Abstract
Purpose:
This study was designed to formulate and evaluate the anti-sebum secretion effects of a topical skin-care cream (w/o emulsion) of sea buckthorn versus its vehicle (Base) as control.
Materials and Methods:
Concentrated sea buckthorn (H.rhamnoides) fruit extract was entrapped in the inner aqueous phase of w/o emulsion. Base containing no extract and a Formulation containing 1% concentrated extract of H.rhamnoides was formulated. Lemon oil was incorporated to the odor. Both the Base and the Formulation were stored at different storage conditions for a period of 4 weeks to predict their stability. Different stability parameters i.e.; physical stability, centrifugation, and pH were monitored at different time intervals. Both the Base and the Formulation were applied to the cheeks of 10 healthy human volunteers (n=10) for a period of 8 weeks.
Result:
The expected organoleptic stability of creams was achieved from 4 weeks in-vitro study period. Odor disappeared with the passage of time due to volatilization of lemon oil. The pH of the Formulation showed significant (P = 0.0002) decline due to high concentration of organic acids present in sea buckthorn. Similarly the Formulation showed statistically significant (P < 0.05) effects on skin sebum secretion.
Conclusion:
The in vitro results showed a good stability over 4 weeks of observation period of both the Base and Formulation and the Formulation has anti sebum secretion effects over 8 weeks of observation period.
Keywords: Acne, emulsion, Hippophae rhamnoides, sebum
Now-a-days herbal extracts are used in the cosmetic preparations for augmenting beauty and attractiveness.[1] Herbal cosmetics are classified on the basis of dosage forms as cream (emulsion), powder, soaps, solutions, etc., and according to the part or organ of the body where it is to be applied as cosmetics for skin, hair, nail, teeth and mouth etc.[2] The use of cosmetics is determined by both their efficacy as well as the minimal risk of skin irritation/skin sensitization that they cause. This is influenced by their Formulation, nature of their use, and quantity and quality of ingredients.[3] Sea buckthorn (Hippophae rhamnoides L.) is a deciduous, dioecious plant with numerous greenish-yellow flowers and bright orange, globular, ellipsoid fruit, belonging to the family Elaeagnaceae.[4] It is native to Europe, India, Nepal, Bhutan, Pakistan, and Afghanistan. Sea buckthorn shrub is 2 m tall with 2–6 cm long leaves.[5] Sea buckthorn juice is an important source of some valuable chemicals such as vitamin C, tocopherol, macronutrients, organic acids, and polyunsaturated fatty acids.[6] It has been used for the treatment of radiation damage, inflammation, burns, eczema, psoriasis, lupus erythematosus, and dermatosus.[7,8] The Formulation of liquid–liquid emulsion is an ordinary practice in food and pharmaceutical industries. Incorporating plant extracts in the form of emulsions is currently drawing more attention in the field of research due to their therapeutic importance.[9] The main advantage of emulsions is that they increase the solubility and bioavailability of therapeutic drugs as well as the ability to favor the topical transport of hydrophilic solute. Topical emulsions also avoid gastrointestinal environment and first pass effect.[10] ABILE EM 90 is suitable for the Formulation of water-in-oil emulsions, w/o/w and o/w/o multiple emulsions. Preparations with ABIL EM90 are highly stable toward heat.[11] Low sebum secretion is considered to be the main feature of fair skin.[12] The skin that is greasy and shiny containing large pores gives an unpleasant appearance. Such a type of skin may be more prone to acne.[13] Increased sebum excretion is one of the main factors involved in the pathophysiology of acne.[14] Acne is the most common skin disease, affecting nearly 80% of people between the ages of 11 and 30.[15] In this study, we formulated a w/o emulsion of H. rhamnoides extract and investigated its effects on the skin sebum secretion over a 8-week study period.
Materials and Methods
Materials
Hippophae rhamnoides berries were purchased from Pak Sea Buckthorn International Skardu, Pakistan. ABIL EM90 was purchased from Franken Chemical, Germany, and methanol, n–hexane, and paraffin oil were purchased from Merk KGaA Darmstadt, Germany. Ethanol was obtained from BDH, England.
Identification of plant
The identification of H. rhamnoides (family: Elaeagnaeae) was performed at Cholistan Institute of Desert Studies (CIDS), The Islamia University of Bahawalpur, Pakistan. The specimen was deposited in the herbarium of Pakistan agricultural research council (PARC), the voucher number is: pharm.3986/Feb 2001.
Apparatus
Centrifuge (Hettich EBA 20, Germany), cold incubator (Sanyo MIR-153, Japan), conductivity meter (WTW COND-197i, Germany), sebumeter MPA 5 (Courage + Khazaka, Germany), digital humidity meter (TES Electronic Corp., Taiwan), electrical balance (Precisa BJ-210, Switzerland), homogenizer (Euro-Star, IKA D 230, Germany), hot incubator (Sanyo MIR-162, Japan), pH meter (WTW pH-197i, Germany), refrigerator (Dawlance, Pakistan), and rotary evaporator (Eyela, Co. Ltd., Japan) were the apparatus used for this study.
Methods
Preparation of extract
Dried fruits of H. rhamnoides were powdered and sequentially extracted in two steps. In the first step, 500 g of the finely ground H. rhamnoides fruit was macerated by a mixture of 2 l of analytical grade methanol and distilled water in the ratio of 1:1 in a glass beaker. The beaker was sealed with aluminum foil and kept at room temperature for 3 days. The beaker was shaken for 10 min after every 12 h. In the second step, the obtained residues were dissolved in 1 l of analytical grade ethanol, n-hexane, and distilled water in a ratio of 1 : 4 : 1. Finally, the macerated material of plant was filtered through 16 layers of muslin cloth for coarse filtration. The coarse filtrate was then filtered through a Whatman # 01 filter paper in order to get particle free extracts. The filtrate obtained in both the steps was evaporated under reduced pressure at 40°C in a rotary vacuum evaporator. The process of evaporation for the first step filtrate was continued till the concentrate reduced to 10% of the initial volume, whereas the process of evaporation for the second step filtrate was continued till the concentrate reduced to 20% of the initial volume. The extracts so obtained were collected in stoppered glass tubes and stored in freezer.
Preparation of emulsions (creams)
In this study, w/o emulsions (Base and Formulation) were prepared by the addition of aqueous phase to the oily phase with continuous agitation.
For the preparation of Formulation, the oily phase consisting of paraffin oil (16%) and the emulsifying agent ABIL EM 90 (5%) was heated up to 70±1°C. The aqueous phase consisting of water (q.s) was heated to the same temperature and then H. rhamnoides extract (1%) was added to it. After this, aqueous phase was added to the oil phase drop by drop with continuous stirring at 2000 rpm by the mechanical mixer for about 15 min until all the aqueous phase was added; few drops of lemon oil were added during the stirring to give a good fragrance. After the complete addition of the aqueous phase, the speed of the mixer was reduced to 1000 rpm for 5 min for homogenization.
Base was prepared by the same procedure but contains no H. rhamnoides fruit extract.
Determination of pH and electrical conductivity
The pH and electrical conductivity values of both freshly prepared emulsion and emulsions kept at various storage conditions were determined with the help of a digital pH and a conductivity meter, respectively.
Stability tests
Stability tests were performed at 8±0.1°C (in refrigerator), 25±0.1°C, 40±0.1°C, and 40±0.1°C with 75% relative humidity (RH) (in incubator). Physical characteristics, i.e., color, creaming, and liquefaction, were noted visually at various intervals for 4 weeks.
Stuy design for product evaluation on skin
One-sided blind study was designed with placebo control in the month of August-September. Ten healthy human volunteers who signed the informed consent, with age in the range 20–35 years were selected. Male volunteers were included in this work as they were easily available with regular under control observations. All the readings were performed at 21±01°C and 40±2% RH conditions.
The experiments were carried out on the cheeks of volunteers. Each volunteer was provided with two creams, Base and Formulation. Each cream was marked ‘right’ or ‘left’ indicating the cheek on which it was applied. The creams were applied by the volunteers themselves, as instructed, for 60 days. Every individual was instructed to come on first, second, third, fourth, sixth, and eighth week for the skin sebum measurements.
Ethical standards
This study was approved by the Board of advance study and Research (BASR), The Islamia University of Bahawalpur and Institutional ethical committee, Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur. The Reference No. is 1663.
Burchard tests (patch tests)
On the first day of skin testing, patch tests were performed on the forearms of each volunteer. A 5 cm × 4 cm region was marked on the forearms. The patch (Bandage disc) for the right forearm was saturated with 1.0 g of Base, whereas the patch for left forearm was saturated with 1.0 g of Formulation. Each of these was applied to the 5 cm × 4 cm marked regions separately on each forearm. The regions were covered with the surgical dressing after application. The patches were removed after 48 h and the forearms were washed with physiological saline.[16] After 48 h, scores were recorded for the presence of erythema (skin redness) using a scale with four points from 0 to 3, where 0 stands for absence of erythema, 1 for mild erythema, 2 for moderate erythema, and 3 stands for severe erythema. Each volunteer was asked to note their irritation/itching toward the patches and then assign a score from the same scale. Average score with respect to volunteers is given in Table 1.
Table 1.
Score given by volunteers to Base and Formulation on the basis of itching/irritation

Panel test
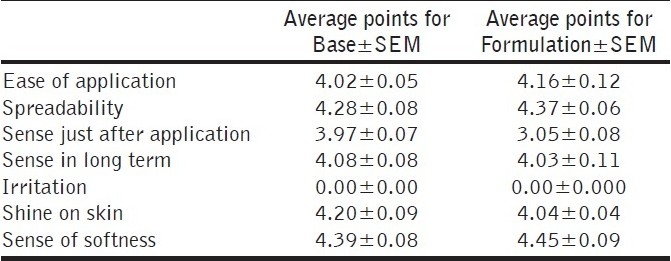
Every individual was provided with a performa prepared previously to test the sensory values of creams. This consisted of seven parameters to be evaluated and every parameter was assigned 11 values from −5 to +5 indicating very bad to very good, respectively. This performa was to be completed independently by each individual at the end of the study period. From the average reply of volunteers it was concluded that Base and Formulation were felt well on the skin, produced a pleasant feeling on application to the skin and produced no irritation on the skin in both the cases, i.e., Base and Formulation, as these were assigned 0.00 point for irritation by all the volunteers. Shine on skin was more for Formulation. This was expected since the Formulation contained essential fatty acids. Similarly, the Formulation led to more softness of the skin than Base.
It was found from paired sample t-test that there was an insignificant difference between the average points of sensitivity for Base and Formulation. It was concluded that there was no immense variation between Base and Formulation regarding the sensory evaluation. Both the creams have similar performance from the sensory point of view [Table 2].
Table 2.
Average values±SEM for panel test by 10 volunteers for Base and Formulation
Mathematical analysis
The percentage changes for the individual values of skin-sebum, taken every week, of volunteers were calculated by the following formula:
percentage change=[(A – B)/B]* …….. (1)
where A is the Individual value of any parameter of first, second, third, fourth, sixth, or eighth week and B is the zero hour value of that parameter.
Statistical analysis
The measured values obtained for pH and sebum secretion were analyzed using SPSS version 12.0 on the PC [paired samples t-test for variation between the two preparations; two-way analysis of variance (ANOVA) for variation between different time intervals while using a 5% level of significance].
Results
Stability testing (centrifugation and phase separation)
Centrifugation tests for Base and Formulation, kept at different storage conditions, were performed using a centrifuge for a period of 4 weeks at different time intervals. No phase separation after centrifugation was found in any of the samples of Base and Formulation kept at 8, 25, and 40°C + 75% RH. However, slight phase separation was observed for the samples of Formulation and Base kept at 40°C after 21 days time period.
pH tests
The pH values of freshly prepared Base and Formulation were 5.61 and 5.18, respectively. Average changes in pH values of Base and Formulation, kept at various storage conditions, occurring from the time of preparation up to fourth week of study period have been determined and are shown in Table 3. The pH values were noted immediately after preparation and then after 12,24,36,48 and 72 h and then first, second, third and fourth weeks.
Table 3.
Average pH values of Base and Formulation kept at 8, 25, 40, and 40°C + 75% RH for a period of 28 days

Skin sebum
Skin sebum secretion was measured before application of creams (0 h readings) and then at first, second, third, fourth, sixth, and eighth weeks of study period by Sebumeter MPA 5 (Courage and Khazaka GmbH Germany). The percent changes occurring in the values for 10 volunteers were calculated, using Equation 1 and they are presented in Table 4.
Table 4.
Percentage of change in skin sebum secretion after application of Base and Formulation for 10 volunteers from 0 h up to eighth week of study period

Discussion
Stability testing
In this work, Base and Formulation were divided into four samples separately and kept at different storage conditions, i.e., at 8°C in a refrigerator, at 25, 40, and 40°C + 75% RH in stability chambers, to study the five main mechanisms that contribute to emulsion instability, i.e., (1) creaming (2) flocculation (3) Ostwald ripening (4) coalescence, and (5) phase separation. The samples at different storage conditions were observed for a period of 28 days at definite time intervals. The samples were observed with respect to change in color, liquefaction, and phase separation.
Color
The freshly prepared Base and Formulation were colored white and yellowish, respectively. No color change was observed in both Base and Formulation during the stability period of 28 days. This showed that the emulsions were stable at different storage conditions, i.e. 8, 25, 40, and 40°C + 75% RH during the 28-day period.
Liquefaction
A slight liquefaction was observed in Base and Formulation kept at 40°C on 14th, 21st, and 28th days of the study period, whereas no liquefaction was observed in both Base and Formulation kept at 8, 25, and 40°C + 75% RH during the whole study period of 28 days.
Phase separation
All the samples of Base and Formulations were stable at all storage conditions, i.e., 8, 25, 40, and 40°C + 75% RH throughout the study period of 28 days.
No oily phase separation was observed even at higher temperatures during the study period. This may be attributed to a number of stability factors such as using ABIL EM 90, a heat stable emulsifying agent.[12]
Centrifugation test
In this study centrifugation test was performed for the samples of Base and Formulation kept at different storage conditions up to a period of 28 days at definite time intervals. No phase separation on centrifugation was observed in any of the samples kept at different storage conditions, i.e., 8, 25, 40, and 40°C + 75% RH up to 28thday of observation. This indicated that the emulsions were stable at all the storage conditions for 28 days. It may be due the proper homogenization speed during emulsion that the Formulation prevented the Base and the Formulation breakage during stress testing.[17]
pH test
In this work, the pH values of freshly prepared Base and Formulation were 5.61 and 5.18, respectively, which is within the range of skin pH. The pH values of the samples of Base kept at different storage conditions, i.e., 8, 25, 40, and 40°C + 75% RH were found to show slight reduction continuously till the 28th day with some variations. At the end of study, pH values of the samples of Base at 8, 25, 40, and 40°C + 75% RH were 4.98, 4.84, 4.70, and 5.10, respectively. Whereas pH values of the samples of Formulation kept at 8, 25, 40, and 40°C + 75% RH showed a reasonable reduction till the end of study period. The pH values of samples of Formulation kept at 8, 25, 40, and 40°C + 75% RH at the end of study period were 4.28, 4.07, 3.98, and 4.05, respectively.
By applying two-way ANOVA at 5% level of significance, it was found that the change in pH of different samples of Base and Formulation was significant (P< 0.05) at different levels of both time and temperature. When List Significant Different (LSD) test was performed to check the individual average effects of the pH of samples of Base at different temperatures with the passage of time by taking average pH values of Zero hour at different temperatures as standard, it showed significant (P< 0.05) changes. Again, when LSD test was performed to check the individual average effect of the pH of samples of Formulation at different temperatures with the passage of time by taking average pH values of 0 h at different temperatures as standard, it showed significant (P< 0.05) changes.
Acidic species were produced as the juice of H. rhamnoides has high concentration (30–36 mg/1000 g) of organic acids such as quinic acid (18–19 g/1000 gm), due to which the Formulation showed reasonable decline in pH with passage of time.[18]
Skin sebum content
In this research work, the creams were evaluated for skin sebum secretion. Sebum was measured every week for 8 weeks. It was concluded that the Base has a variable increasing tendency on skin sebum, whereas the Formulation decreased sebum secretion regularly throughout the study period.
With the help of ANOVA it was found that there was an insignificant (P=0.843) effect of Base with respect to time, whereas in the case of Formulation it was evident that it has significant (P=0.000) effect with respect to time .When the paired sample t test was applied it was found that the Base and Formulation showed significant (P< 0.05) variations with regard to the skin sebum secretion except second and third weeks.
Type 1-α reductase converts testosterone into more potent dihydrotestosterone, which results in the enlargement of sebaceous gland, leading to secretion of high level of sebum.[19] The polyphenol plant extract regulates the extreme sebum secretion. Topically applied oleic and linoleic acids have proved to inhibit type 1-α reductase.[13] Polyphenols in H. rhamnoides include flavonols, catechins, proanthocyanidins, and chlorogenic acids, whereas the main fatty acids of H. rhamnoides are palmitoleic acid, palmitic acid, linoleic acid, and oleic acid. Thus, the Formulation regulated sebum secretion by inhibiting type 1-α reductase.[20,21]
Conclusion
A stable w/o emulsion containing the extract of H. rhamnoids can be formulated. It was observed that the Formulation produced a pronounced decrease in sebum secretion of the skin showing that the Formulation has antiacne effects as excess sebum is one of the most contributable causes of acne.[22] An antiacne targeted study needs to be conducted in future.
Acknowledgments
The authors thanks the Higher Education Commission of Pakistan for financial support and The Department of Pharmacy, The Islamia University of Bahawalpur, for moral support.
Footnotes
Source of Support: The Higher Education Commission of Pakistan,
Conflict of Interest: None declared.
References
- 1.Mukherjee PK. Evaluation of Indian traditional medicine. J Drug Inf. 2002;35:631–40. [Google Scholar]
- 2.Kalia AN. Text book of Industrial Pharmacognosy. 2nd ed. New Delhi: CBS Publishers; 2005. Herbal Cosmtics. [Google Scholar]
- 3.Badiu D, Roncea F, Rosoiu N. Formulation and pharmaceutical evaluation of three w/o emulsions with mythilus galloprovincialis and rapana venosa lipid extracts. Farmacia. 2009;57:212–7. [Google Scholar]
- 4.Heber D. PDR for Herbal Medicines. 4th ed. Montvale: Thomson Healthcare; 2007. [Google Scholar]
- 5.Rizvi MA, Saeed A, Zubairy N. 1st ed. Karachi: Hamdard Institute of Advance Studies and Research; 2007. Medicinal plants History, Cultivation and Uses. [Google Scholar]
- 6.Zeb A. Chemical and nutritional constituents of sea buckthorn juice. Pak J Nutr. 2006;3:99–106. [Google Scholar]
- 7.Negi PS, Chauhan AS, Sadia GA, Rohinishree YS. Antioxidant and antibacterial activities of various sea buckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92:119–24. [Google Scholar]
- 8.Guliyev VB, Gul M, Yildirim A. Hippophae rhamnoides L: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effect. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:291–307. doi: 10.1016/j.jchromb.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Maa YF, Hsu C. Liquid-liquid Emulsification by Rotor/stator homogenization. J Control Release. 1996;38:219–28. [Google Scholar]
- 10.Marti-Mestres G, Nielloud F. Emelsion in health care applications-An overview. J Dispers Sci Technol. 2002;23:419–39. [Google Scholar]
- 11.Evonik ABIL® EM90, Emulsifier for the formulation of cosmetic w/o creams and lotions. 2008. [last cited on 2009 Apr 10]. Available from: http://www.evonik.com/personal-care .
- 12.Cheng Y, Dong Y, Wang J, Dong M, Zou Y, Ren D, et al. Moisturizing and anti-sebum secretion effects of cosmetic application on human facial skin. J Cosmet Sci. 2009;60:7–14. [PubMed] [Google Scholar]
- 13.Dobrev H. Clinical and instrumental study of efficacy of a new sebum control cream. J Cosmet Dermatol. 2007;6:113–8. doi: 10.1111/j.1473-2165.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;12:360–6. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Leyden JJ. Therapy for acne vulgaris. N Engl J Med. 1997;336:1156–62. doi: 10.1056/NEJM199704173361607. [DOI] [PubMed] [Google Scholar]
- 16.Rowe RC, Sheskey PJ, Weller PJ. Handbook of Pharmaceutical Excipients. 4th ed. London: Pharmaceutical Press; 2003. Pharmacokinetics and Drug Disposition. [Google Scholar]
- 17.Nour AH, Yunus RM. Stability investigation of water-in-crude oil emulsion. J Appl Sci. 2006;6:2895–900. [Google Scholar]
- 18.Arimboor R, Venugoplan R, Sarinkumar K, Arumughan C, Sawhney RC. Integrated processing of fresh Indian sea buckthorn (hippophae rhamnoides) berries and chemical evaluation of products. J Sci Food Agric. 2006;86:2345–53. [Google Scholar]
- 19.Mehling A, Buchwald-Werner S. Natural actives for impure skin. SOFW J. 2004;130:2–6. [Google Scholar]
- 20.Rösch D, Bergmann M, Knorr D, Kroh LW. Structure-antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J Agric Food Chem. 2003;51:4233–9. doi: 10.1021/jf0300339. [DOI] [PubMed] [Google Scholar]
- 21.Cakir A. Essential oil and fatty acid composition of the fruit of Hippophae rhamnoides L.(sea Buckthorn) and Myyrtus communis L, from Turkey. Biochem Syst Ecol. 2004;32:809–16. [Google Scholar]
- 22.Kim MK, Choi SY, Byun HJ, Huh CH, Park KC, Patel RA, et al. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch Dermatol Res. 2006;298:113–9. doi: 10.1007/s00403-006-0666-0. [DOI] [PubMed] [Google Scholar]


