Abstract
Morphometric changes in the general population of Nissl-stained neurons in area 9 of the dorsolateral prefrontal cortex have been reported in major depressive disorder (MDD) and schizophrenia. These alterations include lamina-specific reductions in the packing density of neuronal somata in MDD, increases or reductions in the density of neuronal somata in schizophrenia, and reductions in average size of neuronal somata in both MDD and schizophrenia. These changes are prominent in deep layer III, where pyramidal excitatory neurons establishing cortico-cortical association connections are localized. To test whether deep layer III pyramidal neurons are differentially affected in MDD or schizophrenia, an antibody was used that labels both phosphorylated and non-phosphorylated forms of the 200 kD neurofilament protein (NF200) in pyramidal cells of layer III in area 9. The packing density and somal size of NF200-immunoreactive (IR) pyramidal neurons were measured in area 9 in 13 subjects with nonpsychotic MDD, 11 subjects with schizophrenia and 13 psychiatrically normal controls. Analysis of covariance did not reveal a difference in packing density among groups. However, the mean size of NF200-IR somata was significantly larger in subjects with schizophrenia than in controls. These results indicate that this neuronal subpopulation does not contribute to the smaller average size of neuronal somata in layer III of prefrontal cortical area 9 in schizophrenia or MDD. In addition, the enlarged somal size in schizophrenia as compared to controls suggests that NF200 neurons may contribute differentially to unique cognitive disturbances present in schizophrenia and not in MDD subjects.
Keywords: Postmortem, Immunohistochemistry, Brodmann’s area 9, Human, Psychiatry
1. Introduction
In major depressive disorder (MDD) and schizophrenia both structural and functional neuroimaging studies reveal similar alterations in the volume and metabolic activity of the human dorsolateral prefrontal cortex (dlPFC) (Goodwin, 1997; Kennedy et al., 1997; Steffens and Krishnan, 1998; Sweeney et al., 1998; Henn and Braus, 1999; Lim et al., 1999; McCarley et al., 1999; Drevets, 2000; Manoach et al., 2000). Histopathological research using a Nissl-stain to identify virtually all neurons demonstrates that these alterations include lamina-specific reductions in the packing density of neuronal somata in MDD, lamina-dependent alterations of neuronal density in schizophrenia, and reductions in neuronal size in both MDD and schizophrenia (Selemon et al., 1995; Rajkowska et al., 1998; Selemon et al., 1998; Rajkowska et al., 1999; Cotter et al., 2001). These changes are prominent in deep cortical layer III (sublayers IIIb and IIIc), the site of many pyramidal excitatory neurons establishing long range cortico-cortical connections (Carmichael and Price, 1995; Kritzer and Goldman-Rakic, 1995; Barbas and Rempel-Clower, 1997; Barbas, 2000; Cavada et al., 2000). Pyramidal neurons also receive projections from neurons of the mediodorsal nucleus of the thalamus (Barbas, 2000), and these thalamic cell bodies appear to be reduced in number in some (Pakkenberg, 1990; Popken et al., 2000; Byne et al., 2002) but not all (Cullen et al., 2003) studies of schizophrenia. The connection pattern in deep cortical layer III pyramidal neurons suggests that pathological changes may occur in these pyramidal cells that are associated with psychiatrically-relevant dysfunction of prefrontal circuits. In addition, since some of the cognitive anomalies in MDD differ from those in schizophrenia, there may be disease-specific differences in the pathology of area 9 pyramidal neurons and the corresponding circuits in which these cells are involved. In particular, the average neuronal soma size is significantly reduced in sublayers b and c of layer III in cortical area 9 of subjects with schizophrenia (Rajkowska et al., 1998). An independent study found a similar reduction in the size of pyramidal neurons in deep layer III of area 9 in schizophrenia (Pierri et al., 2001). In contrast, in MDD there is no significant change in average neuronal soma size in sublayers IIIb and IIIc, and the average neuronal soma size was significantly decreased only for the undivided layer III (Rajkowska et al., 1999). Furthermore, the density of the largest neuronal somata in layer III, which mainly correspond to pyramidal cells located in sublayer IIIc, is significantly reduced in MDD (Rajkowska et al., 1999). The above data in postmortem tissue suggest that changes in specific populations of pyramidal cells in cortical layer III may be differentially involved in the pathophysiology of schizophrenia and depression.
A specific population of deep layer III pyramidal neurons with long cortico-cortical projections has been detected in the dlPFC of humans and other primates using antibodies raised against the various protein subunits of neurofilaments (Hof et al., 1995, 1996; Nimchinsky et al., 1996; Hof et al., 2000). For example, in Alzheimer’s disease (AD), the packing density of this neuronal subpopulation is dramatically reduced (Hof et al., 1990) and this loss is likely to contribute to the severe cognitive deficits displayed by AD patients. Since cognitive dysfunction is prominent among the psychiatric symptoms of schizophrenia patients, Pierri et al. (2003) assessed the changes in somal size and packing density of the same population of neurons in schizophrenia by using an antibody specific for the non-phosphorylated 160 and 200 kD subunits of neurofilaments (Pierri et al., 2003) and the three-dimensional optical disector probe. This immunohistochemical study, in contrast to the morphometry studies based on Nissl-staining (Rajkowska et al., 1998; Pierri et al., 2001) using the same stereological principles, did not find significant changes in the packing density or size of specifically labeled pyramidal neurons in the left hemisphere in subjects with schizophrenia (Pierri et al., 2003). Interestingly, in the study by Pierri et al. (2003), the group of subjects with schizophrenia included four schizoaffective subjects, while some of the previous studies of the general population of pyramidal cells identified with Nissl-staining did not include schizoaffective subjects (Rajkowska et al., 1995; Selemon et al., 1998). In addition, the antibody used by Pierri et al. (2003) does not recognize the phosphorylated forms of neurofilaments, raising the possibility that labeling with antibodies that detect both phosphorylated and non-phosphorylated forms of neurofilaments may still reveal differences between psychiatric subjects and normal control subjects. A more recent study by Law and Harrison (2003) in schizophrenia, bipolar disorder and MDD used three different antibodies to neurofilaments: two antibodies to non-phosphorylated forms of neurofilaments (SMI32 and FNP7) and the same antibody (N200) as the one used in the present report (this antibody labels both phosphorylated and non-phosphorylated neurofilaments). These authors did not find differences in neuronal areal density or in the average cross-sectional area of individual immunoreactive neuronal cell bodies when comparing controls to each of the groups with psychiatric diagnosis. However, in Law and Harrison (2003) only 2-dimensional indirect counting and measuring methods, and combined left and right hemispheres were used. Studies of individual hemispheres, however, are important because neuroimaging and neuropathological research has detected differences in histological and metabolic parameters in psychiatric groups when examining on the left hemisphere (Baxter et al., 1989; Erbas et al., 1992; Kato et al., 1995; Passero et al., 1995; Buchsbaum et al., 1996; Zaidel et al., 1997; Rajkowska et al., 1999; Pierri et al., 2001; Rajkowska et al., 2001, 2002; Pierri et al., 2003). Potential neuropathology in neurofilament-IR pyramidal neurons in cortical layer III of the left dorsolateral prefrontal cortex has not yet been identified in MDD. Accordingly, the present study examined possible changes in somal size and packing density of neurons labeled with an antibody that binds both phosphorylated and non-phosphorylated forms of the 200 kD subunit of neurofilaments (NF200) in layer III of the left cortical area 9 in postmortem brains of non-psychiatric control subjects, subjects with MDD and subjects with schizophrenia. In addition, we sought to ascertain whether the putative pathology of NF200-immunoreactive (NF200-IR) neurons of layer III in cortical area 9 would allow for a distinction between subjects diagnosed with MDD and those diagnosed with schizophrenia. To maximize the comparability of the present study with the report of Pierri et al. (2003) in which neurofilament immunoreactive neurons in left cortical layer III were examined in schizophrenia and control subjects, packing densities and cell volumes in the prefrontal cortex were determined using morphometric methods and analyses based on Pierri et al. (2003).
2. Material and methods
2.1. Human subjects
The studies reported here were carried out on human postmortem brain tissue collected from autopsies performed at the Cuyahoga County Coroner’s Office in Cleveland, OH, USA. Retrospective psychiatric assessments based on informant interviews were conducted for all subjects using a structured clinical interview. These studies were performed in accordance with the Institutional Review Board policies and written consent was obtained from the next-of-kin. Consensus diagnoses of MDD and schizophrenia were made in accordance with the Diagnostic and Statistic Manual of Mental Disorders-Revised (DSM-IV) (American Psychiatric Association, 1995). Other information collected included hospital medical records, medical or substance abuse illnesses, medication history and postmortem toxicology. Subjects were excluded from the study if there was evidence of head trauma, a neurologic disease or a psychoactive substance use disorder within the last year of life. Only brains were included with a postmortem interval (PMI, time from death to tissue fixation) of less than 30 h and a fixation time no longer than 51 months (Table 1). Other details about the diagnostic procedures and methods for collecting information on the subjects are provided elsewhere (Rajkowska et al., 1999). In the group with schizophrenia, only two subjects (#28 and #36, Table 1)did not have a prescription for an antipsychotic drug within the last month of life, while all of them had prescriptions at some time for antipsychotic medication. Two of the subjects with schizophrenia had a prescription for antidepressant medications during the last month prior to death (one of them for amoxapine, #27, and the other one for trazodone and nortriptyline, #31). In the group with MDD, three subjects had no history of prescription for either antidepressant or antipsychotic medications (#20, #22, and #26). Three subjects with MDD had a prescription for antipsychotic medication (#16, #18, and #23), one of them within the last month prior to death (#18).
Table 1.
Characteristics of subjects in the three study groups
| AGEa/GENDER/RACE | PMI | TF | Cause of death | Duration of disordera | Age of onseta | |
|---|---|---|---|---|---|---|
| Controls | ||||||
| 1 | 47/M/C | 17 | 7.0 | N | ||
| 2 | 30/F/C | 9 | 29.0 | N | ||
| 3 | 51/M/C | 28 | 24.5 | N | ||
| 4 | 46/F/C | 24 | 25.5 | H | ||
| 5 | 27/F/C | 15 | 21.0 | N | ||
| 6 | 50/F/C | 27 | 11.0 | N | ||
| 7 | 42/M/C | 20 | 11.9 | N | ||
| 8 | 24/M/AAm | 15 | 24.0 | H | ||
| 9 | 71/M/C | 24 | 5.0 | N | ||
| 10 | 69/M/C | 18 | 49.0 | N | ||
| 11 | 77/M/C | 24 | 43.1 | N | ||
| 12 | 52/M/C | 17 | 45.9 | N | ||
| 13 | 23/F/C | 11 | 23.0 | Acc | ||
| Mean±SD | Age 46.8±17.9 | 19.2±6.0 | 24.6±14.3 | |||
| Major depression | ||||||
| 14 | 54/M/C | 23 | 12.9 | Acc | 3 | 51 |
| 15 | 42/F/C | 24 | 7.0 | S | 26 | 15 |
| 16 | 34/F/C | 27 | 10.8 | S | 20 | 14 |
| 17 | 40/F/C | 25 | 14.0 | N | 5 | 35 |
| 18 | 63/F/C | 24 | 6.8 | N | 30 | 33 |
| 19 | 42/M/C | 20 | 16.0 | S | 0.25 | 41 |
| 20 | 30/M/AAm | 18 | 6.7 | S | 3 | 27 |
| 21 | 73/M/C | 10 | 16.2 | S | 5 | 68 |
| 22 | 78/F/C | 25 | 9.0 | S | 5 | 73 |
| 23 | 74/M/C | 25 | 17.0 | S | 24 | 50 |
| 24 | 86/M/C | 21 | 5.6 | S | 20 | 56 |
| 25 | 73/F/C | 17 | 14.9 | N | 50 | 23 |
| 26 | 45/M/C | 29 | 14.5 | S | 7 | 38 |
| Mean±SD | Age 56.5±18.9 | 22.2±5.0 | 11.6±4.2 | 15.3±14.6 | 40.3±18.7 | |
| Schizophrenia | ||||||
| 27 | 45/F/C | 6 | 33.7 | N | 27 | 18 |
| 28 | 23/F/C | 26 | 43.7 | S | 5 | 18 |
| 29 | 32/F/AAm | 24 | 50.5 | S | 10 | 22 |
| 30 | 48/F/C | 18 | 26.4 | N | 17 | 31 |
| 31 | 64/F/C | 12 | 42.1 | N | 40 | 24 |
| 32 | 45/M/AAm | 15 | 33.5 | S | 20 | 25 |
| 33 | 64/F/C | 25 | 19.0 | N | 20 | 44 |
| 34 | 48/F/C | 29 | 19.7 | S | 18 | 30 |
| 35 | 55/M/AAm | 24 | 34.8 | N | 24 | 31 |
| 36 | 58/M/C | 24 | 19.8 | N | 23 | 35 |
| 37 | 24/M/AAm | 24 | 10.0 | S | 2 | 22 |
| Mean±SD | Age 46.0±14.5 | 20.6±7.0 | 30.3±12.4 | 18.7±10.6 | 27.3±7.9 | |
ABBREVIATIONS AAm=African American; Acc=accident; C=Caucasian; F=female; H=homicide; M=male; N=natural; PMI=postmortem interval (hours); S=suicide; TF=time in formalin (months).
In years.
2.2. Tissue sampling
The tissue samples were obtained from the left prefrontal cortex of 13 subjects retrospectively diagnosed with MDD (nonpsychotic), 11 subjects diagnosed with schizophrenia and 13 psychiatrically normal controls (Table 1). Tissues obtained at autopsy were fixed in phosphate buffered formalin (10%) as previously described (Rajkowska et al., 1999). Morphometric parameters of immunoreactive neurons were measured in the left dlPFC (Brodmann’s area 9). Previously established cytoarchitectonic criteria for area 9 were used to select the cortical regions of interest for the present study (Rajkowska and Goldman-Rakic, 1995a,b).
2.3. Morphometry
Blocks of tissue (2 × 2 cm) from the dlPFC were embedded in celloidin, and cut into 40 μm thick sections. Three sections evenly spaced at 400 μm apart were chosen from each subject to be immunostained with a monoclonal antibody that labels phosphorylated and non-phosphorylated forms of the 200 kD protein of neurofilaments (clone N52, Catalog number N0142, Sigma-Aldrich, St. Louis, MO) (Shaw et al., 1986; Franke et al., 1991). The antibody was used on the sections at a 1 : 400 dilution according to an immunohistochemical protocol previously described (Miguel-Hidalgo and Rajkowska, 1999). For many cases, sections used for neurofilament immunohistochemistry were adjacent to or within 100 μm of the Nissl-stained sections that were used in our previous morphometric study demonstrating changes in neuronal and glial cell packing density in MDD (Rajkowska et al., 1999). For five cases not included in studies previous to the present one, sections at cytoarchitectonically equivalent levels were Nissl-stained and adjacent sections were used for immunohistochemical staining.
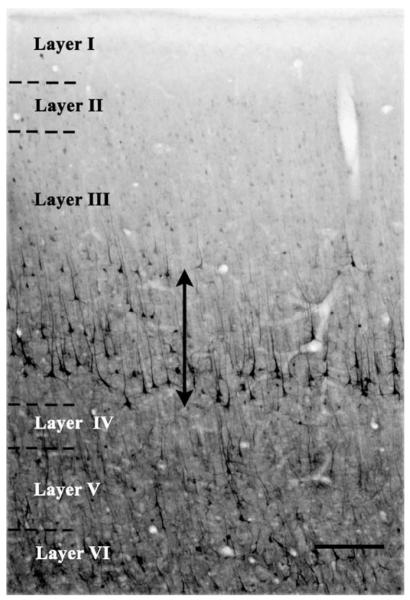
Nissl-stained sections were then used to draw the boundaries between individual cortical layers. These laminar boundaries were imposed as guides on the NF200 immunostained sections to determine the laminar distribution of NF200-IR cells according to the following procedure. To transfer the boundaries to the immunostained sections under the microscope we first obtained prints of the region of interest in the Nissl and the NF200 immunostained sections and drew lines at the cortical surface and the border between layer VI and the white matter (this border is easily visible both in Nissl and immunostained sections). The inner and outer boundaries of layer III were next drawn in the Nissl sections. Taking into account the thickness of the cortex (cortical surface to VI–white matter border) in the immunostained section, the inner and outer the borders of layer III were marked at their proportionally corresponding distances to the surface. Another border marking the middle of layer III was obtained by marking the middle points between the outer and inner borders at 5 locations along the sampled region of layer III. A region of interest was drawn in each section that included the inner half of layer III (the half adjacent to layer IV) in area 9 (Fig. 1). This defined region of interest allowed the inclusion of most of the NF200-IR neuronal somata of layer III. NF200-IR somata were counted within each section by sampling with a 3-dimensional counting box (15 μm depth, 73 μm length and 51 μm width). Guard zones were 1 μm (from the top and from the bottom of the box). After dehydration and coverslipping the final thickness of the sections was 17–18 μm. No significant difference between cohorts was detected in the final thickness of the sections. The location of the boxes within the region of interest in each section was determined using a grid of constant dimensions and random orientation. Counting of neurons within the counting box was performed according to stereological rules of the disector probe (Gundersen and Jensen, 1987; Gundersen et al., 1988).
Fig. 1.
NF200 immunoreactivity in a section through area 9 of the human prefrontal cortex. Note immunoreactive somata mostly localized to the ventral half of layer III (next to layer IV). The vertical line ending with arrowheads shows the dorsal and ventral borders of the region of layer III where NF200-immunoreactive neurons were counted and measured. Calibration bar: 250 μm.
The implementation and the textual description of the methods of morphometric measurements and statistical analysis of data applied in the present study to packing density and soma size of pyramidal neurons is largely based on Pierri et al. (2003).
An estimate of the size of neuronal soma for each diagnosis group in deep layer III was obtained by applying the nucleator probe (Gundersen, 1988) to each of the NF200-IR neurons counted, at a magnification of ×630 with an oil immersion objective of 1.4 numerical aperture, implemented with the commercial Stereo Investigator software (Microbright-field, Inc.). To implement the nucleator probe the center of each immunoreactive soma was defined at the confluence within the soma of the axis of the apical dendrite and the axes of the other major dendrites (Fig. 2), assuming that the apical dendrites of the measured neurons are isotropically oriented in a perpendicular direction towards the brain pial surface. As amply discussed by Pierri et al. (2003), the use of the isotropic nucleator to calculate unbiased estimates of actual cell volume involves sampling in blocks cut at random orientations and ideally would require sampling throughout the cortical area of interest. Due to technical limitations in the present study, only sections cut in a coronal plane were used and consequently a bias could have been introduced in the estimate of the cell volume. However, since the same plane of cutting and the same sampling methods were used for all diagnostic groups the bias would likely have occurred in all groups. There was no significant difference in the average number of neurons counted or measured for size in the control, MDD and schizophrenia cohorts. The mean coefficients of error (standard error of the natural logarithms of the volume of cells bodies in each subject) were not significantly different between control (0.073±0.0137), MDD (0.064±0.0121) and schizophrenia (0.088±0.085) subjects.
Fig. 2.
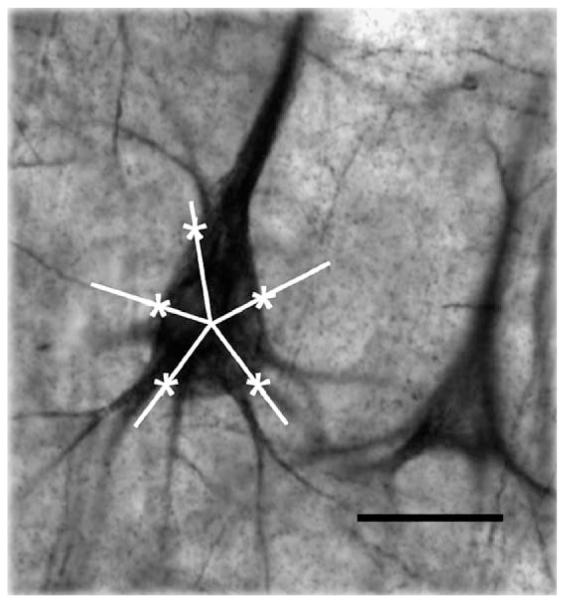
Estimation of somal size with the nucleator. The photomicrograph shows a NF200-immunoreactive neuron of area 9 with an overlay of five equally spaced rays centered within its soma. The intersections of each ray with the outline of the soma were marked to obtain an estimate of somal size using the stereological nucleator probe. Calibration bar: 25 μm.
2.4. Statistics
There was no significant difference (ANOVA, F(2,34)=0.710, p =0.499) in postmortem interval between control (19.154±5.956 h), MDD (21.923±4.821), and schizophrenia (20.636±7.004) subjects. Likewise there was no significant difference in brain pH: control (6.70±0.214), MDD (6.551±0.256), schizophrenia (6.491±0,411). In the schizophrenia group 3 subjects had pH values below 6, while no values below 6 were present in the groups with MDD and control subjects. The remaining 8 subjects with schizophrenia had values within the range observed in the other groups. In the control and MDD groups all values of pH were higher than 6.22 but smaller than 7 with the exception of one control subject (pH 7.01). Although there was no significant correlation between PMI or time in formalin and size or packing density of neurons in each group, ANOVA revealed significant differences (F(2,34)=9.239, p <0.001) in the average time in formalin between control (23.836±14.339), MDD (11.232±3.942) and schizophrenia subjects (30.258±12.448). The difference was significant between MDD and either schizophrenia or control subjects, but not between control and schizophrenia subjects. To control for any influence of time in formalin, PMI, pH and age, each of the dependent variables (neuronal density and somal volume) was compared among groups using analysis of covariance (ANCOVA) with PMI, time in formalin, pH and age at the time of death as covariates. Since a correlation with pH was found in the schizophrenia group and this group contained three very low pH values, an additional ANCOVA was performed with the same covariates as before, but excluding from the analysis the subjects in the schizophrenia group with values of pH lower than 6 (values lower than 6 were absent in the other groups).
Somal size was statistically analyzed using the log-transformed estimates of somal volume (Pierri et al., 2003). For the description of somal volume for each subject, we back-transformed the log-transformed values of somal volumes, which produce estimates of the median somal volumes. From these back-transformed values, the 25% and 75% quartiles were obtained and used in the description of the distribution of somal size values for each group (Pierri et al., 2003). Summary values of cell volumes for each diagnostic group are reported without adjustment for the covariates.
3. Results
3.1. Qualitative description
The pattern of distribution of immunoreactivity for the 200 kD subunit of neurofilaments (NF 200) (phosphorylated plus non-phosphorylated) in the gray matter of sections through human cortical area 9 in this study is comparable to that previously described in postmortem Alzheimer and control human brains and in non-human primates using the same antibody as in the present study (Law and Harrison, 2003; Miguel-Hidalgo and Rajkowska, 1999) or antibodies to non-phosphorylated forms of neurofilament subunits (Hof et al., 1990, 1996; Hof, 1997; Law and Harrison, 2003). In area 9 the majority of cells with NF200-IR positive somata are located in the inner half of layer III adjacent to layer IV (Fig. 1). The apical dendrites of these neurons extend perpendicularly towards the brain surface up to layer II. More sporadically, neuronal somata are also labeled in layer V and VI among a relatively dense mesh of NF200-IR processes, some of which, in layer VI, were continuous with NF200-IR axons in the white matter. Although no attempt was made to quantify the intensity (optical density) of immunoreactivity, there were no apparent differences in the intensity of immunostaining between the three groups under study.
3.2. Packing density of NF200-IR neuronal somata
The packing density of NF200-IR somata located in the inner half (adjacent to layer IV) of layer III was estimated following systematic random sampling with 3-dimensional counting boxes in three sections per subject using the counting rules of the optical disector probe. The borders between cortical layers were determined in Nissl-stained sections adjacent to those immunostained for NF200 using cytoarchitectonic criteria (Rajkowska and Goldman-Rakic, 1995a,b; Rajkowska et al., 1999). Analysis of covariance with age, postmortem delay, brain pH and time in fixative as covariates showed that there was no significant difference [F(2,30)=0.366, p =0.697] in the packing density of NF200-IR cells between the three cohorts (cells × 103/mm3±standard error, control 8.385±0.555, MDD 10.330±0.811, schizophrenia 7.655±1.163), although there was a tendency for lower density of NF200-IR somata in schizophrenia as compared to MDD (Fig. 3). The results were not changed by excluding from the ANCOVA the three schizophrenia subjects with low pH levels. There was no significant correlation between age at the time of death, age at onset of disease, duration of disease, gender or race and the packing density of NF200 somata.
Fig. 3.
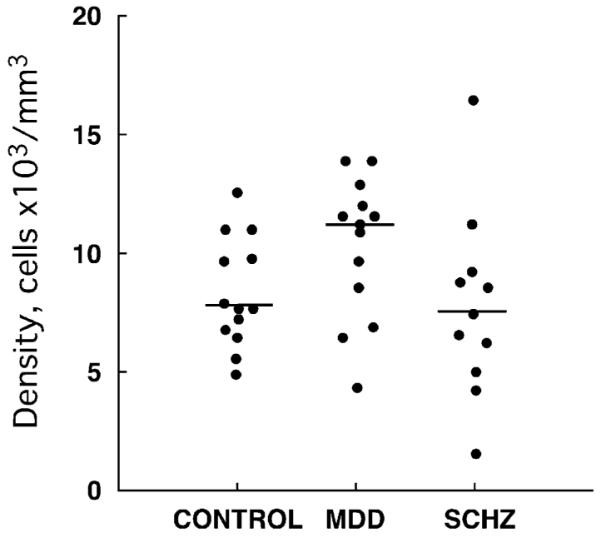
Plots showing the distribution of individual values for packing density of NF200-immunoreactive neurons in the control, major depressive disorder (MDD), and schizophrenia (SCHZ) groups. The horizontal bars represent the median values of packing density for each diagnostic group.
3.3. Size of NF200-IR somata
The volumes of individual NF200-IR neuronal somata (the same somata counted to obtain the packing density) were estimated with a nucleator stereological probe (Fig. 2). Analysis of covariance with age, PMI, brain pH and time in fixative as covariates revealed a significant difference between the three cohorts [F(2, 30)=4.898, p =0.014](Fig. 4). Post hoc comparisons using univariate contrast analysis of the log-transformed values of somal volumes showed that the geometric mean of the volume of NF200-immunoreactive somata in schizophrenic subjects (4513 μm3; 25% quartile 3996 μm3; 75% quartile 4728 μm3) was significantly larger [F(1,30)=9.26, p <0.005] than that of control subjects (2842 μm3; 25% quartile 2360 μm3; 75% quartile 3825μm3) but not different [F(1,30)=1.09, p =0.304] from that in the MDD group (3415 μm3, 25% quartile 2840 μm3, 75% quartile 4061 μm3). The somal volume in the MDD group was not significantly different from the control group [F(1,30)=2.99, p =0.090]. After exclusion from the analysis of the three subjects in the schizophrenia group with low brain pH (see Materials and methods) ANCOVA still showed a significant effect of diagnosis on soma size [F(2,27)=4.156, p =0.027], and the soma size in the schizophrenia group was still larger than in controls [F(1,27)=6.250, p =0.019], but not different from MDD subjects [F(1,27)=0.177, p =0.677]. There was no significant correlation between age at the time of death, age at onset of disease, duration of disease, gender or race and the size of NF200-IR neuronal somata.
Fig. 4.
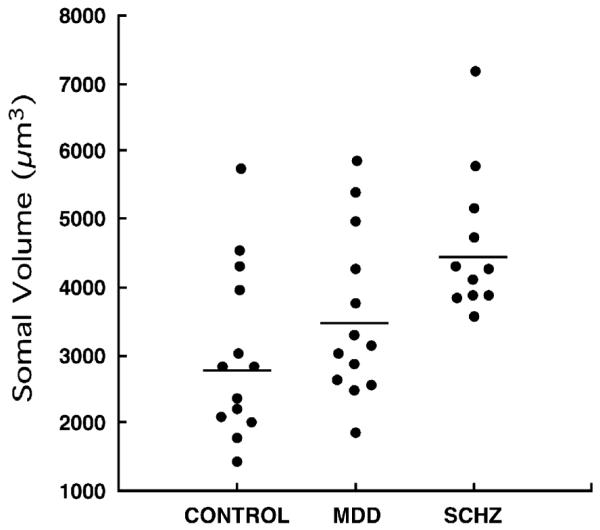
Plots showing the distribution of the individual geometric means of somal volumes of NF200-immunoreactive neurons in the control, major depressive disorder (MDD), and schizophrenia (SCHZ) groups. The horizontal bars represent geometric means for each diagnostic group.
4. Discussion
4.1. Packing density of NF200-IR neurons
The present findings indicate that there is no difference in the packing density of NF200-IR pyramidal neurons in area 9 of the dlPFC between control subjects, and those with schizophrenia or MDD. Since there is evidence in Nissl-stained material supporting that the packing density of pyramidal cells in area 9 is altered in depression and schizophrenia (Rajkowska et al., 1998; Selemon et al., 1998; Rajkowska et al., 1999; Pierri et al., 2001; Rajkowska and Selemon, 2003), the present findings suggest that other populations of pyramidal and non-pyramidal cells might contribute to a greater extent than NF200-IR pyramidal neurons to the average changes in packing density detected in studies on Nissl-stained material. The findings reported here also suggest that neurofilament-related cytoskeletal deficiencies in a particular subpopulation of pyramidal neurons might not have a relevant role in the functional disturbances in prefrontal circuits, which are considered one of the main anatomical substrates for cognitive and emotional deficits in schizophrenia and depression. A note of caution must be considered in establishing a firm conclusion for comparisons of MDD to control subjects, because MDD subjects in the present study were an average of 10 years older than the other subjects. Nevertheless, since our analysis of packing densities and cell body size included age as a covariate, the lack of significant changes in NF200 neurons of MDD subjects as compared to controls is still consistent with morphological and cytoskeletal changes in NF200 neuronal somata not playing a pathophysiological role in MDD. The present data of unchanged density of NF200-IR neurons of deep cortical layer III are also consistent with mounting evidence that prefrontal cognitive impairment related to schizophrenia or MDD is not a result of neurodegenerative mechanisms like those involved in cognitive deficits characteristic of Alzheimer’s Disease (AD). In AD, Hof and colleagues report a dramatic loss of non-phosphorylated NF200-IR pyramidal cells in deep layer III of cortical area 9 and a lower intensity in the staining of the remaining neurons (Hof et al., 1990; Hof and Morrison, 1990; Hof, 1997). The loss of those pyramidal neurons, which are considered to furnish long cortico-cortical connections, supports the possibility of a cortical isolation syndrome (Hof et al., 1990) in AD as one of the bases for its devastating cognitive deficits. Accordingly, loss of layer III NF200-IR neurons supporting cortico-cortical connections does not appear to be a factor in the physiopathology of MDD and schizophrenia.
4.2. Somal Size of NF200-IR neurons
The larger size of NF200-IR somata found in the present study for subjects with schizophrenia as compared to control subjects differs from a previously reported lack of differences in the soma size of neurofilament-IR neurons between other cohorts of schizophrenia subjects and their controls (Pierri et al., 2003; Law and Harrison, 2003). In the present study, examination of the distribution of frequencies of somal sizes shows that the higher proportion of larger NF200-IR somata in schizophrenia is at the expense of a lower proportion of cells with small sizes (Fig. 5). The apparent discrepancy between the present study and the work of Pierri et al. (2003) may result in part from the differences in the composition of the group of subjects with schizophrenia. Pierri et al. (2003) included four schizoaffective subjects while no schizoaffective subjects were included in the present study. Although exclusion of these four subjects by the Pierri et al. (2003) study does not ensure that they would have found a significant difference between subjects with schizophrenia and control subjects, substitution of schizoaffective subjects by other subjects with schizophrenia may have produced different results. This is possible because the highest value of soma size was found in a subject with schizophrenia (Pierri et al., 2003). Another source of discrepancy between Pierri et al. (2003) and the present study may be the use in the present study of an antibody (NF200) that labels both the phosphorylated and the non-phosphorylated forms of 200 kD subunit of neurofilaments. In contrast, Pierri et al. (2003) used an antibody (SMI32) that only labels non-phosphorylated protein subunits of neurofilaments and the labeling includes the 200 and the 160 kD subunit of neurofilaments. The NF200 antibody, which labels both phosphorylated and non-phosphorylated neurofilaments (Shaw et al., 1986; Franke et al., 1991), may reveal more neurofilament binding sites within the soma, dendrites and axons as opposed to antibodies directed only to non-phosphorylated neurofilaments. Non-phosphorylated filaments are differentially transported within the soma and axon as compared to phosphorylated filaments (Pant and Veeranna, 1995; Jung and Shea, 2004). Interestingly, inhibitors of Cdk5 kinase (that phosphorylates the side-arms of NF200) accelerate the transport of neurofilaments, and it has been suggested that phosphorylation slows the transport of neurofilaments due to pausing of neurofilament movement (Ackerley et al., 2003). Since NF200 stains both phosphorylated and non-phosphorylated neurofilaments it is possible that an alteration of the phosphorylation of neurofilaments affecting their transport results in an apparent larger size of immunoreactive somata. An alteration in the neurofilament phosphorylation pattern is possible in schizophrenia because glycogen synthase kinase3h (GSK-3β) phosphorylates specific sites in the side-arms of NF200 (Guidato et al., 1996). The level and the activity of this enzyme have been found reduced in the prefrontal cortex in schizophrenia (Beasley et al., 2001; Kozlovsky et al., 2001). It remains to be tested whether those reductions might be related to changes in NF200 phosphorylation and the distribution of the immunoreactivity for NF200, and reflected in the apparent size of immunolabeled pyramidal cell bodies. In summary, the difference in the specificity of the antibodies used may be crucial because measurements of apparent soma size on the basis of immunoreactivity are likely to depend not only on the actual size of the soma, but also on the extent of NF200 labeling of each soma and its primary dendrites which might be influenced by its phosphorylation pattern. Alternatively, since no significant differences are reported here in the packing density of NF200-IR neurons and there were no obvious differences in the intensity of staining between groups, the larger somal size found in schizophrenia might result from pathology of cellular compartments other than the neurofilaments. That is, subcellular mechanisms not necessarily related to the cytoskeleton might produce a swelling of the soma on this particular neuronal population. How these changes may be related to schizophrenia remains to be explored. It cannot be ruled out that a larger size of NF200-IR pyramidal cells in schizophrenia and possibly in depression may be related to pathology in smaller NF200-IR pyramidal cells (that for example may lose their NF200 immunoreactivity) and in non-pyramidal cells connecting with brain regions different from those related to NF200-IR neurons. Larger neurons with NF200 immunoreactivity and with longer ipsilateral intrahemispheric projections may be spared from pathology or even increase the size of their somata as a compensatory (maybe aberrant) mechanism to alterations in surrounding neurons. In the future, further comparative studies with specific markers for the NF200-negative neuronal populations in layer III are in order to understand the exact contribution of these populations to the pathophysiology of MDD and schizophrenia. In summary, the lack of difference in the packing density of neurons between control, MDD and schizophrenia subjects and the larger average size of those neurons in schizophrenia would support the notion that populations of pyramidal cells not labeled by NF200 antibodies and local-circuit, non-pyramidal cells may be more directly involved in the pathophysiology of psychiatric disorders than NF200 positive neurons, and that the changes in size of these neurons are related to other factors.
Fig. 5.
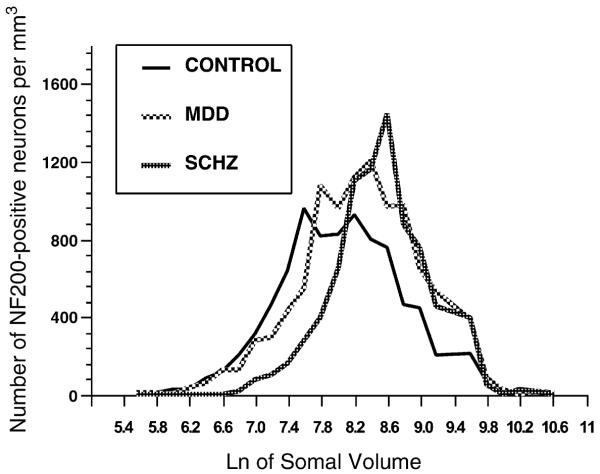
Frequency histogram for the packing density of neurons in the ventral half of layer III according to the natural log of the somal volume representing the distribution of somata at different somal sizes for controls, major depressive disorder (MDD) and schizophrenia (SCHZ) subjects.
An influence of brain pH in the apparent size of NF200-IR neurons is possible because the three subjects with the lowest values of pH were in the schizophrenia group, and these subjects had the largest values of somal volume. This is consistent with previous studies suggesting a correlation between the shape of immunoreactive somata and brain pH at autopsy (Law and Harrison, 2003). If the correlation of pH and soma size is not linear (for example if it is only manifest at lower pH values) it would difficult to detect such correlation in the control and MDD groups because in these groups there were no values smaller than pH 6. In fact, there was no correlation between pH in the MDD and control groups, and, when excluding the three subjects with low pH values, pH was not correlated with size in the schizophrenia group either. Furthermore, the diagnosis effect in soma size was still statistically significant after exclusion of the three subjects with low pH. It is interesting that the lowest values of pH occurred in the schizophrenia group and that for those schizophrenia subjects with higher brain pH (which was actually within the high range of pH in the other two groups) the somal volume was still larger than in controls. Our study suggests some relationship between acidity of the brain on the apparent size of neurofilament immunoreactive somata, particularly at low pH values. Thus, it seems that pH is a variable that should be controlled for in studies of neurofilaments in the human brain. On the other hand since metabolic differences are known to exist between the prefrontal cortex of normal and schizophrenic subjects future studies should determine whether functional alterations in the prefrontal cortex of a subgroup of schizophrenic patients results in lower pH values measured postmortem.
4.3. Possible influence of medication
Pharmacological treatment may have an influence on cytomorphological parameters of NF200-IR neurons. Antipsychotic medications taken by subjects with schizophrenia have been found to produce changes in the volume of central nervous system structures such as the striatum and prefrontal cortex (Chakos et al., 1995; Frazier et al., 1996; Lee et al., 1999; Scheepers et al., 2001). In the present study the larger average somal size in NF200-IR neurons in schizophrenia and MDD might be not only an attribute of schizophrenia but also be influenced by antipsychotic medication. However, in the subjects with schizophrenia that were not being medicated with antipsychotics for at least one month before death, the average size of the NF200-IR somata was larger than the average size in the control and MDD groups, and not different from the average value of the remaining schizophrenics. In addition, in the MDD group, somal size was lower in subjects that had been taking antipsychotic medication than in the group with schizophrenia. These results are rather consistent with a direct association between schizophrenia and a larger average size of NF200-IR somata in deep cortical layer III and do not support a causative role of medication in the observed group differences.
Acknowledgments
The authors gratefully acknowledge the work of James C. Overholser, Ph.D., George Jurjus, M.D., and Lisa Konick in the establishment of retrospective psychiatric diagnoses. The excellent assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH, is greatly appreciated, as is the cooperation and support of the next-of-kin of the deceased. This work was supported by grants from the National Institute of Mental Health (MH63187, MH61578 and MH60451).
References
- Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CC. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J. Cell Biol. 2003;161:489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, D.C: 1995. [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb. Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BM, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Beasley C, Cotter D, Khan N, Pollard C, Sheppard P, Varndell I, Lovestone S, Anderton B, Everall I. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci. Lett. 2001;302:117–120. doi: 10.1016/s0304-3940(01)01688-3. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Someya T, Teng C. Ying, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE. PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am. J. Psychiatry. 1996;153:191–199. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am. J. Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Parkinson N, Craven R, Crow TJ, Esiri MM, Harrison PJ. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr. Res. 2003;60:157–166. doi: 10.1016/s0920-9964(02)00297-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders: implications for a neural model of major depression. Biol. Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Erbas B, Bekdik C, Erbengi G, Enunlu T, Aytac S, Kumbasar H, Dogan Y. Regional cerebral blood flow changes in chronic alcoholism using Tc-99m HMPAO SPECT. Comparison with CT parameters. Clin. Nucl. Med. 1992;17:123–127. doi: 10.1097/00003072-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Franke FE, Schachenmayr W, Osborn M, Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am. J. Pathol. 1991;139:67–79. [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband_Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am. J. Psychiatry. 1996;153:564–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- Goodwin G. Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J. Psychopharmacol. (Oxf.) 1997;11:115–122. doi: 10.1177/026988119701100204. [DOI] [PubMed] [Google Scholar]
- Guidato S, Tsai LH, Woodgett J, Miller CC. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J. Neurochem. 1996;66:1698–1706. doi: 10.1046/j.1471-4159.1996.66041698.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. The nucleator. J. Microsc. 1988;151(Pt. 1):3–21. doi: 10.1111/j.1365-2818.1988.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Henn FA, Braus DF. Structural neuroimaging in schizophrenia. An integrative view of neuromorphology. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249(Suppl 4):48–56. doi: 10.1007/pl00014185. [DOI] [PubMed] [Google Scholar]
- Hof PR. Morphology and neurochemical characteristics of the vulnerable neurons in brain aging and Alzheimer’s disease. Eur. Neurol. 1997;37:71–81. doi: 10.1159/000117414. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: II. Primary and secondary visual cortex. J. Comp. Neurol. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Hof PR, Mufson EJ, Morrison JH. Human orbitofrontal cortex: cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 1995;359:48–68. doi: 10.1002/cne.903590105. [DOI] [PubMed] [Google Scholar]
- Hof PR, Ungerleider LG, Webster MJ, Gattass R, Adams MM, Sailstad CA, Morrison JH. Neurofilament protein is differentially distributed in subpopulations of cortico-cortical projection neurons in the macaque monkey visual pathways. J. Comp. Neurol. 1996;376:112–127. doi: 10.1002/(SICI)1096-9861(19961202)376:1<112::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Nimchinsky EA, Erwin JM. Neurochemical and cellular specializations in the mammalian neocortex reflect phylogenetic relationships: evidence from primates, cetaceans, and artiodactyls. Brain Behav. Evol. 2000;55:300–310. doi: 10.1159/000006665. [DOI] [PubMed] [Google Scholar]
- Jung C, Shea TB. Neurofilament subunits undergo more rapid translocation within retinas than in optic axons. Mol. Brain Res. 2004;122:188–192. doi: 10.1016/j.molbrainres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kato T, Shioiri T, Murashita J, Hamakawa H, Takahashi Y, Inubushi T, Takahashi S. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol. Med. 1995;25:557–566. doi: 10.1017/s003329170003347x. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Javanmard M, Vaccarino F. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can. J. Psychiatry. 1997;42:467–475. doi: 10.1177/070674379704200502. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr. Res. 2001;52:101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Law AJ, Harrison PJ. The distribution and morphology of prefrontal cortex pyramidal neurons identified using anti-neurofilament antibodies SMI32, N200 and FNP7. Normative data and a comparison in subjects with schizophrenia, bipolar disorder or major depression. J. Psychiatr. Res. 2003;37:487–499. doi: 10.1016/s0022-3956(03)00075-x. [DOI] [PubMed] [Google Scholar]
- Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, Kinon BJ, Bilder R, Creese I, Lieberman JA. Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci. 1999;64:1595–1602. doi: 10.1016/s0024-3205(99)00106-x. [DOI] [PubMed] [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch. Gen. Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol. Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fisher IA, Shenton ME. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar cytoarchitecture in celloidin sections from the human cerebral cortex. J. Neurosci. Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Hof PR, Young WG, Morrison JH. Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J. Comp. Neurol. 1996;374:136–160. doi: 10.1002/(SICI)1096-9861(19961007)374:1<136::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch. Gen. Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Pant HC, Veeranna Neurofilament phosphorylation. Biochem. Cell. Biol. 1995;73:575–592. doi: 10.1139/o95-063. [DOI] [PubMed] [Google Scholar]
- Passero S, Nardini M, Battistini N. Regional cerebral blood flow changes following chronic administration of antidepressant drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1995;19:627–636. doi: 10.1016/0278-5846(95)00107-7. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch. Gen. Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Somal size of prefrontal cortical pyramidal neurons in schizophrenia. Differential effects across neuronal subpopulations. Biol. Psychiatry. 2003;54:111–120. doi: 10.1016/s0006-3223(03)00294-4. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr., Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Quantitative criteria for distinguishing areas 9 and 46. Cereb. Cortex. 1995a;4:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46. Cereb. Cortex. 1995b;4:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr. Mol. Med. 2003;3:427–436. doi: 10.2174/1566524033479663. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Reduction in neuronal sizes in prefrontal cortex of schizophrenics and Huntington patients. Schizophr. Res. 1995;15:31. [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon L. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol. Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2002;57:127–138. doi: 10.1016/s0920-9964(02)00339-0. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, de_Wied CC, Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon L, Rajkowska G, Goldman-Rakic P. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a 3-Dimensional, stereologic counting method. J. Comp. Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Shaw G, Osborn M, Weber K. Reactivity of a panel of neurofilament antibodies on phosphorylated and dephosphorylated neurofilaments. Eur. J. Cell Biol. 1986;42:1–9. [PubMed] [Google Scholar]
- Steffens D, Krishnan K. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol. Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Strojwas MH, Mann JJ, Thase ME. Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biol. Psychiatry. 1998;43:584–594. doi: 10.1016/s0006-3223(97)00485-x. [DOI] [PubMed] [Google Scholar]
- Zaidel DW, Esiri MM, Harrison PJ. Size, shape, and orientation of neurons in the left and right hippocampus: investigation of normal asymmetries and alterations in schizophrenia. Am. J. Psychiatry. 1997;154:812–818. doi: 10.1176/ajp.154.6.812. [DOI] [PubMed] [Google Scholar]