Abstract
Nicotinamide reduces ischemic brain injury in adult rats. Can similar brain protection be seen in newborn animals? Seven-day-old rat pups had the right carotid artery permanently ligated followed by 2.5 h of 8% oxygen. Nicotinamide 250 or 500 mg/kg was administered i.p. 5 min after reoxygenation, with a second dose given at 6 h after the first. Brain damage was evaluated by weight deficit of the right hemisphere at 22 days following hypoxia. Nicotinamide 500 mg/kg reduced brain weight loss from 24.6 ± 3.6% in vehicle pups (n = 28) to 11.9 ± 2.6% in the treated pups (n = 29, P < 0.01), but treatment with 250 mg/kg did not affect brain weight. Nicotinamide 500 mg/kg also improved behavior in rotarod performance. Levels of 8-isoprostaglandin F2α measured in the cortex by enzyme immune assay 16 h after reoxygenation was 115 ± 7 pg/g in the shams (n = 6), 175 ± 17 pg/g in the 500 mg/kg nicotinamide treated (n = 7), and 320 ± 79 pg/g in the vehicle treated pups (n = 7, P < 0.05 versus sham, P < 0.05 versus nicotinamide). Nicotinamide reduced the increase in caspase-3 activity caused by hypoxic ischemia (P < 0.01). Nicotinamide reduces brain injury in the neonatal rat, possibly by reducing oxidative stress and caspase-3 activity.
Keywords: Stroke, Free radicals, Caspase-3, 8-Isoprostane F2α, Neuroprotection
1. Introduction
Nicotinamide (Vitamin B3) is a form of niacin and readily enters the brain [36]. Nicotinamide is the precursor for the coenzyme β-nicotinamide adenine dinucleotide (NAD+) and is considered to be an essential nutrient for cell growth and function (for review, see [26]). Recently, nicotinamide has been reported to exert a number of pharmacological effects including prevention of ATP depletion [22,24,42], inhibition of poly(ADP-ribose) polymerase (PARP) [22,23,42] and lipid peroxidation [10,23,29], anti-inflammatory activity [40] and prevention of apoptosis [24,29]. In adult rats nicotinamide protects against both early necrotic cell death in the core and delayed apoptosis like cell death in the penumbra in stroke models [41]. Apoptotic mechanisms are most important in fetal and neonatal animals, since these ages represent the peak of apoptotic activity in the normal brain [19,30]. Caspase-3 is the chief effector caspase in the apoptotic cascade, and a common final pathway for both the intrinsic, mitochondrial pathway and the receptor mediated pathway. Nicotinamide directly modulates mitochondrial membrane potential and pore formation to prevent cytochrome c release and caspase-3 and caspase-9 like activities [10]. In adult animals nicotinamide protects against free radical injury to the brain [10,23,29]. Oxidants and free radicals can initiate apoptosis by stimulating mitochondrial pore formation [35]. Oxidants and free radicals can cause single stranded DNA breaks, which can activate poly(ADP-ribose) polymerase [5,12]. Treatments with nicotinamide are neuroprotective in adult rat transient cerebral ischemia [4,28,41] and permanent focal cerebral ischemia [3,34]. Nicotinamide is also effective in adult rats even when given 6 h after injury [4,41]. Does nicotinamide have similar pharmacological effects and neuroprotective effects in newborn animals to that seen in adult animal? These questions have not been adequately addressed.
Hypoxic–ischemic injury is an important cause of death and disability in newborn humans. The developmental stage of the brain of the 7-day-old rat pup resembles that of term newborn humans [31]. Therefore, study of the role of neuroprotective agents in the neonatal hypoxic–ischemic rat model may provide important information pertinent to the development of treatment for perinatal hypoxic–ischemic brain damage. The neonatal rat hypoxic–ischemic model [32] has been well characterized and extensively used to assess synthetic neuroprotective agents (for review, see [1,2]). Clinical brain injury is caused by hypoxia or ischemia rather than the combination. However, hypoxia of a clinically relevant severity will cause ischemia by inhibiting heart function. Cellular hypoxia is the end point of both hypoxia and ischemia. The ischemia in the Rice model does not cause damage unless combined with hypoxia [32]. In addition, the long-term survival of rats from the Rice model allows study of very important late effect [39]. We have used this hypoxic–ischemic model to evaluate the neuroprotective potency of several drugs [13–15]. The purpose of the present study was to determine whether treatment with nicotinamide would reduce brain injury in newborn rats and to evaluate the effects of nicotinamide on oxygen free radicals and caspase-3 by using the neonatal rat hypoxic ischemic model. This has not previously been tested.
2. Materials and methods
2.1. Animal protocol and drug treatment
Our institutional committee on animal use approved this protocol. Rats were cared for in accordance with the National Institute of Health guidelines. Seven-day-old Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) weighing 12–17 g of either sex were anesthetized with isoflurane and had the right common carotid artery isolated from the nerve and vein, and permanently doubly ligated. The wound was infiltrated with marcaine, a long acting local anesthetic, at the end of the surgery to prevent postoperative pain. The whole procedure took less than 7 min. The pups were returned to their dam for at least 3 h recovery after surgery. The pups were then placed in sealed jars in a 37 °C water bath and subjected to a warmed, humidified mix of 8% oxygen and 92% nitrogen delivered at 4 l/min for 2.5 h. To assess the neuroprotective effect of nicotinamide, pups were randomized to treatment with 500 mg/kg nicotinamide (n = 29), or vehicle (saline, n = 28); or 250 mg/kg nicotinamide (n = 22), or vehicle (saline, n = 23) i.p. immediately after the hypoxia with a second identical dose given 6 h later. The dose was chosen from the adult rat literature [3,4,17,28,34]. To maintain brain concentration we chose to inject a second identical dose of nicotinamide at 6 h after the first injection. Pups were returned to their dams and allowed to recover and grow for 22 days. They were weighed prior to injury and again at 4, 7, 11, 14 and 22 days after injury. Rectal temperature was taken with a 36 gauge flexible thermocouple (Omega Engineering Inc., Stamford, CT, USA) in a sub-set of these pups (five treated with 500 mg/kg of nicotinomide and five from the corresponding vehicle group) prior to dosing and at 0.13, 0.25 0.5, 0.75, 1, 2, 3, 4 and 6 h after treatment. In order to standardize the measurement and reduce the variance, temperature measurements were taken 15 min after removal from the nest into a 25 °C room.
2.2. Gross brain damage grading
Rat pups were anesthetized with pentobarbital and decapitated 22 days after hypoxic exposure. The brains were removed, scored and weighed by an observer blind to the code. Brains were scored normal, mild, moderate or severe by the method of Palmer et al. [31]. “Normal (1)” meant no reduction in the size of the right hemisphere, “mild (2)” meant visible reduction in right hemisphere size, “moderate (3)” meant large reduction in hemisphere size with a visible infarct in the right parietal area, and “severe (4)” meant near total destruction of the hemisphere. After removing the cerebellum and brain stem, the brain was divided into two hemispheres and weighed by an observer blinded to the experimental group of the pups. Results are presented as a percent loss of right hemispheric weight relative to the left [(left − right)/left × 100]. The loss of hemispheric weight can be used to measure brain damage in this model if enough time after injury has elapsed to allow resorption of the dead brain tissue [32]. Missing brain weight can be converted to missing brain volume as measured by TTC staining [41] or similar method as brain weighs approximately 1 g/ml. However TTC staining which is usually done at 3 days after injury will miss the injury to the brain if it is delayed [39].
2.3. Neuro-functional assessment
In a separate experiment, motor coordination was evaluated with rotarod tests (Rotamex, Columbus Instruments, Columbus, OH, USA) conducted 26–30 days after hypoxic exposure by a blinded experimenter. Rotarod testing was conducted as previously described with modifications [11] on 9 pups treated with 500 mg/kg of nicotinamide and 10 pups treated with vehicle. After a practice run at 5 rpm the animals were placed in individual lanes with dark Plexiglas spacers between them and the rotarod was set at 10 rpm. Latency to first fall was recorded for each animal. For each animal the trial was completed after 180 s or three falls.
2.4. Measurement of 8-isoPGF2α
A third set of experiments was performed to determine the effect of nicotinamide on lipid peroxidation using a reliable, sensitive and specific enzyme immunoassay for measuring 8-isoPGF2α [37]. Using the above neonatal hypoxic–ischemic procedure, the rat pups were treated with 500 mg/kg of nicotinamide by i.p. injection at 5 min after hypoxia, with a second dose given 6 h after the first. Levels of 8-isoPGF2α were measured in the cerebral cortex in nicotinamide treated (n = 7), vehicle treated (n = 7) and sham operation groups (n = 6) at 16 h after hypoxia. Eight-isoPGF2α levels peak at 24 h following brain injury [18].
The cortex in lesioned hemispheres was separately dissected on ice and frozen at −80 °C. Eight-isoPGF2α was assessed as described by Hoffman et al. [18]. Briefly, the assay uses an 8-isoprostane-acetylcholinesterase conjugate that competes with 8-isoPGF2α for binding sites on the rabbit monoclonal antibodies against 8-isoPGF2α attached to the plate. When more 8-isoPGF2α is present, less of the conjugate is bound and thus the concentration of 8-isoPGF2α can be determined from the activity of the conjugated enzyme using hyperbolic standard curves measured with each batch of samples. Samples were purified using a C-18 Sep-Pac, evaporated with N2 and reconstituted with enzyme immunoassay buffer and added to a 96-well plate for EIA analysis using an 8-iso-PGF2α EIA kit from Cayman Chemical (Ann Arbor, MI, USA). The concentrations of 8-iso-PGF2α were determined colorimetrically with a microplate reader at 405 nm and calculated using a four-parameter logistic standard curve. The estimated amount of 8-isoPGF2α in the tissue was then calculated in pg/g of brain sample.
2.5. Measurement of caspase-3 activity
A fourth set of experiments was performed to determine the effect of nicotinamide on caspase-3 activity. The pups were anesthetized with 50 mg/kg pentobarbital at 8 or 24 h after hypoxia and then perfused through the left ventricle of the heart with phosphate buffered saline (pH 7.4). Caspase-3 levels in the brain peak at 24–36 h in this model Chen et al. [8]. The cortex in both lesioned and unlesioned hemispheres were separately dissected and homogenized in lysis buffer and caspase-3 activitywas measured with a kit (CaspACE™ Assay System, Promega, WI, USA) using the method described by Chen et al. [8]. Briefly supernate of brain specimen were reacted with acetyl-Asp-Glu-Val-Asp-p-nitroanilide (DEVD-pNA) and the released p-nitroanilide was measured colorimetrically. Protein concentration was determined by the method of Bradford [6] and activity was normalized per mg protein. Caspase-3 activity was measured in the contralateral and the ipsilateral cortex of the 500 mg/kg nicotinamide treated groups, the corresponding vehicle group and a sham group.
2.6. Statistics
Results are presented as mean ± S.E.M. Statistical comparisons were calculated for continuous variables using two tailed t-tests or analysis of variance (ANOVA) with or without repeated measures using Newman–Keuls test to adjust for multiple comparisons in ANOVA and the Bonferoni correction for multiple comparisons with the repeated measures analysis. Ordinal variables were compared using the Mann–Whiney or Kruskal–Wallis tests.
3. Results
3.1. Neuroprotective effects of nicotinamide
Four of the 33 pups (12%) in the 500 mg/kg of nicotinamide group died. Three of the 31 pups (10%) in the vehicle group died. Three of the 25 pups (12%) in the 250 mg/kg of nicotinamide group died. Two of the 25 pups (8%) in the corresponding vehicle group died. All of pups died between 1 and 7 days after reoxygenation. The mortality was not significantly different between nicotinamide treated and vehicle groups.
Right hemisphere weight 22 days after injury was reduced by 24.6 ± 3.6% in the 28 vehicle treated pups, and by 11.9 ± 2.6% in the 29 pups treated with 500 mg/kg nicotinamide (P < 0.01). Right hemisphere weight 22 days after injury was reduced by 16.2 ± 4.4% in the 22 pups treated with 250 mg/kg nicotinamide and by 25.2 ± 4.8% in the corresponding vehicle group (P = 0.18 versus vehicle). Left hemisphere weight is unchanged by hypoxia [31] (vehicle 509 ± 6 mg, 500 mg/kg nicotinamide 492 ± 6 mg, P > 0.05; 250 mg/kg nicotinamide 518 ± 7 mg, vehicle 516 ± 7.2 mg, P > 0.05).
Brain score by blinded observer 22 days after injury in the vehicle treated pups was normal in 9/28 (32%), mild in 7/28 (25%), moderate in 8/28 (29%), and severe in 4/28 (14%). Brain score in the pups treated with 500 mg/kg of nicotinamide immediately after hypoxia was normal in 16/29 (55%), mild in 8/29 (28%), moderate in 5/29 (17%), and severe in 0/29 (0%). Relative to the vehicle group, the 500 mg/kg treatment group has less moderate and severe injuries and more normal and mild brain injuries (P < 0.05). Brain score in the pups treated with 250 mg/kg of nicotinamide immediately after hypoxia was normal in 13/22 (59%), mild in 4/22 (18%), moderate in 5/22 (23%), and severe in 0/22 (0%). The corresponding vehicle group was scored as normal in 10/23 (43%), mild in 5/23 (22%), moderate in 2/23 (9%), and severe in 6/23 (26%). Relative to the vehicle group, the 250 mg/kg treatment group has less severe injuries and more normals (P = 0.19) but this trend did not reach statistical significance.
3.2. Effect of nicotinamide on coordinated motor ability
Treatment with nicotinamide improved coordinated motor behavior. The time till first fall from the rotorod at 10 rpm was 180 ± 0 s in the 500 mg/kg nicotinamide treated group (n = 9), and 122 ± 25 s in the vehicle treated controls (n = 10, P < 0.05). The number of falls off the rotarod was reduced in the nicotinamide treated group compared with vehicle group, but this trend did not reach statistical significance (P = 0.07).
3.3. Effect of nicotinamide on 8-isoPGF2α
The levels of 8-isoPGF2α in right hemisphere cortex at 16 h after hypoxia were significantly higher in vehicle group (320 ± 79 pg/g, n = 7) than in sham group (115 ± 7 pg/g, n = 6, F(2,17) = 4.55, P < 0.05). Treatment with 500 mg/kg of nicotinamide significantly reduced a hypoxia-induced increase in brain 8-isoPGF2α (175 ± 17 pg/g, n = 7) compared with vehicle group (P < 0.05). There was no significant difference between sham group and nicotinamide treated group.
3.4. Effect of nicotinamide on caspase-3 activity
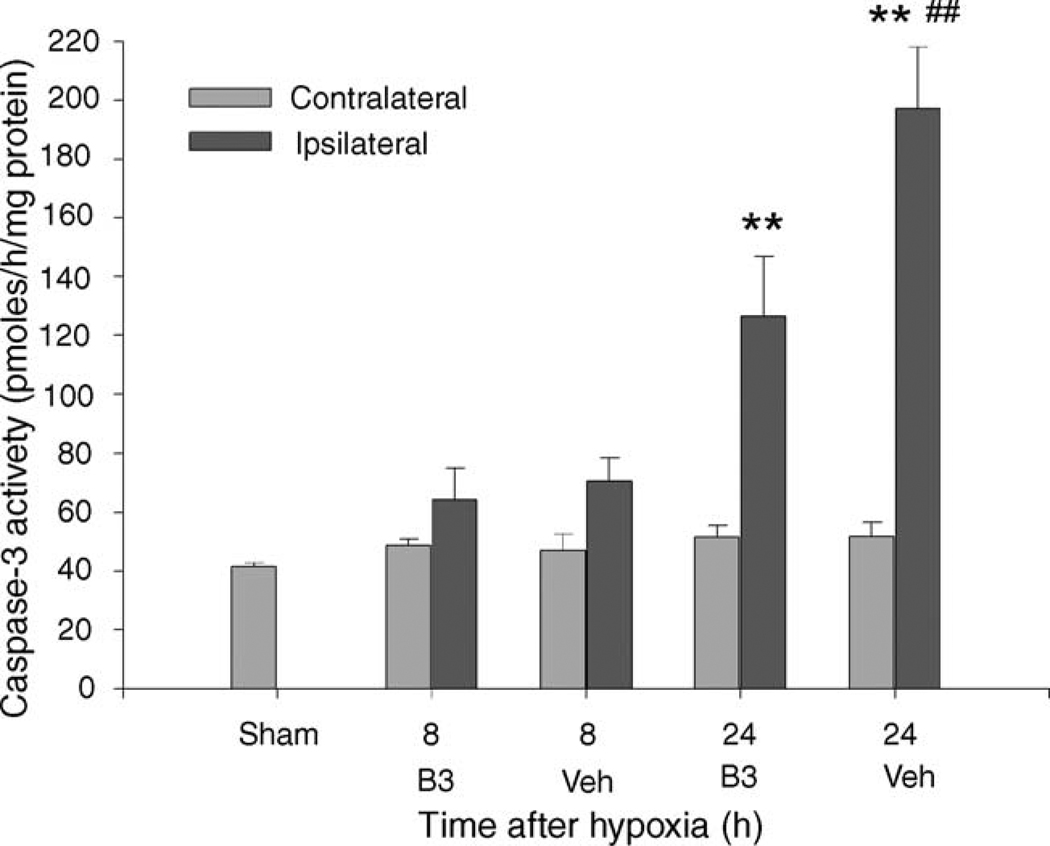
Caspase-3 activity in the cortex was significantly increased at 24 h after hypoxia (F(8,48) = 20.8, P < 0.01 versus shams and versus contralateral hemisphere, n = 6 or 7 for each treatment group and time), but not at 8 h after hypoxia. The nicotinamide treated group’s caspase-3 activity was significantly lower than that in the vehicle treated group (P < 0.01, Fig. 1).
Fig. 1.
In this figure the caspase-3 activity in pmoles/h/mg protein, mean ± S.E.M., is graphed versus the treatment group and the time after reoxygenation the sampling for caspase 3 activity was taken. The light bars are the sham group (n = 7) and the contralateral (left) hemisphere. The dark bars are the ipsilateral (right) hemisphere and are split into the nicotinamide (B3) and vehicle (Veh) treated groups. Nicotinamide pups were treated with 500 mg/kg nicotinamide 5 min after the end of the hypoxic period and 6 h later. Vehicle group pups were given an equal volume of saline. Measurements taken at 8 h after hypoxia (B3, n = 6; Veh, n = 6) were not significantly elevated relative to the shams. Measurements taken 24 h after hypoxia (B3, n = 7; Veh, n = 6) in the ipsilateral (right) hemisphere cortex were significantly increased, **P < 0.01, both relative to the shams and relative to contralateral hemisphere. The difference between the nicotinamide and the vehicle treated groups was also statistically significant at 24 h. ##P < 0.01 vs. nicotinamide. Hypoxic ischemia increases caspase-3 activity, and nicotinamide reduces this increase.
3.5. Rectal temperature and body weight
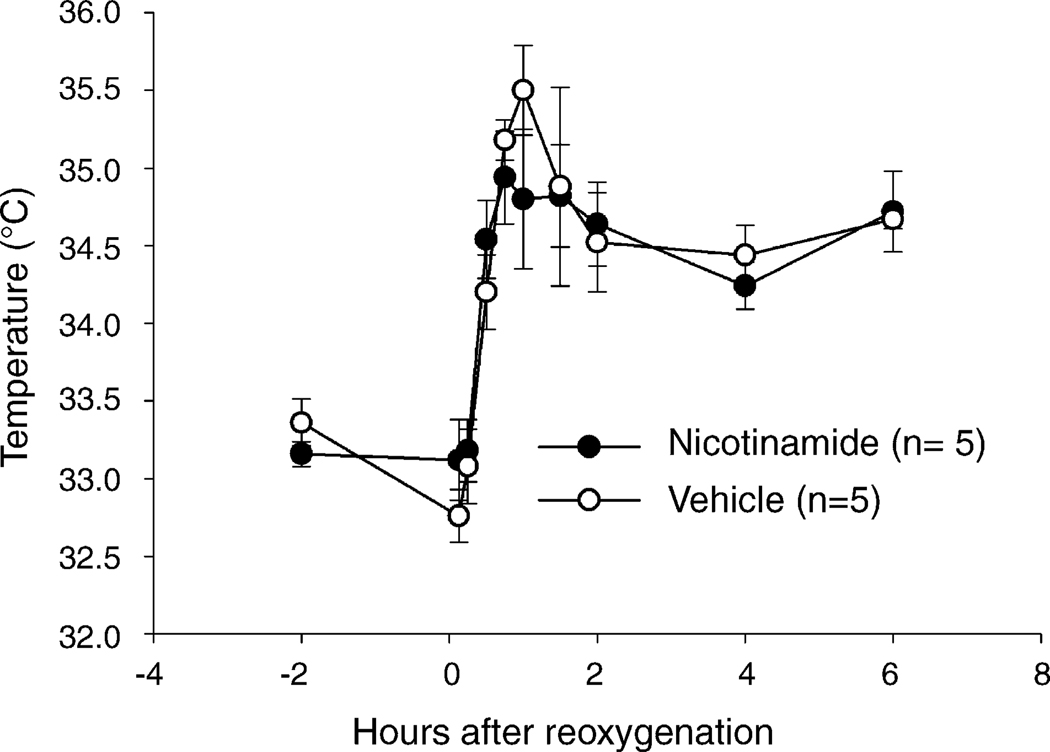
There were no significant differences in rectal temperature between the vehicle and the 500 mg/kg nicotinamide group (group and group by time differences P > 0.05, Fig. 2).
Fig. 2.
Rectal temperature in rat pups treated with 500 mg/kg nicotinamide (open circles, n = 5) or vehicle (closed circles, n = 5), mean ± S.E.M. There were no statistically significant group or group by time differences.
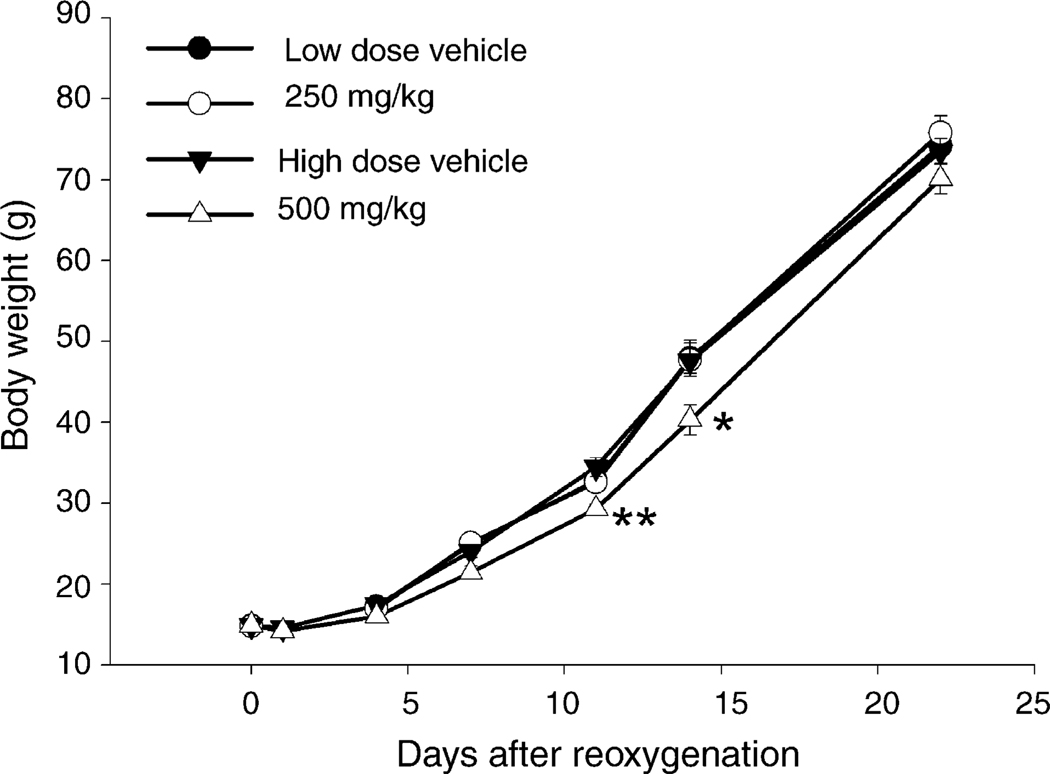
Body weight of the 500 mg/kg nicotinamide treated group was significantly lower than the vehicle group at 11 and 14 days (F(l,55) = 16.1, P < 0.01, Fig. 3), but not at 1, 4, 7 or 22 days. Body weights increased significantly with time in both groups. Body weight in the 250 mg/kg group was essentially identical to that in the vehicle group.
Fig. 3.
Body weight for vehicle (n = 23), dark circles, 250 mg/kg nicotinamide (n = 22), light circles, 500 mg/kg nicotinamide treated pups (n = 28), light triangles, and corresponding vehicle group (n = 29) dark triangles. Data are expressed as the mean ± S.E.M., *P < 0.05, **P < 0.01 vs. the vehicle group. Five-hundred milligrams per kilogram of nicotinamide given i.p. at reoxygenation and 6 h later reduced body weight at 11 and 14 days after reoxygenation.
4. Discussion
Nicotinamide when given at a dose of 500 mg/kg reduces the severity of hypoxic–ischemic brain injury in newborn animals. Measurement of injury by loss of brain weight or volume, by gross pathological examination score and by neurofunctional assessment of motor coordination on the rotorod, all show a neuroprotective effect of nicotinamide. Similar results have been described in adult animals [3,4,17,27,28,33,34,41]. Delayed neuronal injury sometimes requires a prolonged period to develop [39]. Three weeks following the insult, hemispheric hypotrophy is present with a resultant weight difference between the injured and the non-injured hemisphere. There is a high degree of correspondence between the weight deficit of the injured hemisphere and the histologically evaluated loss of brain tissue [1,13,15,16,38]. Thus, weighing can assess the degree of brain damage.
Nicotinamide is potent and effective neuroprotectant at a dose of 500 mg/kg in the rat pup. This dose is the same as that used in many reports showing nicotinamide to be neuroprotective in adult rats [3,4,17,28,34]. It has been shown that treatment after injury with 50, 100, 250, 500, 750 and 1000 mg/kg of nicotinamide is neuroprotective in adult rats only at the dose of 500 mg/kg [3,34]. It has also been shown that a single dose of 500 mg/kg of nicotinamide significantly improved neurological outcome and reduces infarct volume [4,28]. Hoane et al. [17] reported that treatment with 500 mg/kg of nicotinamide at 15 min and second dose at 24 h following brain injury, significantly improved behavioral outcome, reduced the lesion size and reduced the expression of glial fibrillar acidic protein. Our present data confirm their results. Other studies have reported that nicotinamide at doses ranging from 250 to 1000 mg/kg showed significantly reduced infarct size in the permanent focal cerebral ischemia adult rat model [41]. However, unlike in the model of adult rat brain injury [41], a dose of 250 mg/kg of nicotinamide showed no neuroprotective effect in our study. The reason for this difference is unclear but may relate to differences in animal age or other variation in the protocols.
Similar neuroprotective effects were also observed with nicotinamide on motor coordination as assessed by the rotarod test at 26–30 days after hypoxia. The rotarod test is commonly used in the context of motor coordination and balance [11,20]. Treatment with nicotinamide significantly improved coordinated motor behaviors. This study is consistent with the previous research that has shown that administration of nicotinamide improved motor function following brain injury [4,17,28].
It has been reported that treatment with 500 mg/kg of nicotinamide in adult rats improved weight gain [28], or did not effect weight gain [4]. In the present study the data showed that treatment with nicotinamide had side effects at the dose of 500 mg/kg as evidenced by a small temporary decrease in growth. Kang-Lee et al. [21] found that chronic nicotinamide administration in 4–6-week-old rats results in impaired growth because of a methyl-group deficiency state due to greatly increased need for methylation of nicotinamide. In the current study at a dose of 250 mg/kg nicotinamide did not effect growth, but it was also not significantly neuroprotective.
Nicotinamide has many possible mechanisms for neuroprotection. In adult animals nicotinamide prevents depolarization of the mitochondrial membrane, prevents pore formation in the mitochondrial membrane and reduces release of cytochrome c into the cytoplasm [9,10] and reduces activation of caspase-9, which reduces the activation of caspase-3, all of which may be secondary to nicotinamides effect on oxidants and free radicals. Nicotinamide in adult animals inhibits poly(ADP-ribose) polymerase, reduces apoptotic DNA fragmentation, and phosphatidyl serine exteriorization [25]. In the present study the data showed that nicotinamide reduced the increase in caspase-3 activity caused by hypoxic ischemia. This result confirm previous reports that nicotinamide reduces caspase 1, 3 and 8 activation in vitro [9,10]. Oxygen free radical reactions lead to the oxidation of lipids, proteins and polysaccharides and to DNA damage [7]. Eight-isoPGF2α is a stable prostaglandin-like compound that is produced by free radical mediated oxidation of arachidonic acid [37]. Measurement of 8-iso PGF2α is a good way to measure oxidant/free radical formation in vivo [18,37]. In our newborn rats nicotinamide reduces hypoxic ischemia induced elevation in tissue levels of 8-iso-PGF2α in the brain, which is evidence for protection against oxidants and free radicals. Nicotinamide in our newborn rats reduces hypoxic ischemia induced caspase-3 activation, which is evidence for the participation of the caspase mediated apoptotic cascade in nicotinamide’s protection against hypoxic ischemic brain injury in the newborn. The importance of other mechanism described in adult animals in the neuroprotective action of nicotinamide in the immature animal need further study. Since apoptosis physiologically functions to remove non functioning or improperly functioning neurons, it is reassuring that nicotinamide, which probably works through apoptotic channels not only preserves brain substance but also preserves brain function.
In conclusion our findings indicate that nicotinamide reduces the severity of hypoxic–ischemic brain injury in newborn animals. The results also indicate that the suppression of free radicals and caspase-3 activity after hypoxic ischemia by nicotinamide possibly mediated this neuroprotection. Our findings suggest that nicotinamide may be a new treatment avenue for hypoxia ischemia brain injury in newborn.
References
- 1.Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H. Evaluation of brain damage in rat model of neonatal hypoxic-ischemia. J. Neurosci. Meth. 1990;35:253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- 2.Ashwal S, Pearce WJ. Animal models of neonatal stroke. Curr. Opin Pediatr. 2001 13;:506–516. doi: 10.1097/00008480-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ayoub IA, Lee EJ, Ogilvy CS, Beal MF, Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal ischemia in Wistar rats. Neurosci. Lett. 1999;259:21–24. doi: 10.1016/s0304-3940(98)00881-7. [DOI] [PubMed] [Google Scholar]
- 4.Ayoub IA, Maynard KI. Therapeutic window for nicotinamide following transient focal cerebral ischemia. NeuroReport. 2002;13:213–216. doi: 10.1097/00001756-200202110-00008. [DOI] [PubMed] [Google Scholar]
- 5.Banasik M, Komura H, Shimoyama M, Ueda K. Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADP-ribosyl) transferase. J. Biol. Chem. 1992;267:1569–1575. [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Buonocore G, Perrone S, Bracci R. Free radicals and brain damage in the newborn. Biol. Neonate. 2001;79:180–186. doi: 10.1159/000047088. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J. Neurosci. 1998;18:4915–4929. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong ZZ, Lin S, Maiese K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J. Vasc. Res. 2002;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- 10.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J. Cereb. Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 11.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurologic deficits in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 12.Endres M, Wang Z, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Fratkin JD, LeBlanc MH. Treatment with tamoxifen reduces hypoxic-ischemic brain injury in neonatal rats. Eur. J. Pharmacol. 2004;484:65–74. doi: 10.1016/j.ejphar.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, LeBlanc MH. Drug-induced hypothermia begun 5 min after injury with a poly(ADP-ribose) polymerase inhibitor reduces hypoxic brain injury in rat pups. Crit. Care Med. 2002;30:2420–2424. doi: 10.1097/00003246-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Liu Y-M, Fratkins JD, LeBlanc MH. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain Res. Bull. 2005;66:120–127. doi: 10.1016/j.brainresbull.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Hagberg H, Gilland E, Diemer NH, Andiné P. Hypoxia-ischemia in the neonatal rat brain: histopathology after post-treatment with NMDA and non-NMDA receptor antagonists. Biol. Neonate. 1994;66:205–213. doi: 10.1159/000244109. [DOI] [PubMed] [Google Scholar]
- 17.Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J. Neurotrauma. 2003;20:1189–1199. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman SW, Roof RL, Stein DG. A reliable and sensative enzyme immunoassay method for measuring 8-isoprostaglandin F2α: a marker for lipid peroxidation after experimental brain injury. J. Neurosci. Meth. 1996;68:133–136. doi: 10.1016/0165-0270(96)00014-3. [DOI] [PubMed] [Google Scholar]
- 19.Hu BR, Liu CL, Ouyang Y, Blomgen K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxic-ischemia declines during brain maturation. J. Cereb. Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Jones BJ, Roberts DJ. The quantitative measurement of motor incoordination in naive mice using an accelerating rotarod. J. Pharm. Pharm. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 21.Kang-Lee YA, McKee RW, Wright SM, Swendseid ME, Jenden DJ, Jope RS. Metabolic effects of nicotinamide administration in rats. J. Nutr. 1983;113:215–221. doi: 10.1093/jn/113.2.215. [DOI] [PubMed] [Google Scholar]
- 22.Klaidman L, Morales M, Kern S, Yang J, Chang ML, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- 23.Klaidman LK, Mukherjee SK, Adams JD., Jr Oxidative changes in brain pyridine nucleotides and neuroprotection using nicotinamide. Biochim. Biophys. Acta. 2001;1525:136–148. doi: 10.1016/s0304-4165(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 24.Klaidman LK, Mukherjee SK, Hutchin TP, Adams JD., Jr Nicotinamide as a precursor for NAD+ prevents apoptosis in the mouse brain induced by tertiary-butylhydroperoxide. Neurosci. Lett. 1996;206:5–8. doi: 10.1016/0304-3940(96)12446-0. [DOI] [PubMed] [Google Scholar]
- 25.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J. Cereb. Blood Flow Met. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 27.Maynard KI, Ayoub IA, Shen CC. Delayed multidose treatment with nicotinamide extends the degree and duration of neuroprotection by reducing infarction and improving behavioral scores up to two weeks following transient focal cerebral ischemia in wistar rats. Ann. N.Y. Acad. Sci. 2001;939:416–424. doi: 10.1111/j.1749-6632.2001.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 28.Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin B3) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke. 2000;31:1679–1685. doi: 10.1161/01.str.31.7.1679. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee SK, Klaidman LK, Yasharel R, Adams JD., Jr Increased brain NAD prevents neuronal apoptosis in vivo. Eur. J. Pharmacol. 1997;330:27–34. doi: 10.1016/s0014-2999(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 30.Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programed cell death. Dev. Neurosci. 2001;23:186–191. doi: 10.1159/000046141. [DOI] [PubMed] [Google Scholar]
- 31.Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxicishemic brain damage with alopurinol. Pediatr. Res. 1990;27:332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 33.Sadanaga-Akiyoshi F, Yoa H, Tanuma S, Nakahara T, Hong JS, Ibayashi S, Uchimura H, Fujishima M. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in NAD+ levels in ischemic core and penumbra. Neurochem. Res. 2003;28:1227–1234. doi: 10.1023/a:1024236614015. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara Y, Mitha AP, Ogilvy CS, Maynard KI. Post-treatment with nicotinamide (vitamin B3) reduces the infarct volume following permanent focal ischemia in female Sprague-Dawley and Wistar rats. Neurosci. Lett. 2000;281:111–114. doi: 10.1016/s0304-3940(00)00854-5. [DOI] [PubMed] [Google Scholar]
- 35.Sastry PS, Rao KS. Apoptosis and the nervous system. J. Neurosci. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 36.Spector R. Niacin and niacinamide transport in the central nervous system, in vivo studies. J. Neurochem. 1979;33:895–904. doi: 10.1111/j.1471-4159.1979.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 37.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 38.Towfighi J, Housman C, Vannucci RC, Heitjan DF. Effect of unilateral perinatal hypoxic-ischemic brain damage on the gross development of opposite cerebral hemisphere. Biol. Neonate. 1994;65:108–118. doi: 10.1159/000244036. [DOI] [PubMed] [Google Scholar]
- 39.Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev. 1997;19:326–338. doi: 10.1016/s0387-7604(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 40.Ungerstedt JS, Blomback M, Soderdtom T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003;131:48–52. doi: 10.1046/j.1365-2249.2003.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, Chan P, Adams JD., Jr Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol. Biochem. Behav. 2002;73:901–910. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Klaidman LK, Nalbandian A, Oliver J, Chang ML, Chan PH, Adams JD., Jr The effects of nicotinamide on energy metabolism following transient focal cerebral ischemia in Wistar rats. Neurosci. Lett. 2002;333:91–94. doi: 10.1016/s0304-3940(02)01005-4. [DOI] [PubMed] [Google Scholar]