Abstract
This study examined the metabolism of arachidonic acid (AA) by cytochrome P-450 enzymes in isolated glomeruli and the effects of selective inhibitors of the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) and epoxyeicosatetraenoic acids (EETs) on glomerular permeability to albumin (Palb). Glomeruli avidly produced 20-HETE, EETs, dihydroxyeicosatetraenoic acids (diHETEs), and HETEs when incubated with exogenous AA. N-hydroxy-N’-(4-butyl-2-methylphenyl)formamidine (HET0016; 10 μM) selectively inhibited the formation of 20-HETE by 95% and increased Palb from 0.00 ± 0.08 to 0.73 ± 0.10 (n = 43 glomeruli, 4 rats). Addition of a 20-HETE mimetic, 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (20-5,14-HEDE; 1 μM) opposed the effects of HET0016 (10 μM) to increase Palb (0.21 ± 0.10, n = 36 glomeruli, 4 rats). Preincubation of glomeruli with exogenous AA to increase basal production of 20-HETE had a similar effect. We also examined the effect of an epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MSPPOH; 5 μM), on Palb. MSPPOH (5 μM) significantly increased Palb but had no effect on the synthesis of EETs in glomeruli incubated with AA. However, MSPPOH (5 μM) selectively reduced epoxygenase activity by 50% in glomeruli incubated without added AA. Pretreatment with 8,9-EET (100 nM) attenuated the effects of MSPPOH (5 μM) on Palb. These results indicate that glomeruli produce 20-HETE, EETs, diHETEs, and HETEs and that endogenously formed 20-HETE and EETs play an essential role in the maintenance of the glomerular permeability barrier to albumin.
Keywords: glomeruli, HET0016, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide, glomerular permeability to albumin, 20-hydroxyeicosatetraenoic acid, epoxyeicosatetraenoic acids
Hypertension- and diabetes-induced nephropathies are the leading causes of end-stage renal disease (3, 16, 22). Elevations in glomerular capillary pressure are thought to contribute to the development of proteinuria in these conditions (5, 6, 11, 21, 24, 31), but the mechanisms involved are not well understood. A rise in glomerular capillary pressure may contribute to the development of renal injury by increasing the production of transforming growth factor-β (TGF-β) (1, 18). TGF-β is now known to increase glomerular permeability to albumin (Palb), and this is associated with a fall in the endogenous formation of 20-HETE (4). Exogenous administration of a stable 20-HETE mimetic opposes the effects of TGF-β to increase Palb (4). Similarly, 20-HETE has been reported to oppose the increase in Palb following administration of puromycin (12). However, it remains to be determined to what extent cytochrome P-450 (CYP) metabolites of arachidonic acid (AA) are produced by glomeruli and whether they contribute to the maintenance of the glomerular permeability barrier to albumin. Therefore, in the present study, we profiled the CYP metabolites of AA produced by isolated glomeruli in the presence and absence of added substrate. We then determined the effects of selective inhibition of the endogenous formation of 20-hydroxyeicosatetraenoic acid (20-HETE) with N-hydroxy-N’-(4-butyl-2-methylphenyl)formamidine (HET0016) (13) and of epoxyeicosatetraenoic acids (EETs) with N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MSPPOH) (26) on Palb.
METHODS
General
Experiments were performed on 52 male Sprague-Dawley rats weighing between 225 and 350 g, purchased from Taconic Farms (Germantown, NY). The rats were housed in the Animal Care Facility at the Medical College of Wisconsin, which is approved by the American Association for the Accreditation of Laboratory Animal Care. The rats had free access to food and water throughout the study. All protocols were approved by the Animal Care Committee of the Medical College of Wisconsin.
Metabolism of AA in isolated glomeruli
Glomeruli were isolated from the kidneys of rats as previously described (4, 17, 19). Aliquots of the glomeruli were incubated in 1 ml of GIBCO RPMI medium 1640 (Invitrogen, Grand Island, NY) in the presence and absence of a saturating concentration of AA (42 μM; Amersham Biosciences, Piscataway, NJ) and 1 mM NADPH for 60 min at 37°C. We also determined the effects of HET0016 (10 μM), an inhibitor of the synthesis of 20-HETE (13), and MSPPOH (5 and 20 μM), an inhibitor of the formation of EETs (26), on the metabolism of AA by isolated glomeruli. The incubations were stopped by acidification to pH 3.5 with formic acid, glomeruli were homogenized, and the homogenate was extracted twice with 3 ml of ethyl acetate after the addition of 2 ng of an internal standard, d6-20-HETE (Cayman Chemicals, Ann Arbor, MI). The organic phase was collected and dried under nitrogen. The samples were reconstituted with 50% methanol and water, and the metabolites were separated by HPLC on a Betabasic C18 column (150 × 2.1 mm, 3 μM; Thermo Hypersil-Keystone, Belletonte, PA) at a flow rate of 0.2 ml/min, using an isocratic elution starting from a 51:9:40:0.01 mixture of acetonitrile-methanol-water-acetic acid for 30 min, followed by a step change to 68:13:19:0.01 acetonitrile-methanol-water-acetic acid and water for 15 min. The effluent was ionized using a negative ion electrospray, and the peaks eluting with a mass-to-charge ratio (m/z) of 319 > 301 (HETEs and EETs), 337 > 319 [dihydroxyeicosatetraenoic acids (diHETEs)], or 323>270 (internal standard) were monitored using an Applied Biosystems 3000 triple quadrupole mass spectrometer (Foster City, CA). The ratios of ion abundances in the peaks of interest versus those seen in the internal standard were determined and compared with standard curves generated over a range from 0.2 to 1.0 ng for 20-HETE and from 1.0 to 10 ng for the other metabolites. Values are expressed as picomoles of product formed per minute per milligram of protein.
Measurement of Palb
Glomeruli were isolated using the sieving method in a medium containing 5 g/dl of albumin (4, 17, 19). Palb was determined by measuring the change in glomerular volume (ΔV) after exchange of the bath with fresh medium containing 1% albumin (4, 17, 19). Palb was calculated as 1 − (ΔVexperimental/ΔVcontrol), where ΔVcontrol was taken as the mean change in volume measured in all glomeruli treated with vehicle. The glomeruli were subjected to seven different treatments. In group 1, the glomeruli were incubated with vehicle. In group 2, the glomeruli were treated with HET0016 (10 μM). In group 3, the effects of a stable 20-HETE agonist, 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (20-5,14-HEDE; 1 μM) (2, 29) on the Palb response to HET0016 (10 μM) were determined. In group 4, the effects of preincubation of the glomeruli with a saturating concentration of AA (42 μM) for 15 min at 37°C on the Palb response to HET0016 (10 μM) were determined. In groups 5 and 6, the glomeruli were incubated with MSPPOH at concentrations of 5 and 20 μM, respectively. In group 7, the effects of 8,9-EET (100 nM) on the Palb response to MSPPOH (5 μM) were determined. A minimum of five glomeruli from each rat were studied, and these experiments were performed using a minimum of three different rats per treatment group.
Statistical methods
Data are means ± SE. Significance of differences in mean values was determined using an unpaired t-test and one-way ANOVA followed by Dunn’s post hoc test. A P value <0.05 was considered to be statistically significant.
RESULTS
Glomerular metabolism of AA
Glomeruli incubated with a saturating concentration of AA (42 μM) produced large peaks with a m/z of 319 and retention times corresponding to 20-HETE, 15-HETE, 12-HETE, 8-HETE, 5-HETE, 14,15-EET, 11,12-EET, and 8,9-EET, as well as peaks with a m/z of 337 with retention times corresponding to 14,15-diHETE, 11,12-diHETE, and 8,9-diHETE (Fig. 1,A and B). The largest peak, which eluted at 19 min, produced secondary ions following fragmentation at m/z of 301, 275, 273, 257, and 245. This pattern is identical to the tandem mass spectrometry (MS/MS) spectrum generated using a 20-HETE standard. Glomeruli incubated in the absence of exogenous AA also produced a similar profile of metabolites, but the rate of the production of 20-HETE and the other metabolites was 10–100 times lower than that seen when glomeruli were incubated in the presence of AA (Fig. 2).
Fig. 1.

Profile of the metabolites formed by isolated glomeruli incubated with arachidonic acid (AA; 42 μM) in the presence of NADPH (1 mM). A: representative liquid chromatography/mass spectroscopy chromatogram showing that isolated glomeruli produce 20-hydroxyeicosatetraenoic acid (20-HETE), dihydroxyeicosatetraenoic acids (diHETEs), and other HETEs when incubated with AA. B: the same chromatogram as in A with the scale expanded to show that epoxyeicosatetraenoic acids (EETs) are also produced by isolated glomeruli, but the levels are much lower than that seen for 20-HETE. cps, counts per second.
Fig. 2.

Production of 20-HETE, EETs, diHETEs, and HETEs by isolated glomeruli incubated in the presence (A) and in the absence (B) of exogenous AA. Values are means ± SE. *Significantly different from the corresponding values in glomeruli incubated with AA. CYP450, cytochrome P-450.
Effects of HET0016 on the metabolism of AA in isolated glomeruli and on Palb
HET0016 (10 μM) selectively reduced the synthesis of 20-HETE by >95% and had no effect on the formation of EETs, diHETEs, and HETEs in glomeruli incubated in the presence of exogenous AA (Fig. 3A). HET0016 significantly increased Palb from 0.00 ± 0.08 to 0.73 ± 0.10 (Fig. 3B).
Fig. 3.

Effects of HET0016 (10 μM) on the CYP-dependent metabolism of AA by isolated glomeruli and on glomerular permeability to albumin (Palb). A: effects of HET0016 (10 μM) on the formation of 20-HETE, EETs, diHETEs, and other HETES in glomeruli incubated with exogenous AA. B: effects of HET0016 (10 μM) on Palb. Glomeruli were incubated with vehicle or HET0016 (10 μM) for 15 min at 37°C, and changes in Palb were determined. Numbers in parentheses indicate the number of rats and glomeruli studied per group. Values are means ± SE. *Significantly different from the corresponding value in the vehicle-treated group.
Effects of a 20-HETE agonist and exogenous AA on the Palb response to HET0016
The results of these experiments are presented in Fig. 4. Addition of the stable 20-HETE mimetic 20-5,14-HEDE (1 μM) had no effect on baseline Palb, but it attenuated the increase in Palb produced by HET0016 by >70% (Fig. 4A). Similar results were obtained when the glomeruli were preincubated with AA to stimulate the endogenous formation of 20-HETE before the addition of HET0016 (Fig. 4B).
Fig. 4.

Effects of a stable 20-HETE mimetic, 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (20-5,14-HEDE), and preincubation of glomeruli with AA for 15 min to elevate the endogenous production of 20-HETE on the changes in Palb produced by HET0016. Glomeruli were incubated with vehicle or HET0016 (10 μM) for 15 min at 37°C, and changes in Palb were determined. The effects of preincubation of glomeruli with a stable 20-HETE agonist, 20-5,14-HEDE (1 μM; A) or AA (42 μM; B) for 15 min at 37°C to raise the endogenous production of 20-HETE on the Palb response to HET0016. Numbers in parentheses indicate the number of rats and glomeruli studied per group. Values are means ± SE. *Significantly different from the corresponding value in glomeruli treated with vehicle within a group. †Significant difference after HET0016 from the corresponding value in glomeruli treated with AA or 20-5,14-HEDE vs. value in the control group.
Effects of MSPPOH on Palb and the metabolism of AA in isolated glomeruli
MSPPOH at concentrations of 5 and 20 μM significantly increased Palb from 0.00 ± 0.06 to 0.61 ± 0.14 and 0.65 ± 0.09, respectively (Fig. 5A). Preincubation of glomeruli with 8,9-EET reduced the increase in Palb in response to MSPPOH (5 μM). MSPPOH at a concentration of 20 μM reduced the formation of EETs and 20-HETE by ~60% in glomeruli incubated with exogenous AA, but it was not effective at a concentration of 5 μM (Fig. 5B). In glomeruli incubated without exogenous substrate, 5 μM MSPPOH selectively decreased epoxygenase activity by 50% (Fig. 5C).
Fig. 5.
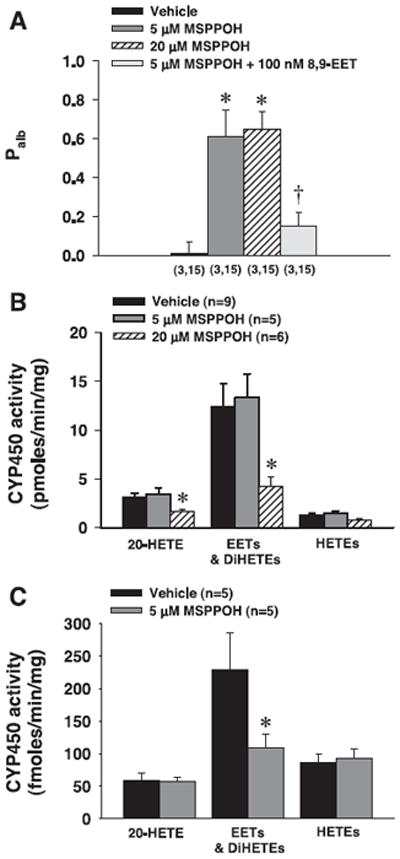
Effects of N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MSPPOH; 5 and 20 μM) and 8, 9-EET (100 nM) in the presence of MSPPOH (5 μM) on Palb and CYP-dependent metabolism of AA by isolated glomeruli. A: effects of MSPPOH on Palb. Glomeruli were pretreated with vehicle or MSPPOH (5 and 20 μM) for 15 min at 37°C. Effects of preincubation of glomeruli with 8,9-EET (100 nM) on the Palb response to MSPPOH (5 μM) were also studied. Numbers in parentheses indicate the number of rats and glomeruli studied per group. B: effects of MSPPOH (5 and 20 μM) on the metabolism of AA in glomeruli incubated with exogenous AA. C: effects of MSPPOH (5 μM) on the metabolism of AA in glomeruli incubated in the absence of exogenous AA. Values are means ± SE. *Significantly different from the corresponding value in glomeruli treated with vehicle. †Significantly different from the corresponding value in glomeruli treated with MSPPOH (5 μM).
DISCUSSION
Previous studies indicated that induction of the renal formation of 20-HETE with fibrates or following the introgression of the CYP4A region chromosome 5 of normotensive rats into the genetic background of Dahl S rats reduces the degree of renal injury and proteinuria during the development of hypertension (7, 14, 20, 25, 27, 28). Moreover, our laboratory recently provided evidence that the increase in Palb produced by TGF-β is associated with a fall in the glomerular production of 20-HETE, and preventing the fall in 20-HETE levels by administration of 20-HETE or a stable 20-HETE mimetic, 20-5,14-HEDE (2, 29), opposes the effects of TGF-β to increase Palb (4). Similarly, 20-HETE has been reported to oppose the increase in Palb produced by puromycin (12). All of these studies suggest that 20-HETE may have a protective role on the glomerular permeability barrier to oppose the development of a proteinuria and glomerular disease (4, 12). However, the role of 20-HETE that is endogenously produced by the glomerulus in the regulation of the glomerular filtration barrier is unknown and was the focus of the present study.
Isolated glomeruli incubated with exogenous AA produced 19- and 20-HETE, along with lesser quantities of 15-, 12-, 8-, and 5-HETE. The glomeruli also produced detectable amounts of 14,15-, 11,12-, and 8,9-EETs, but the levels were quite low compared with the other metabolites formed. Nearly all of the epoxygenase metabolites appeared as diols rather than the epoxides. These results are consistent with the view that soluble epoxide hydrolase is highly expressed in kidney (30). The profile of metabolites were similar when glomeruli were incubated without added substrate, but the rate of production was 10 to 100 times less than when the glomeruli were incubated in the absence of exogenous AA. This finding indicates that isolated glomeruli normally produce 20-HETE and EETs and that the production of these compounds is limited by the turnover of phospholipids and the availability of free AA.
The present finding that 20-HETE is the major CYP metabolite of AA produced by isolated rat glomeruli of the rat is consistent with previous observations that mRNA for the CYP4A1, -2, -3, and -8 isoforms are expressed in microdissected glomeruli and that the expression of CYP4A protein in the glomerulus is almost as high as that found in the proximal tubule and higher than the expression in renal microvessels (9, 10).
We next evaluated the importance of endogenously formed 20-HETE in the regulation of Palb. HET0016 (10 μM) selectively inhibited the formation of 20-HETE by isolated glomeruli and markedly increased Palb. Preventing the fall in 20-HETE levels in the glomerulus by adding a 20-HETE mimetic attenuated the effects of HET0016 to increase Palb. Preincubation of the glomeruli with AA, to increase the production and tissue levels of 20-HETE, also reduced the ability of HET0016 to increase Palb. These experiments indicated that the effects of HET0016 on Palb is due to a fall in the levels of 20-HETE in the glomerulus and not to a nonspecific effect of this compound. Overall, these results are the first to indicate that the sustained production of 20-HETE in the glomerulus is required to maintain the glomerular permeability barrier to albumin. We were a bit surprised that such a high concentration of HET0016 was needed to inhibit 20-HETE production in these experiments, since the reported IC50 for this compound to inhibit 20-HETE production in rat renal microsomes is 35 nM (13). However, in follow-up experiments, we determined using mass spectrometry that HET0016 is >98% bound to the albumin used to isolate the glomeruli and measure Palb.
Additional experiments were performed to examine the role of the endogenous production of epoxygenase metabolites in the regulation of Palb. MSPPOH increased Palb by about the same extent as HET0016. At a concentration of 20 μM, this compound reduced the formation of both EETs and 20-HETE in glomeruli incubated with AA by ~60%. However, at the lower concentration it had no effect on the formation of EETs or 20-HETE. These findings are consistent with previous reports indicating that MSPPOH is a competitive inhibitor of epoxygenase activity with an IC50 of ~13 μM. At higher concentrations, it also competes for the metabolism of AA by other enzymes and inhibits the formation of 20-HETE (26).
Additional experiments were performed to try to understand why the low concentration of MSPPOH increased Palb apparently without inhibiting epoxygenase activity in glomeruli incubated with exogenous AA. Since MSPPOH is a competitive inhibitor of the metabolism of AA, and Palb was measured in the absence of exogenous AA, we wondered whether MSPPOH might inhibit epoxygenase activity in glomeruli that were incubated in the absence of exogenous substrate when the free AA concentration was low. Under these conditions, MSPPOH (5 μM) selectively reduced the formation of EETs and DiHETEs by 50% (Fig. 5C). The addition of exogenous 8,9-EET attenuated the effects of MSPPOH (5 μM) to increase Palb. This suggests the effects of MSPPOH on Palb are due to its ability to lower the levels of EETs in the glomerulus and not to a nonspecific effect of this inhibitor. Overall, these data suggest that EETs or diHETEs are also produced by the glomerulus and that they normally contribute to the regulation of Palb.
The cell types in the glomerulus that express CYP enzymes and produce EETs and 20-HETE and the mechanisms by which these products influence the glomerular protein permeability barrier remain to be determined. Possible mechanisms include changes in the shape of podocytes or capillary endothelial cells, phosphorylation or dephosphorylation of proteins such as nephrin and podocin in podocytes to alter the size of the slit pores, or changes in the phosphorylation state of ICAM and other proteins important for adherence of the foot processes of podocytes to the glomerular basement membrane. In this regard, 20-HETE has been reported to activate the PKC and MAPK signaling pathways in renal arteries (23). Nowicki et al. (15) demonstrated that 20-HETE inhibits Na+,K+-ATPase in the proximal tubule by activating the PKC pathway. Likewise, Imig et al. (8) and others have demonstrated that EETs dilate the afferent arteriole through activation of the cAMP-PKA signaling pathway, which is also known to alter Palb. Thus 20-HETE and EETs activate many signaling pathways that have been shown to influence the function of podocytes and the glomerular permeability barrier to albumin. Further work is needed to determine the pathways involved now that it is clear that these compounds play a key role as autocrine factors in the regulation of Palb.
Perspectives
Previous studies have indicated that increasing the renal formation of 20-HETE and/or EETs protects against the development of hypertension-induced proteinuria and glomerular disease (7, 14, 20, 25, 27, 28) and that exogenous administration of 20-HETE opposes the effects of TGF-β and puromycin to increase Palb (4, 12). The present results now extend these findings and indicate that glomeruli normally produce 20-HETE, EETs, diHETEs, and other HETEs and that the endogenous formation of both 20-HETE and EETs plays an essential role in the maintenance of the glomerular permeability barrier to albumin.
Acknowledgments
GRANTS This work was supported by National Institutes of Health Grants DK-38266, HL-29587, and HL-36279, the Robert A. Welch Foundation, and a United Negro College Fund/Merck Postdoctoral Science Research Fellowship (to J. Williams).
References
- 1.Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G. Transforming growth factor-β1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol. 2002;161:2179–2193. doi: 10.1016/s0002-9440(10)64495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 3.August P, Leventhal B, Suthanthiran M. Hypertension-induced organ damage in African Americans: transforming growth factor-β1 excess as a mechanism for increased prevalence. Curr Hypertens Rep. 2000;2:184–191. doi: 10.1007/s11906-000-0080-5. [DOI] [PubMed] [Google Scholar]
- 4.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-β, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension. 2005;45:643–648. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 5.Dunn BR, Zatz R, Rennke HG, Meyer TW, Anderson S, Brenner BM. Prevention of glomerular capillary hypertension in experimental diabetes mellitus obviates functional and structural glomerular injury. J Hypertens Suppl. 1986;4:S251–S254. [PubMed] [Google Scholar]
- 6.Dworkin LD, Feiner HD, Randazzo J. Glomerular hypertension and injury in desoxycorticosterone-salt rats on antihypertensive therapy. Kidney Int. 1987;31:718–724. doi: 10.1038/ki.1987.57. [DOI] [PubMed] [Google Scholar]
- 7.Hoagland KM, Alonso-Galicia M, Fenoy FJ, Jacob HJ, Garret MR, Roman RJ. Altered blood pressure and renal function in chromosome 5 congenic Dahl salt-sensitive rats. J Hypertens. 2000;18:S10. [Google Scholar]
- 8.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck JR. Afferent arteriolar vasodilation to the sulfonimide analog of 11,12-epoxyeicosatrienoic acid involves protein kinase A. Hypertension. 1999;33:408–413. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 9.Ito O, Alonso-Galicia M, Hopp KA, Roman RJ. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol Renal Physiol. 1998;274:F395–F404. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- 10.Ito O, Roman RJ. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1749–R1757. doi: 10.1152/ajpregu.1999.276.6.R1749. [DOI] [PubMed] [Google Scholar]
- 11.Iversen BM, Amann K, Kvam FI, Wang X, Ofstad J. Increased glomerular capillary pressure and size mediate glomerulosclerosis in SHR juxtamedullary cortex. Am J Physiol Renal Physiol. 1998;274:F365–F373. doi: 10.1152/ajprenal.1998.274.2.F365. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy ET, Sharma R, Sharma M. Protective effect of 20-hydroxyeicosatetraenoic acid (20-HETE) on glomerular protein permeability barrier. Kidney Int. 2005;67:152–156. doi: 10.1111/j.1523-1755.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller DN, Theuer J, Shagdarsuren E, Kaergel E, Honeck H, Park JK, Markovic M, Barbosa-Sicard E, Dechend R, Wellner M, Kirsch T, Fiebeler A, Rothe M, Haller H, Luft FC, Schunck WH. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–532. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer BF. Impaired renal autoregulation: implications for the genesis of hypertension and hypertension-induced renal injury. Am J Med Sci. 2001;321:388–400. doi: 10.1097/00000441-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- 18.Shankland SJ, Ly H, Thai K, Scholey JW. Increased glomerular capillary pressure alters glomerular cytokine expression. Circ Res. 1994;75:844–853. doi: 10.1161/01.res.75.5.844. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-β1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int. 2000;58:131–136. doi: 10.1046/j.1523-1755.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Shatara RK, Quest DW, Wilson TW. Fenofibrate lowers blood pressure in two genetic models of hypertension. Can J Physiol Pharmacol. 2000;78:367–371. [PubMed] [Google Scholar]
- 21.Simons JL, Provoost AP, Anderson S, Rennke HG, Troy JL, Brenner BM. Modulation of glomerular hypertension defines susceptibility to progressive glomerular injury. Kidney Int. 1994;46:396–404. doi: 10.1038/ki.1994.287. [DOI] [PubMed] [Google Scholar]
- 22.Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365–370. doi: 10.1111/j.0894-0959.2004.17346.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 24.Van Dokkum RP, Alonso-Galicia M, Provoost AP, Jacob HJ, Roman RJ. Impaired autoregulation of renal blood flow in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol. 1999;276:R189–R196. doi: 10.1152/ajpregu.1999.276.1.R189. [DOI] [PubMed] [Google Scholar]
- 25.Vera T, Taylor M, Bohman Q, Flasch A, Roman RJ, Stec DE. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension. 2005;45:730–735. doi: 10.1161/01.HYP.0000153317.06072.2e. [DOI] [PubMed] [Google Scholar]
- 26.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 27.Williams JM, Zhao X, Wang MH, Imig JD, Pollock DM. Peroxisome proliferator-activated receptor-α activation reduces salt-dependent hypertension during chronic endothelin B receptor blockade. Hypertension. 2005;46:366–371. doi: 10.1161/01.HYP.0000172755.25382.fc. [DOI] [PubMed] [Google Scholar]
- 28.Wilson TW, Alonso-Galicia M, Roman RJ. Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension. 1998;31:225–231. doi: 10.1161/01.hyp.31.1.225. [DOI] [PubMed] [Google Scholar]
- 29.Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR. 20-Hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem. 2003;11:2803–2821. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol. 2004;286:F720–F726. doi: 10.1152/ajprenal.00165.2003. [DOI] [PubMed] [Google Scholar]
- 31.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]


