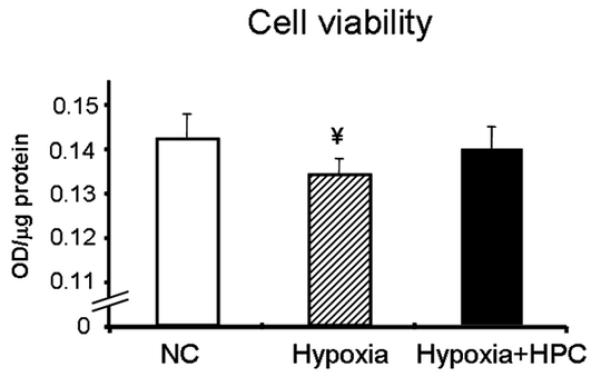
Cell survival was assessed using the MTT assay under hypoxic conditions (1% O2, 4% CO2, 95%N for 24 hours), in cells with and without hypoxic preconditioning (HPC). Hypoxia resulted in decreased survival of cardiomyoblasts, while HPC cells had increased survival compared to untreated cells. ¥p<0.05 compared to control and Hypoxia+HPC.
Assessment of oxidative stress
Cells under control conditions had minimal expression of the oxidative stress markers DCF, due to low levels of peroxide production. In response to hypoxia, however, there was an increase in fluorescence staining to DCF (Figure 2), which was blocked by the addition of the endogenous scavenger enzyme catalase (demonstrating the specificity of the oxidative stress measurement, data not shown). The majority of the peroxides are produced in the cytoplasm, thus DCF signal was observed in the cell cytoplasm (Figure 2, left). DCF interacts with the production of a large number of peroxides7, 19, thus, as expected, blockade with catalase (which mainly metabolizes H2O2) only partially decreased the amount of peroxide levels. Most importantly, when cells were pre-conditioned with HPC prior to exposure to prolonged hypoxia, the production of peroxides was significantly reduced and not different from control cells (Figure 2), suggesting preservation of oxidant status. Similarly, hypoxia was associated with increased conversion of DHE to ethidium (Figure 2B), also suggesting an increase in the production of ROS. As expected, DHE signal co-localized with DAPI staining, was he DHE signal was originated in the cell nucleus (ethidium intercalates with DNA, Figure 2 right). Treatment with SOD decreased the conversion of DHE (less ROS reactions), providing evidence that these measurements reflect changes in oxidative stress of these cells (data not shown). Importantly, as with DCF, HPC decreased the amount of ROS reactions (Figure 2).
Figure 2. Assessment of oxidative stress.
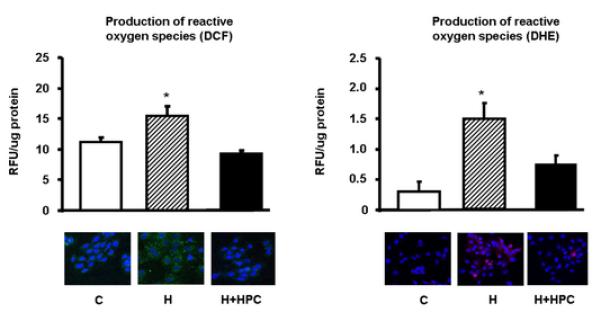
left, top, Representative fluorescence staining of the oxidative stress conversion of 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA); bottom, Fluorescence quantification of the conversion of DCF (expressed as fluorescence/μg protein). Right, top, Representative fluorescence staining of the oxidative stress conversion of Dihydroethidium (DHE); bottom, Quantification of the percent area where DHE staining was present, normalized by the number of nuclei in each microscopic field analyzed. *p<0.05 compared to control and HPC.
To understand whether the increase in oxidative stress was related to a decrease in ROS metabolism or an increase in oxidative stress production, we assessed the protein expression of one of the main ROS producers (NAD(P)H oxidase) and found that under hypoxia, cells had an increase in the expression of NAD(P)H subfraction p47phox (Figure 2) and altered the balance of endogenous scavenger enzymes, as shown by the increase in catalase and Mn-SOD (Figure 2). . Furthermore, HPC normalized expression of the two endogenous scavenger enzymes but did alter the expression of NAD(P)H p47phox.
All put together, HPC normalized the oxidative stress balance altered by hypoxia, mostly by normalizing the metabolism of ROS, while not directly modulating the expression of pro-oxidant enzymes like NAD(P)H.
Studies in living subjects
Thirteen Sprague-Dawley rats (weight: 130±14g, heart rate: 320±5bpm) were imaged with HiRes US (frequency 30MHz). There was no morbidity (as assessed by changes in respiratory rate or heart rate) or mortality associated with the procedure. In addition, no changes in the electrocardiogram (lead II) were observed during the procedure. As expected, there were no differences in LVEF between the groups (control: 80.5±1.6% vs. HPC: 77.5±6%, p=0.38).
Animals from both groups were followed up for 11 days or until no BLI signal was detected in the myocardial area (Figure 3). Baseline cell viability was similar in both groups (control: 1.20×106, HPC: 1.07×106 p/sec/cm2/sr, p=0.36). Compared to day 1, the detected signal in the HPC group was significantly higher compared to the control group (p<0.05), and that difference was observed for the first 3 days (expressed as percent change from baseline-day 1-, Figure 3). After that, cells in the HPC group still had higher viability than cells in the control group, but this did not reach statistical significance. It is important to mention that for the remaining days (after day 5) the detected myocardial signal was not different than background.
Figure 3. Assessment of oxidative stress.
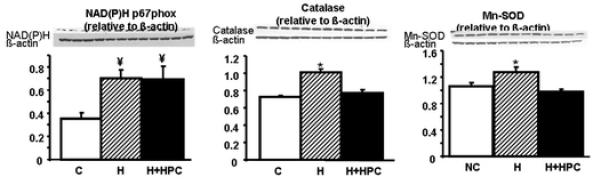
Protein expression bands and densitometric analysis of NAD(P)H p47phox (left), the endogenous scavenger enzymes catalase (middle) and manganese-SOD (Mn-SOD, right). Under hypoxic conditions, there was increased protein expression of NAD(P)H p47phox, together with increased expression of catalase, and Mn-SOD. All of these suggest a pro-oxidant state a pro-oxidant state, what was normalized by HPC. *p<0.05 compared to control and HPC, ¥p<0.05 compared to control.
Discussion
In the present study we showed that the beneficial effect of HPC on cell survival is associated preservation of oxidative status, an effect that can be monitored using molecular imaging in the living subject.
Previous studies have shown that HPC can be used to improved stem cell survival after transplantation. Wang et al demonstrated that HPC increases the mobility and therapeutic potential of stem cells after transplantation6. Most of the studies that examined the role of HPC in stem cell survival focus on the role of survival genes, like Akt and bcl-26. However, the role of other mechanisms like oxidative stress was not known. Oxidative stress has been linked to cell survival, both in native cells as well as transplanted cells. In this study we confirm the observations that hypoxia is associated with an alteration of the oxidant balance. These results are in concert with other studies that showed that hypoxia was associated with an increase in the expression of scavenger enzymes20. However, it is important to mention that alternative pathophysiologic conditions may results in decrease (not increase) of scavenger enzymes. In other words, oxidant status is a fine time-dependent balance of a number of factors, and care should be exercise when applying results from one study to other pathophysiologic situations.
Previous studies have shown that HPC can be beneficial in the preservation of stem cell survival after transplantation. Furthermore, studies have demonstrated that HPC increased the expression of survival genes, and suggested that it is through that mechanism that survival is preserved. In the current, we confirmed the beneficial effect of HPC on cell survival and further extend those observations by demonstrating the important role of oxidative stress in stem cell survival. Furthermore, we provide evidence that maintenance of oxidative stress balance after HPC is achieved by reduced production of the oxidant by-product H2O2 and the normalization of the expression of Mn-SOD.
Oxidative stress has also been linked to other cell function (beyond survival) like stem cell differentiation and many other cell functions, and it has been proposed that a certain degree of oxidative stress is physiologic and needed for “normal” cell function. In fact, cells under normal conditions have a “baseline” level of oxidative stress, as measured by pro-oxidant enzymes, amount of ROS, or scavenger enzyme levels/activity. In this study, we provide evidence that increased oxidative stress affects cell survival and mitochondrial function, and that HPC is beneficial in preserving the level of oxidant stress. However, this study was not designed to test some of the other biological functions linked to oxidative stress, and thus conclusions should not be drawn.
The second important point in this study is the beneficial effect of HPC on transplanted cell survival can be monitored non-invasively directly in the living subject. In previous studies, we showed that the signal obtained from cells that stably express reporter genes (using optical imaging as the imaging modality) directly correlates with the amount of viable cells13. For these studies, we chose to use the firefly luciferase reporter gene system, because it allows fast and high throughput imaging of cell survival, and it can be adapted for clinical use.
In the present study we use a prolonged hypoxic challenge to stress cardiomyoblasts, and we showed that such challenge results in changes in cell survival and increased oxidative stress. However, it is possible that the challenge experienced by cells in the living subject may go beyond hypoxia, as it has been postulated that other pathophysiological mechanisms, like inflammation, immune response, etc, may also play a role and be partly responsible of the poor cell survival observed after transplantation. In other words, the degree of change observed in cell culture and in the living subject should not be compared, as they may not represent an accurate comparison between the two conditions. Furthermore, it is important to mention that different cell types (e.g. mesenchymal stem cells or iPS cells), from the one used in this study (cardiomyoblast), may have different responses to comparable challenge, and thus different survival. Lastly, it should also be kept in mind that while the HPC protocol used in this study served to prove the concept of its beneficial effect on cell survival and its link to oxidative stress, it is likely that the beneficial effect of HPC can be optimized.
In the current study we delivered cells using an epicardial approach. This route of delivery is the most commonly used route in small animal models4, 5, 21 and has the potential to be used in clinical studies 22. In the present study we used intact unperturbed rats, so results from this study should not be extrapolated to other models of disease (e.g. myocardial ischemia/infarction). Nevertheless, the results reported here open novel avenues to investigate the effect of endogenous metabolic intervention in ischemic myocardium.
Conclusion
This study provides evidence that part of the beneficial response to HPC is due to preservation of the oxidative stress balance. Furthermore, it also shows that BLI has the sufficient molecular sensitivity to monitor cells survival after transplantation to the myocardium. Use of imaging strategies like the one used in this study will be critical as this novel therapies are being translated to the clinical arena.
Figure 4. Longitudinal monitoring and quantification of cell viability in living subjects.

Bioluminescence imaging non-invasive monitoring of cell engraftment and survival in living rats (n=7 in each group). Data is expressed as percent change (%) of maximal radiance compared to day 1. Until day 3, cells that received HPC had higher myocardial engraftment and survival compared to untreated cells (control). After day 3, cell survival was similar between the two groups. Error bars represent SEM. *p<0.05 compared to day 0.
Funding
This work was supported in part by National Health Lung Blood Institute R00 HL88048, and the Mayo Clinic Scholarship Program, Mayo Clinic College of Medicine, Rochester, Minnesota.
Bibliography
- 1.AmericanHeartAssociation [accessed January, 2010];Heart Disease and Stroke Statistics-2009 update. www.americanheart.org.
- 2.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136(5):341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Murtuza B, Beauchamp JR, Smolenski RT, Varela-Carver A, Fukushima S, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. Faseb J. 2004;18(10):1153–1155. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 5.Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108(11):1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, et al. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29(1):74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 7.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108(16):1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Porcel M, Gheysens O, Paulmurugan R, Chen IY, Peterson KM, Willmann JK, et al. Antioxidants Improve Early Survival of Cardiomyoblasts After Transplantation to the Myocardium. Mol Imaging Biol. 2009 doi: 10.1007/s11307-009-0274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16(4):378–387. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 10.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006;6(6):484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 11.Shah K, Jacobs A, Breakefield XO, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11(15):1175–1187. doi: 10.1038/sj.gt.3302278. [DOI] [PubMed] [Google Scholar]
- 12.Wu JC, Tseng JR, Gambhir SS. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11(4):491–505. doi: 10.1016/j.nuclcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Porcel M, Gheysens O, Chen IY, Wu JC, Gambhir SS. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12(6):1142–1147. doi: 10.1016/j.ymthe.2005.07.532. [DOI] [PubMed] [Google Scholar]
- 14.Gheysens O, Lin S, Cao F, Wang D, Chen IY, Rodriguez-Porcel M, et al. Noninvasive evaluation of immunosuppressive drug efficacy on acute donor cell survival. Mol Imaging Biol. 2006;8(3):163–170. doi: 10.1007/s11307-006-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Ksiazek K, Piwocka K, Brzezinska A, Sikora E, Zabel M, Breborowicz A, et al. Early loss of proliferative potential of human peritoneal mesothelial cells in culture: the role of p16INK4a-mediated premature senescence. J Appl Physiol. 2006;100(3):988–995. doi: 10.1152/japplphysiol.01086.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46(4):772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 18.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, et al. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15(4):958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 19.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad M, Zhao X, Kelly MR, Kandhi S, Perez O, Abraham NG, et al. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. American journal of physiology. 2009;297(4):H1453–1461. doi: 10.1152/ajpheart.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, et al. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114(1 Suppl):I174–180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]
- 22.Baklanov DV, de Muinck ED, Simons M, Moodie KL, Arbuckle BE, Thompson CA, et al. Live 3D echo guidance of catheter-based endomyocardial injection. Catheter Cardiovasc Interv. 2005;65(3):340–345. doi: 10.1002/ccd.20379. [DOI] [PubMed] [Google Scholar]


