Abstract
Ischemia/reperfusion injury (IRI) is a common cause of acute kidney injury (AKI) that is associated with a patient mortality of up to 50%. Currently there are not effective pharmacologic therapies for AKI. This Commentary highlights recent evidence indicating that 20-HETE plays an important role in IRI and that drugs that target this pathway have potential as therapeutic agents for AKI.
Ischemia/reperfusion injury (IRI) is a common cause of acute kidney injury (AKI) in a variety of clinical settings ranging from critical illness to renal transplantation.1 AKI increases patient morbidity and is associated with patient mortality of up to 50 %.1 Currently, there are no effective pharmacologic therapies for AKI in humans. Thus, there is a profound need for new treatments for AKI.
Recent studies have reported that HET0016, a selective inhibitor of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis, reduces infarct size in the brain and heart following IRI.2–4 Interestingly, the beneficial effect following IRI to the brain is independent of changes in cerebral blood flow, suggesting that the inhibitors of the synthesis of 20-HETE may have direct neuroprotective actions.4 Until recently, the role of CYP metabolites of arachidonic acid in AKI had not been well characterized. The study by Hoff et al.5 (this issue) indicates that 20-HETE may play a central role in renal IRI, and that agents that target the 20-HETE pathway have the potential to minimize kidney injury.
The study by Hoff et al.5 is important because it provides the first direct evidence that renal 20-HETE levels increase during ischemia. The authors found that the release of 20-HETE increased fourfold following ischemia in an isolated perfused kidney, and that this was attenuated by 50% by inclusion of 100 nm HET0016 in the perfusion solution. Similarly, tissue levels of free 20-HETE increased tenfold following 45 min of warm ischemia in the single remaining kidney of uninephrectomized rats. Pretreatment of the rats with HET0016 attenuated the increase in 20-HETE levels and reduced the degree of IRI by about 50%. Similar effects were seen following an intrarenal artery injection of 6,15-20-HEDE, which antagonizes the vasoconstrictor actions of 20-HETE.6 In contrast, administration of the 20-HETE agonist 5,14-20-HEDE had no effect on the degree of IRI.
However, the study of Hoff et al.5 raises many questions. The first concerns the dose of the compounds and whether they are sufficient to inhibit the synthesis and actions of 20-HETE. Hoff et al.5 pretreated rats with a 50-µg bolus injection of HET0016 into the renal artery. Assuming that renal blood flow was 10 ml/min, the first-pass concentration should have been 20 µm. However, HET0016 is highly bound to plasma proteins (>95 %), so the free concentration that can cross the peritubular capillaries and reach the renal tubules would be closer to 1 µm. This concentration should be sufficient to inhibit 20-HETE production, as the half-maximal inhibitory concentration in rat renal microsomes is less than 32 nm.7 Moreover, Hoff et al.5 show that HET0016 attenuated the increase in 20-HETE levels associated with ischemia. However, after the first pass through the kidney, the drug should redistribute, and the concentration remaining in the kidney may have been insufficient to inhibit 20-HETE synthesis, especially during the reperfusion period.
A better understanding of the pharmacokinetics is also needed to interpret the effects of the 20-HETE antagonist 6,15-20-HEDE on IRI. Hoff et al.5 administered a 20-µg bolus dose into the renal artery, which should produce a first-pass concentration of 6 µm. Like 20-HETE, however, this compound is extensively bound to plasma proteins, so the free concentration was probably less than 1 µm. This compound should also rapidly redistribute and is subject to metabolism by β-oxidation, lipoxygenase, and epoxygenase enzymes. Th us, the free concentration in the kidney during the ischemic and reperfusion periods should have been lower than 1 µm. Regardless of this issue, Hoff et al.5 clearly demonstrated that the compound had a beneficial effect, but it remains to be determined whether the tissue levels of 6,15-20-HEDE were high enough to inhibit the tubular or vascular effects of 20-HETE or whether it acts through some other mechanism.
The final issue concerns the mechanism of action of the 20-HETE in IRI. Hoff et al.5 report that pretreatment of the rats with the 20-HETE antagonist improved reperfusion of the renal medulla and increased tissue PO2 levels. They suggest that the effects of both 6,15-20-HEDE and HET0016 might be secondary to blockade of the vasoconstrictor actions of 20-HETE. However, the concentration of 20-HETE in the kidney only rose from 6 to 60 nm during the ischemic period. The authors indicate that the threshold concentration for the renal vasoconstrictor actions of 20-HETE is 1 nm.8 However, this is true only in isolated vessels in the absence of blood or protein, which avidly binds 20-HETE and other lipids. Much higher concentrations (1–10 µm) are typically needed to elicit vasoconstriction in vivo.9 Moreover, although 20-HETE is a potent vasoconstrictor, it is not very efficacious, and maximal concentrations of 20-HETE reduce the diameter of isolated vessels by only 14%.8 If 20-HETE elevates renal vascular tone following IRI, then blockade of the synthesis or actions of 20-HETE with HET0016 or 6,15-20-HEDE should have produced a profound hyperemia. Instead, 6,15-20-HEDE had no effect on whole-kidney or cortical blood flow and produced only a transient improvement in medullary blood flow. Thus, additional studies are needed to determine whether elevated levels of 20-HETE contribute to IRI by enhancing vasoconstriction in the medullary circulation.
For these reasons, it seems more likely that the beneficial effect of blocking the synthesis and action of 20-HETE might be related to the elevation in PO2 in the postischemic period. The mechanism responsible for this effect is unclear, as the 20-HETE inhibitors did not increase renal perfusion. Furthermore, 20-HETE inhibits sodium transport in the proximal tubule and thick ascending loop of Henle,9 so blockade of 20-HETE activity would be expected to enhance rather than inhibit tubular transport and oxygen utilization. Hoff et al.5 suggest that perhaps the elevation in PO2 might be related to an increase in nitric oxide availability secondary to a fall in reactive oxygen species, since 20-HETE stimulates production of reactive oxygen species both by activating NADPH oxidase and through nitric oxide synthase uncoupling.10 Nitric oxide is known to diminish oxygen utilization through several mechanisms.
Although the results of the study by Hoff et al.5 are consistent with previous findings that inhibitors of 20-HETE synthesis reduce IRI in the heart and brain, it should be noted that these findings are in direct opposition to an earlier study by Regner et al.11 In that study, HET0016 enhanced rather than opposed renal IRI, and systemic administration of the 20-HETE analogs 5,14-20-HEDE and 5,14,20-HEDGE reduced renal IRI and produced a sustained increase in medullary blood flow following ischemia in rats.11 Furthermore, 5,14-20-HEDGE was found to inhibit sodium transport and produced diuresis and natriuresis in normal rats. The authors concluded that the protective effect of the 20-HETE analogs in renal IRI was due to prevention of post ischemic medullary hypoxia through a simultaneous increase in renal medullary blood flow and attenuation of tubular transport and oxygen demand. Indeed, 20-HETE has been shown to increase renal medullary blood flow in rats12 and to inhibit renal tubular Na+ -K+ -ATPase activity and sodium transport in the proximal tubule and medullary thick ascending loop of Henle.9
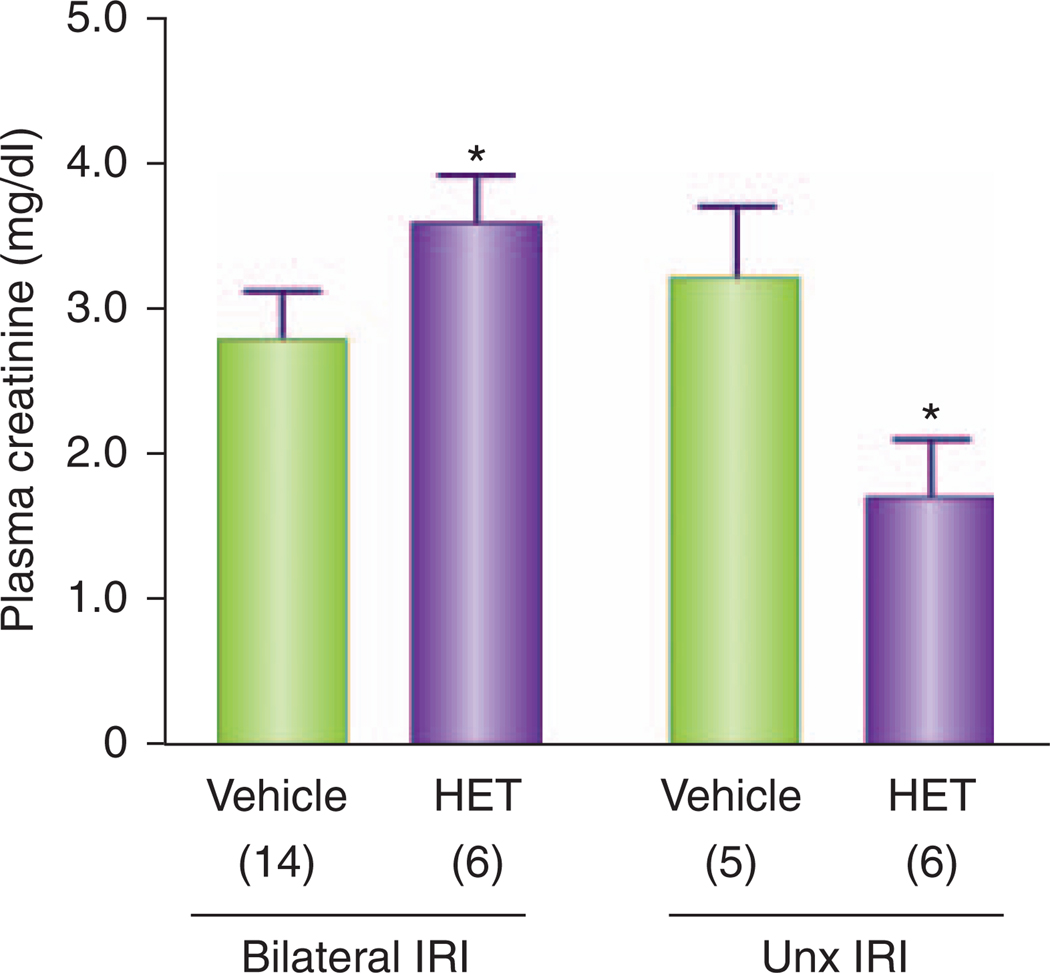
Although there is no obvious way to reconcile the divergent conclusions of these two studies, there are some important differences in the experimental design that should be noted. Regner et al.11 administered HET0016 subcutaneously at a dose of 10 mg/kg, which has been shown to produce blood levels of about 4 µM and selectively inhibits the renal synthesis of 20-HETE for several hours.13 This contrasts with the study of Hoff et al.,5 in which the drug was delivered directly into the renal artery and the kidney was probably exposed to a higher first-pass concentration but a lower concentration during the ischemic and postischemic periods. Similarly, the systemic administration of the 20-HETE analogs 5,14-20-HEDE and 5,14-20-HEDGE by Regner et al.11 produced sustained blood levels of about 1 µm throughout the experiment, whereas the intrarenal injection of 5,14-20-HEDE by Hoff et al.5 probably produced a higher first-pass blood level but much lower levels after the drug redistributed. Finally, the IRI models used in these two studies were very different. Regner et al.11 used Sprague Dawley rats and warm bilateral ischemia for 30 min, whereas Hoff et al.5 studied Lewis rats that were uninephrectomized and then subjected to warm ischemia of the remaining kidney for 45 min. Although it remains to be determined why uninephrectomy alters the response to 20-HETE inhibitors in IRI, the results presented in Figure 1 indicate that this is a potential answer to the difference in the results between the two studies. In our previous study, administration of HET0016 (10 mg/kg, subcutaneously) enhanced renal injury and increased plasma creatinine levels 24 h after 30 min of bilateral ischemia in Sprague Dawley rats.11 On the other hand, we recently confirmed the findings of Hoff et al.5 and show in Figure 1 that HET0016 (10 mg/kg, subcutaneously) does reduce IRI when given to uninephrectomized rats subjected to 45 min of renal ischemia.
Figure 1. Contrasting effects of HET0016, 24 h after 30 min of bilateral renal IRI, or after acute uninephrectomy and 45 min of IRI in the one remaining kidney of Sprague Dawley rats.
The results obtained in the bilateral renal ischemia group were replotted from the study of Regner et al.,11 whereas the results following ischemia in the uninephrectomized rats are original. Asterisks indicate a significant difference in the plasma creatinine concentration measured in the animals treated with HET0016 (10 mg/kg, subcutaneously) versus the corresponding values observed in the vehicle-treated animals. HET, HET0016; IRI, ischemia/reperfusion injury; Unx, uninephrectomized.
Following ischemia, the intracellular Ca2+ concentration increases and activates phospholipase A2 to promote the release of arachidonic acid from membrane phospholipid pools. Arachidonic acid can be metabolized into prostaglandins, leukotrienes, and 5-, 12-, and 15-HETE by cyclooxygenases and lipoxygenases, and into 20-HETE and epoxyeicosatrienoic acids via cytochrome P450 pathways.9 Of these metabolites, 20-HETE has numerous effects on renal tubular and vascular function and activates a number of signaling pathways that could potentiate or oppose renal IRI. In this regard, 20-HETE may exacerbate postischemic reductions in renal blood flow, leading to secondary ischemic injury, as it is a potent vasoconstrictor of preglomerular vessels.8 Prolonged exposure to high concentrations of 20-HETE may also enhance renal IRI by increasing the generation of reactive oxygen species.14 On the other hand, 20-HETE may attenuate renal IRI by mitigating medullary hypoxia, since it increases medullary blood flow and inhibits tubular sodium transport.9,12 Finally, 20-HETE modulates a number of intracellular signal transduction pathways critical to cell fate in renal IRI. Indeed, we recently demonstrated that 20-HETE and 5,14-20-HEDE activates the Raf–MEK–ERK and phosphatidylinositol-3′-kinase–AKT pathways in renal tubular epithelial cells.15 This is intriguing because activation of the ERK1/2 pathway enhances renal epithelial-cell survival in models of renal IRI.16,17 In conclusion, the results of the studies by Hoff et al.,5 Regner et al.,11 and Nilakantan et al.14 indicate that the 20-HETE pathway is activated during renal ischemia and that it has the potential to augment or mitigate IRI through a variety of mechanisms. The results of these studies further suggest that drugs that target the 20-HETE pathway may have a therapeutic potential to treat AKI. In order to translate these therapies to clinical use, more work is needed to determine the optimal dose, route of administration, and timing of administration of either 20-HETE inhibitors or 20-HETE agonists.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants HL036279 and HL029587 (to RJR), the PKD Foundation (to FP), and the Clinical and Translational Science Institute at the Medical College of Wisconsin (to KRR).
Footnotes
DISCLOSURE
The authors declared no competing interests.
REFERENCES
- 1.Lamiere N. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 2.Nithipatikom K, Gross ER, Endsley MP, et al. Inhibition of cytochrome P450ω-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–e71. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- 3.Miyata N, Seki T, Tanaka Y, et al. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther. 2005;314:77–85. doi: 10.1124/jpet.105.083964. [DOI] [PubMed] [Google Scholar]
- 4.Renic M, Klaus JA, Omura T, et al. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–639. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoff U, Lukitsch I, Chaykovska L, et al. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 2011;79:57–65. doi: 10.1038/ki.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Galicia M, Falck JR, Reddy KM, et al. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 7.Miyata N, Taniguchi K, Seki T, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imig JD, Zou AP, Stec DE, et al. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 9.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 10.Roman RJ, Lombard JH. Does 20-hydroxyeicosatetraenoic acid contribute to sex differences in cardiovascular risk by increasing oxidative stress? Hypertension. 2007;50:37–38. doi: 10.1161/HYPERTENSIONAHA.107.090803. [DOI] [PubMed] [Google Scholar]
- 11.Regner KR, Zuk A, Van Why SK, et al. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75:511–517. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyekan AO. Differential effects of 20-hydroxyeicosatetraenoic acid on intrarenal blood flow in the rat. J Pharmacol Exp Ther. 2005;313:1289–1295. doi: 10.1124/jpet.104.080218. [DOI] [PubMed] [Google Scholar]
- 13.Williams JM, Sarkis A, Lopez B, et al. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 14.Nilakantan V, Maenpaa C, Jia G, et al. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol. 2008;294:F562–F570. doi: 10.1152/ajprenal.00387.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbulut T, Regner KR, Roman RJ, et al. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol. 1999;277:F195–F203. doi: 10.1152/ajprenal.1999.277.2.F195. [DOI] [PubMed] [Google Scholar]
- 17.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001;276:11870–11876. doi: 10.1074/jbc.M007518200. [DOI] [PubMed] [Google Scholar]