Abstract
Neurorestorative therapy is the next frontier in the treatment of stroke. An expanding body of evidence supports the theory that after stroke, certain cellular changes occur which resemble early stages of development. Increased expression of developmental proteins in the area brodering the infarct suggest an active repair or reconditioning response to ischemic injury. Neurorestorative therapy targets parenchymal cells (neurons, oligodendrocytes, astrocyes and endothelial cells) to enhance endogenous neurogenesis, angiogenesis, axonal sprouting and synaptogenesis to promote functional recovery. Pharmacological treatments include statins, phosphodiesterase 5 inhibitors, erythropoietin, and nitric oxide donors which have all improved funtional outcome after stroke in the preclinial arena. Thymosin β4 is expressed in both the developing and adult brain and it has been shown to stimulate vasculogenesis, angiogenesis and arteriogenesis in the post-natal and adult murine cardiac myocardium. In this manuscript, we describe our rationale and techniques to test our hypothesis that Thymosin β4 may be a candidate neurorestorative agent.
Keywords: Thymosin β4, Neurorestorative, stroke, neurogenesis
Introduction
Stroke is a major cause of morbidity and mortality worldwide [1]. Most survivors exhibit some form of disability and functional recovery is slow and unpredictable. Over the past two decades, research on treatment of stroke has focused on neuroprotection and revascularization strategies. The only successful clinical trials that has resulted from this research is the use of tissue plasminogen activator (rt-PA) administered within 4.5 hours of symptom onset [2, 3]. Use of t-PA has been limited because of its narrow time dependent treatment window as most stroke patients present to the Emergency Department well beyond 4.5 hours of symptom onset [4, 5]. Moreover, use of rt-PA is complicated by a 6.4% symptomatic intracerbral hemmorhage (ICH) rate which has caused considerable controversy regarding its practical use, particularly amongst Emergency physicians. The restriction on time and potential adverse effects have limited the use of rt-PA to approximately 3% of stroke patients [6, 7]. New strategies need to be designed to produce a treatment for stroke patients that are not limited to a symptom onset time window and produce improved outcomes with minimal side effects. Therapeutic advances would be to initiate treatment many hours or even days after stroke onset with the intent of improving neurological function rather than just simply reducing the area of infarction. Pharmacologically based therapies designed to enhance endogenous neurogenesis to promote functional recovery is the next frontier in stroke research.
Neurorestorative therapy
Recovery recapitulates ontogeny [8]. Genes and proteins that are present and active during development are reactivated after ischemic brain injury. Expression of these developmental genes and proteins after ischemia, especially with limited cerebral metabolic resources, demonstrates a conservation of plasticity that is capable of activation even in an aged adult organism. Examples of increased cerebral protein expression during development and ischemia are structional proteins (nestin and MAP-2), growth factors (VEGF and BDNF) and differeation factors (NeuroD) [9, 10]. In a similar fashion, the number of dendritic spines reach a maximum several years after birth and subsequently decline. Likewise after stroke, the number of dendritic spines and synapses are increased during recovery and pruned [11, 12]. Neurorestorative therapy capitalizes on the these fundamental observations to enhance these brain repair processes
Neurorestorative therapies using cell-based or pharmacological treatments are designed to be administered 24 hours or later after stroke onset to remove the time restraints of thrombolytics thereby enabling most strokes to be treated. Neurorestorative therapy treats the intact tissue and targets specific parenchymal cells (nerual progenitor cells, astrocytes, oligodendrocytes or endothelial cells) to activate endogenous neurogenesis, oligogenesis, or angiogenesis [13]. Cell based therapies currently under investigation are bone-marrow mesenchymal cell, cord blood cells, fetal embryoic cells. Pharmacological treatments include statins, phosphodiesterase 5 inhibitors erythropoietin, and nitric oxide donors [14-18]. These treatments have all demonstrated improved funtional outcome in the preclinial arena.
Stroke induced neurogenesis
In the adult rat brain, neurogenesis occurs primarily in the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) [19]. The SVZ contains a hetergenous group of progenitor or adult stem cells which have the protential of differentiating into neurons, astrocytes or oligodendrocytes. Type A cells are neuroblasts, while a subpopulation of type B cells (astrocytes) are neural stem cells. Slow dividing type B cells generate rapidly dividing type C (progenitor cells) cells that differentiate into type A cells (migrating neuroblasts) [20] Type A cells ultimately travel the rostral migratory stream to the olfactory bulb where they differentiate into inter-neurons throughout rodent life [21]. A small subpopulation (5%) of type C cells in the SVZ become oligoprecursor cells (OPC) [22].
Stroke induces neurogenesis in the SVZ and dentate gyrus [23, 24]. Ischemia induces neurogenesis by activating quiescent stem cells in the SVZ into a proliferative state by a symmetric mode of cellular (blast) division. Doublecortin (DCX) is a microtubular associated protein required for neuroblast migration [25]. Stroke significantly increases the number of DCX expressing neuroblasts in the ischemic SVZ and ischemic boundary region (Figure 1A and 1B) [24].
Figure 1.
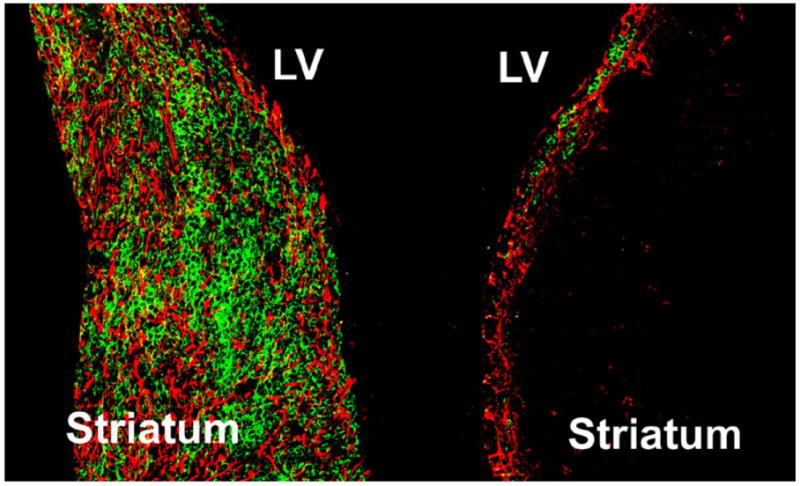
Figure 1 (left) demonstrates neuroblasts (green, DCX positive cells) along with activated astrocytes (red, GFAP (glial fibrillary acidic protein)), in the ischemic SVZ and striatum 14 days after stroke. Figure 1 (right) represents the non-ischemic SVZ hemisphere [24].
Thymosin β4 a potential neurorestorative agent?
Thymosin β4 (Tβ4) is a developmentally expressed 43-amino acid peptide that was originally isolated from thymic extract and exists in numerous tissues and isoforms [26]. Tβ4's fundamental action is to inhibit organization of the actin-cytoskeletin by sequestration of G-actin monomers and thereby enabling cells to migrate [27]. Tβ4 has been shown to promote cardiomyocyte survival and to improve cardiac function after myocardial infarction (MI) in experimental adult mice [28]. Tβ4 has been shown to regulate vasculogenesis, angiogenesis and arteriogenesis in the post-natal and adult murine cardiac myocardium. Vascular progenitor cells isolated from the adult epicardium differentiated into smooth muscle and endothelial cells when cultured in the presence of Tβ4 [29]. It is postulated that Tβ4 may improve cardiac function by acting in conjunction with other angiogenic factors (ie VEGF) to revascularize damage myocardium through stimulation of adult vascular progenitor cells. Additionally, Tβ4 was found to be an essential paracrine factor of embryonic endothelial progenitor cell cardiac protection after acute MI [30].
Tβ4 has been shown to be expressed in both the developing and adult brain [31, 32]. Specifically, Tβ4 has been to be localized in growing neurites of cerebellar granule cells [33, 34]. In many disease states such as focal ischemia, hippocamal denervation and Huntington's disease, gene expression of Tβ4 is upregulated [35-37]. Since actin dynamics contribute a critical role in cell migration and synaptogenesis, speculation exists that upregulation of Tβ4 could be vital to recovery. Given these observations and the robust neurogenesis observed after stroke in the SVZ, Tβ4 could potentially act as a neurorestorative agent by promoting the differentiation and migration of stems cells from the SVZ to the ischemic area of injury.
Experimental Techniques
Embolic stroke rat model
Our embolic stroke rat model has been used to test a variety of thromblytic, antiplatelet, neuroprotective and neurorestorative therapies [17, 38-43]. The model is reproducible and predicted the FDA approved three hour treatment window of rt-PA. The robust neuroprogenitor proliferation and upregulation of DCX after ischemia provides an avenue for studying potential neurorestorative treatments.
Procedure
Male Wistar rats are anesthetized with 3.5% halothane and anesthesia maintained with 1.0% halothane in 70% N2O and 30% O2 using a face mask. Rectal temperature is maintained at 37 ± 0.5 °C throughout the surgical procedure using a feedback regulated water heating system. The right femoral artery is cannulated with a PE-50 catheter (Becton Dickinson and Company, Sparks, MD) for continuous monitoring of blood pressure and measurement of blood gases and the right femoral vein is cannulated with a PE-50 catheter for drug administration.
Preparation of the emboli
Before occlusion of the middle cerebral artery (MCA), the clot must be prepared the previous day from arterial blood from a donor rat. Femoral arterial blood from a donor rat is withdrawn into 20 cm of PE-50 tubing and retained in the tube for 2 hours to clot at room temperature and subsequently maintained at 4 °C for 22 hours. A total of 5 cm of the PE-50 tube containing the clot is cut and attached at each end to a 20-cm PE-10 tube interconnected by a syringe filled with saline. The clot is shifted by continuous alternating movement from one syringe to the other for 5 minutes. A single clot (1.0 μL) is transferred to a modified PE-50 catheter with a 0.3-mm outer diameter filled with saline.
Emboli placement
The MCA is occluded by placement of the embolus at the origin of the MCA. Using an operating microscope (Carl Zeiss, Inc., Thornwood, NY) the right common carotid arteries (CCAs), the right external carotid artery (ECA) and the internal carotid artery (ICA) are isolated via a midline incision. A modified PE-50 catheter with a 0.3-mm outer diameter filled with a 1.0 μL clot, which is attached to a 100-μL Hamilton syringe filled with 0.9% saline, is introduced into the ECA lumen through a small puncture (figure 2A) [40]. A 15-to 16-mm length of catheter is then gently advanced from the ECA into the lumen of the ICA. The clot in the catheter is injected into the ICA along with 2 to 3 μL of 0.9% saline. Finally, the catheter is withdrawn from the right ECA 5 minutes after injection (figure 2B) and the right ECA is ligated
Figure 2.
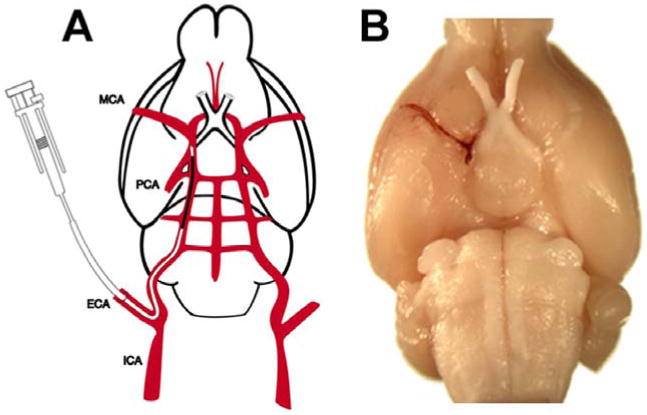
Figure 2A is a schematic drawing of the embolic stroke rat model. Figure 2B demonstrates the clot placed in the MCA [40].
Functional tests
After neurological injury, functional outcome is the most important indicator of drug efficacy. In our rat model, neurological functional testing is routinely performed to access treatment effect. Specific behavioral tests have been developed to test motor and sensory functions [44]. These tests are performed before MCA occlusion and at specific days after MCA occlusions by an investigator who is blinded to the experimental groups. The battery of tests consists of the adhesive-removal test and the modified Neurological Severity Score (mNSS). The adhesive removal test, which measures somatosensory deficit, is performed according to the method developed by Schallert and Whishaw [45]. Briefly, the rats are removed from their home cages so that the adhesive paper dots are firmly and accurately attached. Two small pieces of adhesive-backed paper dots (of equal size, 113.1 mm2) are used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The rats are returned to their cages and typically remove each stimulus one at a time using their teeth. The time required to remove both stimuli from each limb is recorded in 5 trials per day. Before surgery, the rats are trained 5 times a day for 3 days and all rats are able to remove the dots within 10 sec at the end of training.
Measurement of lesion volume
After completion of the functional testing the rats are sacrificed and the brains removed. Rat brains are fixed by transcardial perfusion with saline and embedded in paraffin. Seven brain sections are traced using a microcomputer imaging device (MCID) analysis system (Imaging Research, St. Catharines, Canada). The indirect lesion area, in which the intact area of the ipsilateral hemisphere is subtracted from the area of the contralateral hemisphere, is calculated. Lesion volume is represented as a volume percentage of the lesion compared with the contralateral hemisphere. Measurement of lesion volumes is correlated to functional outcome. However, reduction of lesion volume is not always correlated to improved functional outcome.
Immunohistochemical staining
Standard paraffin blocks are obtained from the center of the lesion, corresponding to coronal coordinates for bregma -1-1 mm. A series of 6 μm thick sections at various levels (100 μm interval) are cut and stained with hematoxylin and eosin (H&E). Tissue sections are incubated in commercially prepared antibodies used to identify specific cellular and vascular cell types. The sections are treated with an ABC kit (Vector laboratories, INC) and diaminobenzidine (DAB) is used as a sensitive chromogen for light microscopy. Coronal sections are digitized using a 40x objective using a three-CCD color video camera (Sony DXC-970MD) interfaced with a MCID system for quantification.
Summary
Tβ4 is a potential neurorestorative agent because of its actin binding properties that may enhance neuroprogenitor cellular migration and differentiation in the area of ischemic injury promoting a brain repair or regenerative process. Tβ4 is presently being tested in our rat model of embolic stroke Tβ4. In future publications, we hope to provide evidence that Tβ4 promotes restorative mechanisms and recovery after stroke and that recovery will not depend on a narrow time window of treatment.
References
- 1.Feigin VL, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.CASPER Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 5.Deng YZ, et al. IV tissue plasminogen activator use in acute stroke: experience from a statewide registry. Neurology. 2006;66:306–312. doi: 10.1212/01.wnl.0000196478.77152.fc. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, et al. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke. 2009;40:3580–3584. doi: 10.1161/STROKEAHA.109.554626. [DOI] [PubMed] [Google Scholar]
- 7.Reeves MJ, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, et al. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980-1971. [DOI] [PubMed] [Google Scholar]
- 11.Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733:142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- 12.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 16.Lu D, et al. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, et al. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006;140:377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gage FH, et al. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligon KL, et al. Olig gene function in CNS development and disease. Glia. 2006;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proc Natl Acad Sci U S A. 1966;56:1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Bock-Marquette I, et al. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 29.Smart N, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 30.Hinkel R, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpintero P, et al. Expression of thymosin beta4 messenger RNA in normal and kainate-treated rat forebrain. Neuroscience. 1999;90:1433–1444. doi: 10.1016/s0306-4522(98)00494-1. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Marquez J, Anadon R. The beta-thymosins, small actin-binding peptides widely expressed in the developing and adult cerebellum. Cerebellum. 2002;1:95–102. doi: 10.1007/BF02941895. [DOI] [PubMed] [Google Scholar]
- 33.van Kesteren RE, et al. Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowth. J Neurosci. 2006;26:152–157. doi: 10.1523/JNEUROSCI.4164-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, et al. The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res. 2008;33:2269–2280. doi: 10.1007/s11064-008-9712-y. [DOI] [PubMed] [Google Scholar]
- 35.Dong JH, et al. Expression of thymosin beta4 mRNA by activated microglia in the denervated hippocampus. Neuroreport. 2005;16:1629–1633. doi: 10.1097/01.wnr.0000183326.21241.48. [DOI] [PubMed] [Google Scholar]
- 36.Sapp E, et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol. 2001;60:161–172. doi: 10.1093/jnen/60.2.161. [DOI] [PubMed] [Google Scholar]
- 37.Vartiainen N, et al. Induction of thymosin beta(4) mRNA following focal brain ischemia. Neuroreport. 1996;7:1613–1616. doi: 10.1097/00001756-199607080-00017. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, et al. Postischemic (6-Hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor PS-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke. 2001;32:2926–2931. doi: 10.1161/hs1201.100207. [DOI] [PubMed] [Google Scholar]
- 39.Zhang RL, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 40.Zhang RL, et al. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang RL, et al. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZG, et al. Postischemic treatment (2-4 h) with anti-CD11b and anti-CD18 monoclonal antibodies are neuroprotective after transient (2 h) focal cerebral ischemia in the rat. Brain Res. 1995;698:79–85. doi: 10.1016/0006-8993(95)00830-j. [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZG, et al. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res. 2001;912:181–194. doi: 10.1016/s0006-8993(01)02735-4. [DOI] [PubMed] [Google Scholar]
- 44.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 45.Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]


