Abstract
Objectives:
The aim of this study was to investigate the antidiabetic activity of Crateva nurvala stem bark (family: Capparidaceae) extracts in alloxan-induced diabetic albino rats. A comparison was made between the action of different extracts of C. nurvala and a known antidiabetic drug glibenclamide (600 μg/kg b. wt.). An oral glucose tolerance test (OGTT) was also performed in diabetic rats.
Materials and Methods:
The petroleum ether, chloroform, alcohol, and aqueous extracts of C. nurvala stem bark were obtained by simple maceration method and were subjected to standardization by following pharmacognostical and phytochemical screening methods. Dose selection was made on the basis of acute oral toxicity study (50–5000 mg/kg b. wt.) as per Organization for Economic Co-operation and Development (OECD) guidelines.
Results and Conclusions:
C. nurvala petroleum ether extract (CNPEE) and ethanolic extract (CNEE) showed significant (P< 0.001) antidiabetic activities. In alloxan-induced model, blood glucose level of these extracts on seventh day of study were CNPEE (126.33±13.703 mg/dl) and CNEE (126.66±13.012 mg/dl) when compared with diabetic control (413.50±4.752 mg/dl) and chloroform extract (320.83±13.516 mg/dl). In OGGT model (glucose loaded rats), CNPEE showed a glucose level of 178.83±3.070 mg/dl after 30 min and 131.66±2.486 mg/dl after 90 min, whereas CNEE showed 173.66±4.224 mg/dl after 30 min and 115.50±3.394 mg/dl after 90 min. These extracts also prevented body weight loss in diabetic rats. The drug has the potential to act as an antidiabetic drug.
Keywords: Alloxan, antidiabetic activity, acute toxicity, Crateva nurvala, phytochemical
Diabetes mellitus is a serious complex chronic condition that is a major source of ill health worldwide. This metabolic disorder is characterized by hyperglycemia and disturbances of carbohydrate, protein, and fat metabolisms, secondary to an absolute or relative lack of the hormone insulin. Besides hyperglycemia, several other factors including dislipidemia or hyperlipidemia are involved in the development of micro and macrovascular complications of diabetes that are the major causes of morbidity and death.[1] According to WHO projections, the prevalence of diabetes is likely to increase by 35%. Currently, there are over 150 million diabetic patients worldwide and this is likely to increase to 300 million or more by the year 2025. Statistical projection about India suggests that the number of diabetics will rise from 15 million in 1995 to 57 million in the year 2025, the highest number of diabetics in the world.[2] Reasons for this rise include increase in sedentary lifestyle, consumption of energy-rich diet, obesity, higher life span, etc. Other regions with greatest number of diabetics are Asia and Africa, where diabetes mellitus rates could rise to twofold to threefold than the present rates.[3]
Evaluation of plant products to treat diabetes mellitus is of growing interest as they contain many bioactive substances with therapeutic potential. In recent years, several authors evaluated and identified the antidiabetic potential of traditionally used Indian medicinal plants using experimental animals. Previous studies confirmed the efficacy of several medicinal plants in diabetes mellitus. Although a large number of medicinal plants have been already tested for their antidiabetic effects, these effects remain to be investigated in several other Indian medicinal plants.[4]
Crateva nurvala (family: Capparidaceae) is a small tree with a much branched head. Leaves are deciduous three foliolate; petioles 3.8–7.6 cm long; leaflets 5–15 ovate, lanceolate or obovate, acute or acuminate, attenuate at the base, entire, glabrous on both surfaces, pale beneath, and reticulately veined. It is usually cultivated in the vicinity of temples in Central India, Bengal, and Assam. Its bark is hot, tasting bitter at first and then sharp sweet, easy to digest, stomachic, laxative, antilithic, anthelmintic, expectorant, and antipyretic. Researches have shown that its bark contains saponins which are especially useful in urinary complaints such as kidney and bladder stones.[5–6]
To our best knowledge, no scientific data regarding the antidiabetic effect of C. nurvala stem bark are available except in the treatise of Ayurvedic medicine. Thus, the present study was undertaken to evaluate the antidiabetic effect of C. nurvala bark in alloxan-induced diabetic rats.
Materials and Methods
Animals
Adult Albino rats of wistar strain (150–200 g) of either sex were procured from Government Veterinary College, Bangalore, and were housed in the animal house of KLES College of Pharmacy, Ankola, with 12 h light and 12 h dark cycles. Standard pellets obtained from Goldmohar rat feed, Mumbai, were used as a basal diet during the experimental period. The control and experimental animals were provided food and drinking water ad libitum.
Chemicals
The chemicals used in the study were: alloxan monohydrate (Spectrochem Pvt. Ltd, Mumbai), glibenclamide (Aventis Pharma Ltd, Verna, Goa), dextrose (Emkay Labs, Mumbai), Tween 80 (S.D. Fine-chem Ltd, Mumbai), and anesthetic ether (Ozone International, Mumbai).
Accu-chek ®active glucometer (Roche Diagnostic Corporation, Mannheim, Germany), blood and glucostrips (Roche Diagnostic Pvt Ltd, Mumbai) were used .All other chemicals and reagents used were of analytical grade.
Plant material
Stem bark of C. nurvala was collected in and around the local forest area of Sirsi in Western Ghats, Karnataka, and authenticated by the Botanist Prof G. S. Naik, Department of Botany, G. C. Science and Art College, Ankola. A voucher herbarium specimen No. GCSAC/CN/01 was also preserved in the same college. The collected bark was dried under shade and powdered to a coarse consistency in grinder mill. The powder was passed through 40 # mesh particle size and stored in an airtight container at room temperature.
Preparation of plant extract
Exactly 2.5 kg of the fresh air-dried, powered crude drug of C. nurvala was extracted with petroleum ether (60–80°C), chloroform, 95% ethanol, and chloroform water I. P. by adopting simple maceration procedure at room temperature for 7 days in a conical flask with occasional shaking and stirring. The extract was filtered and concentrated to dryness at room temperature to avoid the decomposition of the natural metabolites.[7] The yield of the extracts was 1.46, 3.62, 10.65, and 20.45% w/w for petroleum ether, chloroform, ethanol, and water, respectively. All the extracts were preserved in a refrigerator till further use. Preliminary phytochemical analysis was carried out in all the four extracts by different methods of phytochemical analysis.[8] A known volume of extract was suspended in distilled water and was orally administered to the animals by gastric intubation using a force feeding needle, during the experimental period.
Acute oral toxicity studies
Acute oral toxicity studies[9] of the extracts were carried out as per the OECD guidelines, draft guidelines 423 adopted on 17th December, 2001, received from Committee for the Purpose of Supervision and Control of Experiments on Animals (CPCSEA), Ministry of social justice and empowerment, Govt. of India. Administration of the stepwise doses of all four extracts of C. nurvala from 50 mg/kg b. wt. up to the dose 5000 mg/kg b. wt. caused no considerable signs of toxicity in the tested animals. One tenth of upper limit dose were selected as the level for examination of antidiabetic activity.
Experimental models
Oral glucose tolerance test (OGTT)
Fasted rats were divided into six groups of six rats each. Group I served as normal control (NC) and received distilled water with Tween 80. Groups II received standard drug glibenclamide as an aqueous suspension at a dose of 600 μg/kg b. wt. Groups III–VI received different extracts at a dose of 500 μg/kg b. wt. as a fine Tween 80 suspension. After 30 min of extract administration, the rats of all groups were orally treated with 2 g/kg of glucose. Blood samples were collected from the rat tail vein just prior to glucose administration and at 30, 60, and 90 min after glucose loading. Blood glucose levels were measured immediately with the glucometer.[10]
Alloxan-induced diabetic model
Alloxan monohydrate was first weighed individually for each animal according to their weight and then solubilized with 0.2 ml saline just prior to injection. Diabetes was induced by injecting it at a dose of 150 mg/kg b. wt. intraperitonially.[11] After 1 h of alloxan administration, the animals were given feed ad libitum, and 5% dextrose solution was also given in a feeding bottle for a day to overcome the early hypoglycemic phase. The animals were kept under observation and after 48 h blood glucose was measured by glucometer. The diabetic rats (glucose level >300 mg/dl) were separated and divided into six different groups for experimental study, with each group containing six animals.
Experimental design
The animals were divided into seven groups and each group consisted of six rats [Table 1].
Table 1.
Different groups of experimental animals (n=6)
This experimental design was followed in both alloxan-induced model and in OGGT model, but in the latter model normal rats loaded with glucose were used instead of diabetic rats.
Body weight measurement
Body weight was measured totally four times during the course of study period[12] [i.e., before alloxan induction (initial values), and on the first, fourth, and seventh days of the treatment period], using a digital weighing scale obtained from KERN (EMB), Tischwaage, Germany.
Statistical analysis
The results of the study were subjected to one way analysis of variance (ANOVA) followed by Dunnett's t test for multiple comparisons. Values with P< 0.05 were considered significant.
Results and Discussion
Standardization parameters for C. nurvala stem bark were determined and all the parameters were found to be within pharmacopoeial standards limit. Crude powder taken for extraction was of yellowish-brown color with a bitter taste. Loss on drying, total ash, acid insoluble ash, and water soluble ash were found to be 4.37, 9.76, 0.647, and 1.56% w/w, respectively. Thin layer chromatography of C. nurvala stem bark revealed bright brownish red spot (Rf= 0.30), Rf= 0.71 (magenta color), Rf= 0.15, 0.90 (light violet spots) which turn magenta on keeping.
Phytochemical screening of all the extract of C. nurvala showed the presence of various chemical constituents, mainly triterpenoids and flavonoids which may be responsible for its antilithic and antidiabetic properties, respectively. The results obtained were comparable and satisfied the standard literature. In acute toxicity study, all the extracts of C. nurvala stem bark did not show significant toxicity signs when observed for the parameters during the first 4 h and followed by daily observations for 14 days and no mortality was also observed; the drug was found to be safe at the tested dose level of 5000 mg/kg b. wt. To ascertain a scientific base for the usefulness of this plant in the treatment of diabetes, it was decided to evaluate experimental design of antidiabetic activity by following glucose tolerance test and the alloxan-induced model. As expected, in the diabetic control (DC), there was severe hyperglycemia when compared with the normal animals. Compared with the DC, all the four extracts, C. nurvala aqueous extract (CNAE), C. nurvala ethanolic extract (CNEE), C. nurvala chloroform extract (CNCE), and C. nurvala petroleum ether extract (CNPEE) [Table 1], lowered the elevated blood glucose levels only in subacute treatment [Table 2]. It was observed that the standard drug glibenclamide lowered the blood glucose level significantly bringing it nearly back to normal, whereas CNPEE and CNEE significantly (P< 0.01) decreased fasting blood serum glucose in the diabetic rats on third, fifth, and seventh days compared with the initial (0 h) blood serum glucose levels [Figure 1].
Table 2.
Effect of C. nurvala extracts on blood glucose level of alloxan-induced diabetic albino rats after subacute treatment
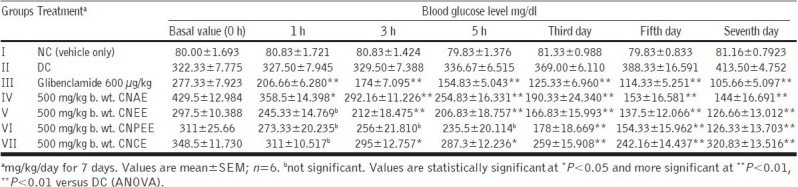
Figure 1.
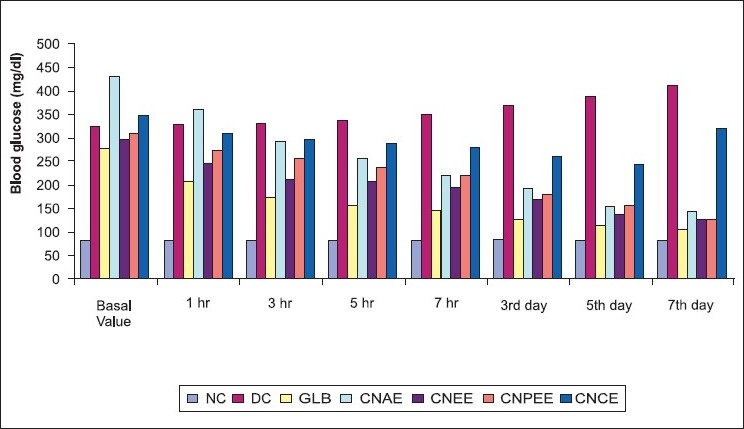
Blood glucose level of alloxan-induced diabetic albino rats after treatment
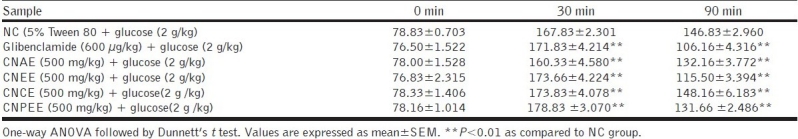
The effects of different extracts on glucose tolerance test in normal rats are shown in Table 3. At 30 min after glucose administration, the peak of blood glucose level increased rapidly from the fasting value and then subsequently decreased. CNEE and CNPEE exhibited remarkable blood glucose lowering effect at 90 min.
Table 3.
Effect of C. nurvala extracts on OGTT in normal rats
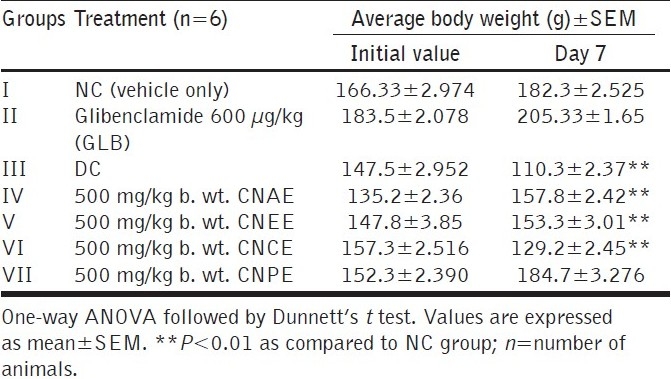
In the present study, diabetic rats had lower body weights, high blood glucose level as compared to the normal rats. In spite of the increased food consumption, loss of body weight due to defect in glucose metabolism and excessive breakdown of tissue protein is a characteristic condition in diabetics. As shown in Table 4, treatment with CNEE and CNPEE improved the average body weights of rats which indicate control over polyphagia and muscle wasting resulted due to hyperglycemic condition.
Table 4.
Effect of various extracts on body weight after treatment in diabetic rats
Alloxan causes massive reduction in insulin release, through the destruction of β-cells of the islets of Langerhans. In our study, we have observed a significant increase in the plasma insulin level when alloxan diabetic rats were treated with CNAE and CNPEE. This could be due to potentiation of the insulin effect of plasma by increasing the pancreatic secretion of insulin from existing β-cells of islets of Langerhans or its release from bound insulin. The significant and consistent antidiabetic effect of CNEE and CNPEE in alloxan diabetic rats may also be due to enhanced glucose utilization by peripheral tissues.
Few researchers such as Alam et al. have reported the antinociceptive effect of the crude ethanolic extract of C. nurvala on mice and showed that its action was peripherally and centrally mediated. Bhaskar et al. have reported the antifertility activity of stem bark of C. nurvala and that their action was due to antizygotic and blasocytotoxicity.[13,14] Neither the exact biological active constitutent(s) responsible for the above said effect nor the exact mode of action of the antidiabetic activity was reported earlier, with the lone observation that it is used in folklore diabetic treatments.
Conclusion
We conclude that the CNEE and CNPEE have potent antidiabetic effects in alloxan-induced diabetic rats. The present investigation has also opened avenues for further research especially with reference to the development of potent formulation for diabetes mellitus from C. nurvala stem bark. Activity guided fractionation, formulation, and its evaluation is in progress and will be available in short period of time.
Acknowledgments
The authors are grateful to Prof G. S. Naik, Department of Botany, G. C. Science and Art College, Ankola, for authenticating the plant material.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Kameswararao B, Kesavulu MM, Apparao C. Evaluation of antidiabetic effect of Momordica cymbalaria fruit in alloxan-diabetic rats. Fitoterapia. 2003;74:7–13. doi: 10.1016/s0367-326x(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 2.Satyanarayana T, Katyayani BM, Hemalatha E, Anjana AM, Chinna EM. Hypoglycemic and antihyperglycemic effect of alcoholic extract of Euphorbia leucophylla and its fractions in normal and in alloxan induced diabetic rats. Pharmacog Mag. 2006;2:244–53. [Google Scholar]
- 3.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Punitha R, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Pongamia pinnata (Linn.) Pierre flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2006;105:39–46. doi: 10.1016/j.jep.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Kirtikar KR, Basu BD. 2nd ed. Vol. 1. Dehradun: International book publisher; 2005. Indian medicinal plants; pp. 190–2. [Google Scholar]
- 6.Nadkarni KM. Vol. 1. Mumbai: Popular Prakashan Pvt; 2005. Indian Materia Medica; pp. 387–8. [Google Scholar]
- 7.New Delhi: Ministry of Health, Government of India; 1982. Pharmacopoeia of India; pp. 948–650. [Google Scholar]
- 8.Khandewal KR. 14th ed. Pune: Nirali Prakashan; 2005. Practical Pharmacognosy; pp. 146–57. [Google Scholar]
- 9.OECD/OCDE, OECD Guidelines for the testing of chemicals, revised draft guidelines 423: Acute Oral toxicity- Acute toxic class method, revised document, CPCSEA, Ministry of Social Justice and Empowerment, Govt. of India. 2000 [Google Scholar]
- 10.Sellamuthu PS, Muniappan BP, Perumal SM, Kandasamy M. Antihyperglycemic effect of mangiferin in streptozotocin induced diabetic rats. J Health Sci. 2009;55:206–14. [Google Scholar]
- 11.Kannur DM, Hukkeri VI, Akki KS. Antidiabetic activity of Caesalpinia bonducella seeds extracts in rats. Fitoterapia. 2006;77:546–9. doi: 10.1016/j.fitote.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Nagappa AN, Thakurdesai PA, Venkat Rao N, Singh J. Antidiabetic activity of Terminalia catappa linn fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 13.Alam MA, Haque ME. Anticociceptive effect of the crude ethanolic extract of Crateva nurvala buch on mice. Bangl J Vet Med. 2006;4:65–8. [Google Scholar]
- 14.Bhaskar VH, Profulla KM, Balakrishnan BR, Balakrishnan N, Sangameswaran B. Evaluation of the anti-fertility activity of stem bark of Crataeva nurvala buch. Afr J Biotech. 2009;8:6453–56. [Google Scholar]


